Abstract
Background: Cardiac involvement is common in Fabry disease (FD) and typically manifests with left ventricular hypertrophy (LVH). Patients with FD are frequently misdiagnosed, and this is mainly related to the lack of disease awareness among clinicians. The aim of this study was to determine whether providing a targeted educational intervention on FD may enhance FD diagnosis. Methods. This research was designed as a single-arm before-and-after intervention study and evaluated the impact of providing a specific training on FD to cardiologists from different Italian centers, without experience in rare diseases. In the 12-month period after the educational intervention, the rate of FD screening and diagnosis was assessed and compared with those conducted in the two years preceding the study initiation. Results: Fifteen cardiologists participated to this study, receiving a theoretical and practical training on FD. In the two previous two years, they conducted 12 FD screening (6/year), and they did not detect any cases of FD. After the training, they performed 45 FD screenings, with an eight-fold rise in the annual screening rate. The screened population (age: 61 ± 11 years, men: 82%) was mainly composed of patients with unexplained LVH (n = 43). There were four new FD diagnoses and, among of them, three had a late-onset GLA variant. After the cascade genetic screening, 11 affected relatives and 8 heterozygous carriers were also detected. Conclusions: A targeted educational intervention for cardiologists allowed the identification of four new families with FD. Enhancing FD awareness is helpful to reduce the diagnostic and therapeutic delay.
1. Introduction
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by mutations in the gene encoding the enzyme a-galactosidase A, resulting in intracellular accumulation of globo-triaosylceramide in different organs, including the heart [1].
At cardiac level, the main phenotypic expression of FD is the development of progressive left ventricular hypertrophy (LVH), which may be the only phenotypic manifestation, mimicking hypertrophic cardiomyopathy (HCM) [2,3].
FD is a rare disease with an incidence reported to range from 1/117.000 to 1/40.000, but these numbers likely underestimate the burden of disease [4,5,6,7]. Registry and cohort studies demonstrate that FD patients are often mis-diagnosed with other disorders, experiencing diagnostic delays of many years [4,5,6,7]. An early diagnosis of FD is a key goal since the beneficial effects of disease-specific therapy critically depend on the stage of the disease in which the treatment is started, with little or no effect in patients with advanced cardiomyopathy or end-stage renal disease [8,9,10].
Contributing factors to the diagnostic delay include not only the rarity of FD and the heterogeneous clinical features, but also the lack of awareness among the wide range of specialists to which patients may initially present [11,12,13]. Thus, targeted educational interventions for health professionals may be a valuable tool to enhance diagnosis of FD and optimize timing of therapeutic interventions [11,12,13].
In this study, we aimed to examine whether providing targeted information on FD to cardiologists with no or little experience of rare diseases and tutoring them in the evaluation of patients with unexplained LVH may improve the diagnostic pathway of FD.
2. Materials and Methods
The present research is a single-arm before-and-after intervention study, promoted and coordinated by the Fondazione Policlinico A. Gemelli IRCCS, Rome.
The study was conducted evaluating the changes in the rate of screening and diagnostic performed by a group of Italian cardiologists, after receiving an educational intervention on FD. These physicians were selected from different Italian spoke centers, without outpatient clinic dedicated to rare diseases, including FD.
At the time of study initiation, we determined the numbers of FD screening and diagnosis conducted by participant cardiologists in the past 2 years.
2.1. Educational Intervention on FD
The training course on FD was articulated in two phases:
- Theoretical phase: this phase was based on 3 online, interactive lessons on FD, performed over one month (each of them lasting 3 h), aimed at raising the disease awareness. More specifically, the topics of the webinars were the following:
- -
- Lesson n. 1: Molecular genetics, epidemiology, clinical presentation, diagnostic work-up and screening and multiorgan involvement.
- -
- Lesson n. 2: Electrocardiographic, echocardiographic, and cardiac magnetic resonance (CMR) characteristics of Fabry cardiomyopathy and its pharmacological management (specific and support therapy).
- -
- Lesson n. 3: Differential diagnosis of Fabry cardiomyopathy with HCM and other HCM phenocopies.
- Tutored phase: Cardiologists have been supported in their daily clinical practice for 5 months. An expert cardiologist from the promotor institution (“Tutor”) was available by video calls for 6 h/5 days a week. They provided interpretative assistance on electrocardiograms (ECG) and echocardiograms when requested and supported the diagnostic work-up of suspected FD cases.
2.2. Active Phase of the Study
After the educational intervention, the active phase of the study started. This phase lasted 12 months and was both prospective and retrospective. The prospective part included disease’ screening performed on patients evaluated for the first time at the participating centers during this period. For the retrospective part, physicians have been invited to check their clinical database and/or outpatient lists, looking for patients with features suggestive of FD.
More specifically, they have been encouraged to screen high-risk patients [1,3], as follows:
- -
- Patients with LVH of unknown etiology (diagnosed in or after the 3rd decade of life) defined by a left ventricular maximum wall thickness (LVMWT) ≥ 13 mm measured on echocardiography or CMR, in absence of male-to-male transmission and co-existent pathologies that could explain the increased LV wall thickness (hypertension, aortic stenosis);
- -
- Patients with LVH (diagnosed in or after the 3rd decade of life) and ECG findings frequently encountered with Fabry cardiomyopathy (short PR interval, right bundle branch block [RBBB], high QRS voltage and remarkable repolarization abnormalities);
- -
- Patients with unexplained LVH (diagnosed in or after the 3rd decade of life) and history of chronic kidney disease (CKD), ischemic stroke or clinical characteristics compatible with FD systemic involvement.
Clinical, electrocardiographic, and echocardiographic data of screened patients were anonymized and collected.
2.3. Diagnostic Kits
The screening of suspected FD patients was conducted by dried filter-paper blood spots (DBSs), as in current clinical practice. Sample analyses were centralized (Centogene, Germany) and included the quantification of blood alfa-galactosidase A enzyme activity and GLA genotyping. In agreement with current recommendations [3], genetic analysis of the GLA gene was performed in male patients with low α-galactosidase A activity and for all women. All patients signed an informed consent for genetic testing.
2.4. Study Endpoint
The power of the targeted information was evaluated through the comparison of annual rates of FD screening and diagnosis performed during the active phase of the study versus those conducted in the two years before the study initiation. The Wilcoxon test was used to evaluate the changes in the annual screening rate following the educational intervention. The test was two-sided, and p values < 0.05 were considered statistically significant.
3. Results
A total of fifteen cardiologists from different Italian institutions participated in the present study and received the educational intervention on FD. In the two years prior to the study, these physicians conducted a total of 12 screenings (6/year), and they did not detect any new diagnoses of FD (as illustrated in Figure 1).
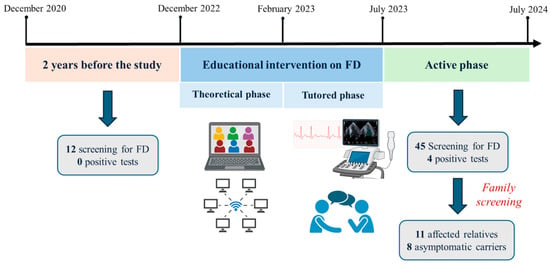
Figure 1.
Study timeline: The impact of educational intervention on FD screening and diagnostic rates. Created in BioRender. Lillo, R. (2025) https://BioRender.com/fez7x5p. Abbreviations. FD: Fabry disease.
3.1. Screened Population
From July 2023 to July 2024 (active phase), the cardiologists performed FD screening on 45 patients, resulting in an eight-fold increase in the annual screening rate in comparison to the two years before the educational intervention (p = 0.018).
The main clinical and echocardiographic features of the screened population (age: 61 ± 11 years, men: 82%) are reported in Table 1. The screenings were all conducted in patients prospectively evaluated by the participant cardiologists, while they did not perform screening on retrospective cases. The testing was performed in patients with (1) LVH due to unknown etiology or judged as disproportionate compared with well-controlled arterial hypertension (n = 43) and (2) in those with mildly increased LV wall thickness (LVMWT between 10 and 13 mm) associated with a typical pattern of late gadolinium enhancement (involving the inferolateral segments and with a mid-wall pattern) on CMR (n = 2). A total of 40% of patients had a history of hypertension, while 36% had CKD (defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2).

Table 1.
Characteristics of the population screened for FD.
3.2. New Diagnosis of Fabry Disease
The screening allowed the identification of four index cases of FD (two men and two women), with a rate of positive screening of 8.8%. The characteristics of patients with FD are reported in Table 2. After the diagnosis, patients were referred for treatment initiation based on current international recommendations [14]. Decisions regarding disease-specific treatment strategies (ERT/chaperone therapy) were made on an individual basis, after multidisciplinary discussion, taking into account several factors including age, gender, disease phenotype and features, renal function, and patient preference.

Table 2.
Characteristics of the patients diagnosed with FD.
Patient 1 was a 70-year-old man admitted to the hospital with fatigue and shortness of breath. The 12-lead electrocardiogram showed a rhythm of atrial fibrillation complicated with a third-degree atrioventricular block and a ventricular rate of 30 beats per minute (Figure 2A), which required an urgent dual-chamber pacemaker implantation. The echocardiographic assessment revealed a severe LVH with a concentric pattern and an LVMWT of 20 mm at the level of the interventricular septum (Figure 2B), preserved left ventricular ejection fraction, severe left atrial enlargement, right ventricular hypertrophy and mildly reduced right ventricular function. The DBS showed a severe reduction in α-galactosidase A activity, and the gene sequencing identified a hemizygous pathogenic variant in the GLA gene: c.644A>G (p. Asn215Ser). CMR (performed with a 6 -week delay after pacemaker implantation) revealed decreased myocardial native T1 values and the presence of LGE in the infero-lateral wall with a subepicardial pattern. After the exclusion of extensive myocardial fibrosis [14], he started enzymatic replacement therapy (ERT).
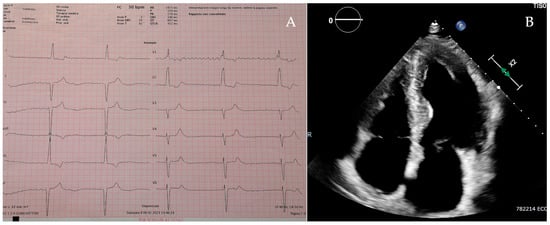
Figure 2.
Patient 1’s electrocardiogram (A) and apical 4-chamber view on the echocardiogram (B).
Genetic testing of the at-risk family members (Figure 3) allowed identification of six relatives affected (five men and one woman), which consequently also initiated disease-specific therapy (five cases of ERT and one of chaperone therapy), and five heterozygous carriers with no signs or symptoms of disease involvement, who are on active surveillance.
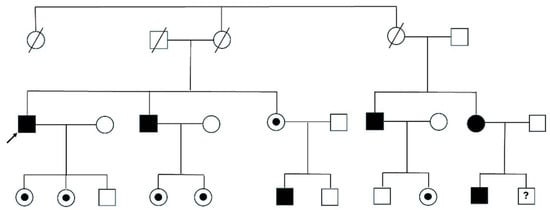
Figure 3.
Pedigree tree of patient 1. Legend. Circles are women and squares are men. Filled symbols are affected patients, while dots refer to heterozygous carriers without phenotypic expression. Dashed symbols are deceased patients; the question mark is a patient not yet tested. The arrow indicates the index patient.
Patient 2 was a 41-year-old woman admitted to the hospital for recurrent ischemic stroke. During the admission, a mild impairment of renal function with proteinuria was documented and the echocardiogram revealed a mild, concentric LVH, with an LVMWT of 14 mm. These red flags raised the suspicion of FD and the DBS confirmed a heterozygous pathogenetic GLA variant c.679C > T (p.Arg227Ter). Lately she started enzymatic replacement therapy, and, following the family cascade screening, one heterozygous carrier (her daughter) was identified.
Patient 3 was a 74-year-old women, followed up for a few years for a supposed diagnosis of HCM. She had a positive familiar history of HCM, in absence of a male-to-male transmission. DBS analysis revealed a heterozygous pathogenetic GLA variant c.644A > G (p. Asn215Ser) and, after the diagnosis, she started enzymatic replacement therapy. The screening of offspring revealed that three sons were also affected, and two daughters were asymptomatic carriers of the disease.
Patient 4 was a 56-year-old man referred for cardiological evaluation due to palpitations. The echocardiogram documented a severe LVH (LVMWT 23 mm). Electrocardiographic features (RBBB and voltage criteria for LVH with secondary repolarization abnormalities) and a positive familiar history of HCM without male-to-male transmission raised the suspicion of FD. DBS analysis confirmed the diagnosis, revealing a severe reduction in α-galactosidase A activity and the pathogenetic GLA variant 5.644A>G (p. Asn215Ser). After the cascade genetic screening, two affected relatives were identified (two brothers) and were also referred for disease-specific therapy initiation.
4. Discussion
In the present study, following a targeted educational intervention on FD, we observed a remarkable rise in the disease screening rate and, importantly, four new families with FD were detected by participant cardiologists.
Despite the diagnostic and therapeutic advances of the last decades, FD remains largely underdiagnosed [4,5,6,7]. An early diagnosis is crucial for FD patients aiming for a timely initiation of disease-specific therapy and slowing of disease progression [11]. Indeed, the efficacy of ERT is closely related to the stage of disease in which the treatment is initiated, with no effect demonstrated in patients with advanced cardiac or renal damage [8,9,10,11].
The lack of disease awareness among the clinicians who face these patients is one of the contributing factors to the diagnostic delay [12]. Thereby educational interventions may be an easy but effective tool to enhance the disease knowledge and consequently improve the diagnostic pathway of FD.
Cardiac involvement is common in FD, and it is the main cause of impaired quality of life and mortality of these patients [3,15,16,17]. The development of a progressive LVH is the main feature of the disease at cardiac level, mimicking HCM [3,16,17]. Thus, cardiologists have a central role in the diagnostic pathway of FD, being the first-line healthcare providers to detect LVH and eventually suspect FD. As underscored by current guidelines [18], FD should always be suspected in HCM cases, when cardiac or extracardiac red-flags are identified. The age of cardiomyopathy onset, familiar history (thereby the pattern of inherence) and electrocardiographic findings are important diagnostic clues, which should always be investigated [19,20,21]. In addition, advanced cardiac imaging techniques, with strain analysis [22,23,24] and T1 mapping [25], have improved the diagnostic approach to FD and are helpful tools for the differential diagnosis from other causes of LVH.
Of importance, cardiac involvement may be the only phenotypic manifestation of FD, and this is typical among patients affected by late-onset or cardiac GLA variants, including the p. Asn215Ser, which are associated with residual α-Gal A activity [26,27]. Notably, in the present study, p. Asn215Ser was the prevalent mutation among the new diagnosis of FD. Indeed, because of the lack of multiorgan involvement, patients with a late-onset phenotype may be more frequently mis-diagnosed with HCM, experiencing diagnostic delays of many years [27]. On the other hand, in patients with classic disease phenotype and renal involvement, LVH may be erroneously attributed to CKD and/or hypertension (such as in patient 3 of the present study).
Of interest, previous studies with systematic searches for FD in patients with unexplained LVH reported a prevalence of the disease varying between 1% and 12% [28,29,30,31]. The rate of positive screening in our study (8.8%) was higher in comparison to that previously reported by Savary and colleagues [12]. Following a targeted educational intervention among 45 French cardiologists, two new FD families were diagnosed, with a 3.4% rate of positive screenings [12]. This discrepancy may potentially be related to the different educational interventions adopted in the two studies. We combined a theoretical phase with a tutored phase and we feel that the chance of discussing real-word clinical cases with experts may have added value for the clinicians approaching this rare disease. Moreover, cardiologists were specifically trained on the key clinical and instrumental red flags of FD and the differential diagnosis with HCM and other phenocopies.
Importantly, the improved diagnosis rate observed in our study translated into concrete patient benefits. All four newly diagnosed index patients were promptly started on disease-specific therapy—either ERT or chaperone therapy—after the diagnosis was confirmed. Moreover, cascade genetic screening enabled the identification of affected at-risk family members, eight of whom were diagnosed with FD and initiated a disease-specific therapy, while six heterozygous carriers were enrolled in regular follow-up. This is particularly relevant since the efficacy of disease-specific treatment critically depends on the timing in which it is initiated, with clear evidence that early therapy can slow progression, prevent irreversible organ damage, and improve long-term outcomes [8,9,10,11]. Accordingly, patients identified by cascade screening were referred for specific treatment as soon as early signs of kidney, heart or brain involvement were identified. Therefore, our findings underscore that targeted educational interventions for cardiologists do not only increase the diagnostic yield but also facilitate timely therapeutic intervention, ultimately improving the management and prognosis of patients affected by FD and their families.
Further studies investigating the impact of targeted educational interventions for other specialists that typically face FD patients, such as nephrologists, are warranted.
Limitations
Firstly, study limitations are linked to the study design, and particularly the lack of a control group and the relatively small sample size of cardiologists that have been enrolled. The cardiologists who participated in this study may have been more motivated and this may be a source of a sample bias. Moreover, it is not possible to draw conclusions on the effects of the training on the long-term rate of FD screening and diagnosis. Despite the fact that we demonstrated a significant rise in the annual screening rate after educational training, statistical testing of the changes in the diagnostic yield was not feasible.
5. Conclusions
Our study shows that a targeted educational intervention for cardiologists substantially increased the screening and diagnosis rates for Fabry disease, leading to the identification of four new affected families within a relatively short timeframe. Thus, raising disease awareness and providing practical guidance to frontline cardiologists may play a key role in reducing diagnostic and therapeutic delays and, lately, in improving the prognosis of patients with FD.
Author Contributions
Conceptualization, R.L. and F.G. (Francesca Graziani); data curation, M.C.M. and C.D.B.; formal analysis, M.C.M.; funding acquisition, F.G. (Francesca Graziani); project administration, M.C.M.; resources, V.M., M.L., A.B.S., L.V.S.T., F.M. and F.d.S.; supervision, E.A., P.C., S.C. and F.B. (Francesco Burzotta); validation, M.C., G.T., F.B. (Federico Biondi), V.G., V.P., F.G. (Francesca Giordana) and B.M.; visualization, I.P., R.S. and S.L.; writing—original draft, M.C.M.; writing—review and editing, R.L., F.G. (Francesca Graziani) and F.B. (Francesco Burzotta). All authors have read and agreed to the published version of the manuscript.
Funding
The present research was funded by Takeda Pharmaceutical Company.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committees of the promotor center (identification number: 3924, 23 June 2022) and participant institutions.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the results of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Rosa Lillo has received advisory board fees from Amicus Therapeutics, Sanofi Genzyme, Takeda and Shire. Francesca Graziani has research grants from Takeda; she has also received advisory board fees from Amicus Therapeutics, Sanofi Genzyme, and Shire. The remaining authors have nothing to disclose in relation to this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| FD | Fabry disease |
| CKD | Chronic kidney disease |
| GLA | Galactosidase alpha |
| HCM | Hypertrophic cardiomyopathy |
| LVMWT | Left ventricular maximum wall thickness |
| LVH | Left ventricular hypertrophy |
References
- Linhart, A.; Elliott, P.M. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart 2007, 93, 528–535. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Elliott, P. Anderson-Fabry disease and the heart. Prog. Cardiovasc. Dis. 2010, 52, 326–335. [Google Scholar] [CrossRef]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Sunder-Plassmann, G.; Fodinger, M. Diagnosis of Fabry disease: The role of screening and case-finding studies. In Fabry Disease:Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006; Chapter 17. [Google Scholar]
- Spada, M.; Pagliardini, S.; Yasuda, M.; Tukel, T.; Thiagarajan, G.; Sakuraba, H.; Ponzone, A.; Desnick, R.J. High incidence of later-onset Fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006, 79, 31–40. [Google Scholar] [CrossRef]
- Hsu, T.R.; Hung, S.C.; Chang, F.P.; Yu, W.C.; Sung, S.H.; Hsu, C.L.; Dzhagalov, I.; Yang, C.F.; Chu, T.H.; Lee, H.J.; et al. Later Onset Fabry Disease, Cardiac Damage Progress in Silence: Experience With a Highly Prevalent Mutation. J. Am. Coll. Cardiol. 2016, 68, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Reisin, R.; Perrin, A.; García-Pavía, P. Time delays in the diagnosis and treatment of Fabry disease. Int. J. Clin. Pract. 2017, 71, e12914. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Niemann, M.; Breunig, F.; Herrmann, S.; Beer, M.; Störk, S.; Voelker, W.; Ertl, G.; Wanner, C.; Strotmann, J. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation 2009, 119, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; Wijburg, F.A.; Wanner, C.; Vaz, F.M.; van Kuilenburg, A.B.; Hughes, D.A.; Biegstraaten, M.; Mehta, A.; Hollak, C.E.; Langeveld, M. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017, 121, 157–161. [Google Scholar] [CrossRef]
- Germain, D.P.; Elliott, P.M.; Falissard, B.; Fomin, V.V.; Hilz, M.J.; Jovanovic, A.; Kantola, I.; Linhart, A.; Mignani, R.; Namdar, M.; et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts. Mol. Genet. Metab. Rep. 2019, 19, 100454. [Google Scholar] [CrossRef]
- Pieroni, M.; Namdar, M.; Olivotto, I.; Desnick, R.J. Anderson-Fabry disease management: Role of the cardiologist. Eur. Heart J. 2024, 45, 1395–1409. [Google Scholar] [CrossRef]
- Savary, A.L.; Morello, R.; Brasse-Lagnel, C.; Milliez, P.; Bekri, S.; Labombarda, F. Enhancing the diagnosis of fabry disease in cardiology with a targeted information: A before-after control- impact study. Open Heart 2017, 4, e000567. [Google Scholar] [CrossRef]
- Thomas, A.S.; Mehta, A.B. Difficulties and barriers in diagnosing Fabry disease: What can be learnt from the literature? Expert. Opin. Med. Diagn. 2013, 7, 589–599. [Google Scholar] [CrossRef]
- Biegstraaten, M.; Arngrímsson, R.; Barbey, F.; Boks, L.; Cecchi, F.; Deegan, P.B.; Feldt-Rasmussen, U.; Geberhiwot, T.; Germain, D.P.; Hendriksz, C.; et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J. Rare Dis. 2015, 10, 36. [Google Scholar] [CrossRef]
- Meucci, M.C.; Lillo, R.; Del Franco, A.; Monda, E.; Iannaccone, G.; Baldassarre, R.; Di Nicola, F.; Parisi, V.; Lombardo, A.; Spinelli, L.; et al. Prognostic Implications of the Extent of Cardiac Damage in Patients With Fabry Disease. J. Am. Coll. Cardiol. 2023, 82, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Del Franco, A.; Iannaccone, G.; Meucci, M.C.; Lillo, R.; Cappelli, F.; Zocchi, C.; Pieroni, M.; Graziani, F.; Olivotto, I. Clinical staging of Anderson-Fabry cardiomyopathy: An operative proposal. Heart Fail. Rev. 2024, 29, 431–444. [Google Scholar] [CrossRef]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A.; Elliott, P.M. European FOS Investigators. Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry Outcome Survey. Eur. Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, G.; Monda, E.; Tramonte, S.; Gragnano, F.; Masarone, D.; Frisso, G.; Esposito, A.; Gravino, R.; Ammendola, E.; Salerno, G.; et al. Prevalence and clinical significance of red flags in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 299, 186–191. [Google Scholar] [CrossRef]
- Maurizi, N.; Monda, E.; Biagini, E.; Field, E.; Passantino, S.; Dall’Aglio, G.; Fumagalli, C.; Antiochos, P.; Skalidis, I.; Pieroni, M.; et al. Hypertrophic cardiomyopathy: Prevalence of disease-specific red flags. Eur. Heart J. 2025, ehaf026. [Google Scholar] [CrossRef]
- Vitale, G.; Ditaranto, R.; Graziani, F.; Tanini, I.; Camporeale, A.; Lillo, R.; Rubino, M.; Panaioli, E.; Di Nicola, F.; Ferrara, V.; et al. Standard ECG for differential diagnosis between Anderson-Fabry disease and hypertrophic cardiomyopathy. Heart 2022, 108, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Huang, W.M.; Wang, W.T.; Hung, S.C.; Sung, S.H.; Chen, C.H.; Yang, Y.J.; Niu, D.M.; Yu, W.C. Reduced global longitudinal strain as a marker for early detection of Fabry cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Meucci, M.C.; Lillo, R.; Lombardo, A.; Lanza, G.A.; Bootsma, M.; Butcher, S.C.; Massetti, M.; Manna, R.; Bax, J.J.; Crea, F.; et al. Comparative analysis of right ventricular strain in Fabry cardiomyopathy and sarcomeric hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 542–551. [Google Scholar] [CrossRef]
- Meucci, M.C.; Lillo, R.; Mango, F.; Marsilia, M.; Iannaccone, G.; Tusa, F.; Luigetti, M.; Biagini, E.; Massetti, M.; Lanza, G.A.; et al. Left atrial structural and functional remodelling in Fabry disease and cardiac amyloidosis: A comparative analysis. Int. J. Cardiol. 2024, 402, 131891. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef]
- Oder, D.; Liu, D.; Hu, K.; Üçeyler, N.; Salinger, T.; Müntze, J.; Lorenz, K.; Kandolf, R.; Gröne, H.J.; Sommer, C.; et al. Alpha-galactosidase A genotype N215S induces a specific cardiac variant of Fabry disease. Circ. Cardiovasc. Genet. 2017, 10, e00169. [Google Scholar] [CrossRef]
- Germain, D.P.; Brand, E.; Burlina, A.; Cecchi, F.; Garman, S.C.; Kempf, J.; Laney, D.A.; Linhart, A.; Marodi, L.; Nicholls, K.; et al. Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: A multicenter Fabry Registry study. Mol. Genet. Genom. Med. 2018, 6, 492–503. [Google Scholar] [CrossRef]
- Monserrat, L.; Gimeno-Blanes, J.R.; Marín, F.; Hermida-Prieto, M.; García-Honrubia, A.; Pérez, I.; Fernández, X.; de Nicolas, R.; de la Morena, G.; Payá, E.; et al. Prevalence of fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2007, 50, 2399–2403. [Google Scholar] [CrossRef]
- Elliott, P.; Baker, R.; Pasquale, F.; Quarta, G.; Ebrahim, H.; Mehta, A.B.; Hughes, D.A. Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: The European Anderson-Fabry Disease survey. Heart 2011, 97, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Pieroni, M.; Morgante, E.; Antuzzi, D.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004, 110, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, B.; Takenaka, T.; Teraguchi, H.; Tei, C.; Lee, P.; McKenna, W.J.; Elliott, P.M. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 2002, 105, 1407–1411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).