A HF-Free Synthesis Method for High-Luminescent Efficiency Narrow-Bandgap Red Phosphor K3AlF6: Mn4+ with NH4HF2 as the Molten Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Properties Characterization
2.3. Device Fabrication and Performance Measurements
3. Results and Discussion
3.1. Structural and Morphological Properties
3.2. Optical Properties
3.3. Device Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, R.; Wang, W.; Zhang, J.; Jiang, S.; Chen, Z.; Li, W.; Yu, X. Compounds. Synthesis and luminescence properties of Li2SnO3: Mn4+ red-emitting phosphor for solid-state lighting. J. Alloys Compd. 2017, 704, 124–130. [Google Scholar] [CrossRef]
- Li, C.; Fang, Z.; Yan, Y.; Li, H.; Luo, X.; Wang, X.; Zhou, P. Study on the Performance of Deep Red to Near-Infrared pc-LEDs by the Simulation Method Considering the Distribution of Phosphor Particles. Micromachines 2024, 15, 1035. [Google Scholar] [CrossRef]
- Liao, C.; Cai, H.; Dai, D.; Zhang, L. Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4: Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux. Solids 2025, 6, 53. [Google Scholar] [CrossRef]
- Deng, T.; Song, E.; Zhou, Y.; Chen, J.; Cheng, Y.; Yuan, J.; Fan, T. The use of a single ammonium acidic salt towards simple green co-precipitation synthesis for Mn4+-activated fluorides. Dalton Trans. 2020, 49, 5823–5831. [Google Scholar] [CrossRef]
- Lian, H.; Huang, Q.; Chen, Y.; Li, K.; Liang, S.; Shang, M.; Liu, M.; Lin, J. Resonance Emission Enhancement (REE) for Narrow Band Red-Emitting A2GeF6:Mn4+ (A = Na, K, Rb, Cs) Phosphors Synthesized via a Precipitation–Cation Exchange Route. Inorg. Chem. 2017, 56, 11900–11910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Z.; Wang, N.; Zhou, Q.; Zhou, J.; Ma, L.; Wang, X.; Xu, Y.; Brik, M.; Dramićanin, M. Single-Crystal Red Phosphors: Enhanced Optical Efficiency and Improved Chemical Stability for wLEDs. Adv. Opt. Mater. 2019, 8, 1901512. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Y.; Li, Z.; Liang, S.; Guan, X.-H.; Yang, Z.-J.; Lian, S.; Zhou, W. Modulating luminescence of K3AlF6:Mn4+ NCs via charge compensation and localized surface plasmon resonance effect. Sci. China Mater. 2025, 68, 1047–1056. [Google Scholar] [CrossRef]
- Senden, T.; Geitenbeek, R.; Meijerink, A. Co-precipitation synthesis and optical properties of Mn4+-doped hexafluoroaluminate w-LED phosphors. Materials 2017, 10, 1322. [Google Scholar] [CrossRef]
- He, S.; Xu, F.; Wu, D.; Wang, Z.; Peng, J.; Ye, X. Engineering. Effects of introducing cations on the luminescence performance of CsPF6: Mn4+ phosphor. Nonferr. Met. Sci. Eng. 2020, 11, 111–117. [Google Scholar]
- Deng, T.; Song, E.; Zhou, Y.; Wang, L.; Ye, S.; Zhang, Q. Stable narrowband red phosphor K3GaF6: Mn4+ derived from hydrous K2GaF5 (H2O) and K2MnF6. J. Mater. Chem. C 2017, 5, 9588–9596. [Google Scholar] [CrossRef]
- Rui, K.; Wen, Z.; Lu, Y.; Jin, J.; Shen, C. One-Step Solvothermal Synthesis of Nanostructured Manganese Fluoride as an Anode for Rechargeable Lithium-Ion Batteries and Insights into the Conversion Mechanism. Adv. Energy Mater. 2014, 5, 1401716. [Google Scholar] [CrossRef]
- Hung, N.; Tung, D.; Dat, L. Synthesis and Waterproofness Improvement of K3AlF6:Mn4+ Phosphor for Warm White Light-emitting Diodes. VNU J. Sci. Math.—Phys. 2021, 37, 101–108. [Google Scholar] [CrossRef]
- King, G.; Abakumov, A.; Woodward, P.; Llobet, A.; Tsirlin, A.; Batuk, D.; Antipov, E. The high-temperature polymorphs of K3AlF6. Inorg. Chem. 2011, 50, 7792–7801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Su, H.; Gao, Y.; Zhang, S.; Li, L.; Hong, F.; Xu, Y.; Yu, D.; Lin, H. A red-emitting phosphor Na3AlF6: Mn4+: Green synthesis, optical characteristics, thermal stability and application in high-performance warm WLED. J. Solid State Chem. 2024, 340, 125016. [Google Scholar] [CrossRef]
- Yi, X.; Li, R.; Zhu, H.; Gao, J.; You, W.; Gong, Z.; Guo, W.; Chen, X. K2NaAlF6: Mn4+ red phosphor: Room-temperature synthesis and electronic/vibronic structures. J. Mater. Chem. C 2018, 6, 2069–2076. [Google Scholar] [CrossRef]
- Qiang, J.; Ruan, H.; Wang, L.; Wang, T.; Lei, J.; Liao, S.; Li, S. Luminescence enhancement of K2LiAlF6: Mn4+ phosphors by Zn2+-mediated charge compensation for fast-response backlighting applications. Inorg. Chem. 2023, 62, 14344–14354. [Google Scholar] [CrossRef]
- Ming, H.; Zhang, J.; Liu, L.; Peng, J.; Du, F.; Ye, X. Technology. Luminescent properties of a Cs3AlF6: Mn4+ red phosphor for warm white light-emitting diodes. J. Solid State Sci. Technol. 2018, 7, R149. [Google Scholar] [CrossRef]
- Deng, T.; Song, E.; Zhou, Y.; Wang, L.; Zhang, Q. Tailoring photoluminescence stability in double perovskite red phosphors A2BAlF6: Mn4+ (A=Rb, Cs; B=K, Rb) via neighboring-cation modulation. J. Mater. Chem. C 2017, 5, 12422–12429. [Google Scholar] [CrossRef]
- Song, E.; Wang, J.; Shi, J.; Deng, T.; Ye, S.; Peng, M.; Wang, J.; Wondraczek, L.; Zhang, Q. Highly Efficient and Thermally Stable K3AlF6:Mn4+ as a Red Phosphor for Ultra-High-Performance Warm White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 8805–8812. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, H. Advances in valence state analysis of manganese in Mn4+-activated red phosphors for white LEDs. Chin. J. Lumin. 2020, 41, 1195–1213. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Zhang, X.; Cao, C.; Yang, L.; Huang, Y.; Liao, S.; Zhang, H. Synthesis, luminescence properties and nephelauxetic effect of nano stick phosphors K3AlF6: Mn4+ for warm white LED. J. Mater. Sci. Mater. Electron. 2019, 30, 1870–1877. [Google Scholar] [CrossRef]
- Tuyet, D.; Hong, V.; Bondzior, B.; Dereń, P.; Velpula, R.; Trung Nguyen, H.; Tuyen, L.; Hung, N.; Nguyen, H. Deep red fluoride dots-in-nanoparticles for high color quality micro white light-emitting diodes. Opt. Express 2020, 28, 26189–26199. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, Y.; Zhang, X.; Zou, R.; Pan, F.; Wang, J.; Wu, M. HF-free hydrothermal route for synthesis of highly efficient narrow-band red emitting phosphor K2Si1-x F6: X Mn4+ for warm white light-emitting diodes. Chem. Mater. 2016, 28, 1495–1502. [Google Scholar] [CrossRef]
- Olchowka, J.; Suta, M.; Wickleder, C. Green synthesis of A2SiF6 (A= Li, Cs) nanoparticles using ionic liquids as solvents and as fluorine sources: A simple approach without HF. Chem.–A Eur. J. 2017, 23, 12092–12095. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Novitskaya, E.; Lam, N.; Sanchez, M.; Kim, Y.; Li, Z.; Im, W.; Graeve, O.; McKittrick, J. Synthesis of Mn4+ activated Na2SiF6 red-emitting phosphors using an ionic liquid. J. Lumin. 2020, 218, 116835. [Google Scholar] [CrossRef]
- Luo, X.; Hou, Z.; Zhou, T.; Xie, R. A universal HF-free synthetic method to highly efficient narrow-band red-emitting A2XF6: Mn4+ (A= K, Na, Rb, Cs; X= Si, Ge, Ti) phosphors. J. Am. Ceram. Soc. 2020, 103, 1018–1026. [Google Scholar] [CrossRef]
- Hou, Z.; Tang, X.; Luo, X.; Zhou, T.; Zhang, L.; Xie, R. A green synthetic route to the highly efficient K2SiF6: Mn4+ narrow-band red phosphor for warm white light-emitting diodes. J. Mater. Chem. C 2018, 6, 2741–2746. [Google Scholar] [CrossRef]
- Stoll, C.; Bandemehr, J.; Kraus, F.; Seibald, M.; Baumann, D.; Schmidberger, M.; Huppertz, H. HF-free synthesis of Li2SiF6: Mn4+: A red-emitting phosphor. Inorg. Chem. 2019, 58, 5518–5523. [Google Scholar] [CrossRef]
- Noh, M.; Yoon, D.; Kim, C.; Lee, S. Organic solvent-assisted synthesis of the K3SiF7: Mn4+ red phosphor with improved morphology and stability. J. Mater. Chem. C 2019, 7, 15014–15020. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, J.; Im, W. Towards green synthesis of Mn4+-doped fluoride phosphors: A review. J. Mater. Res. Technol. 2021, 11, 181–195. [Google Scholar] [CrossRef]
- Wu, Q.; Liao, C.; Pan, J.; Ye, X.; You, W.; Xia, L. HF-free molten salt route for synthesis of highly efficient and water-resistant K2SiF6: Mn4+ for warm white LED. J. Am. Ceram. Soc. 2020, 103, 6901–6912. [Google Scholar] [CrossRef]
- Ilton, E.; Post, J.; Heaney, P.; Ling, F.; Kerisit, S. XPS determination of Mn oxidation states in Mn (hydr) oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, J.; Wang, Y.; Li, S.; Luan, D.; Lou, X. Formation of CuMn Prussian blue analog double-shelled nanoboxes toward long-life Zn-ion batteries. Angew. Chem. Int. Ed. 2022, 61, e202212031. [Google Scholar] [CrossRef]
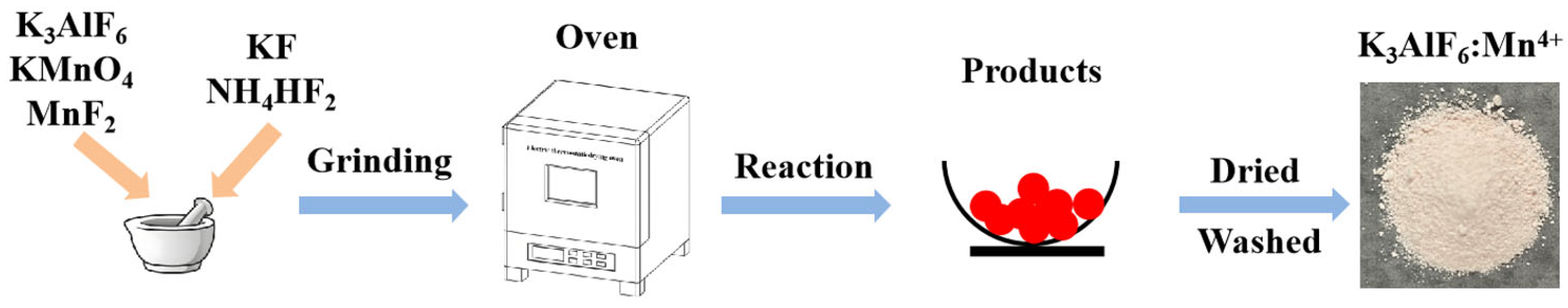
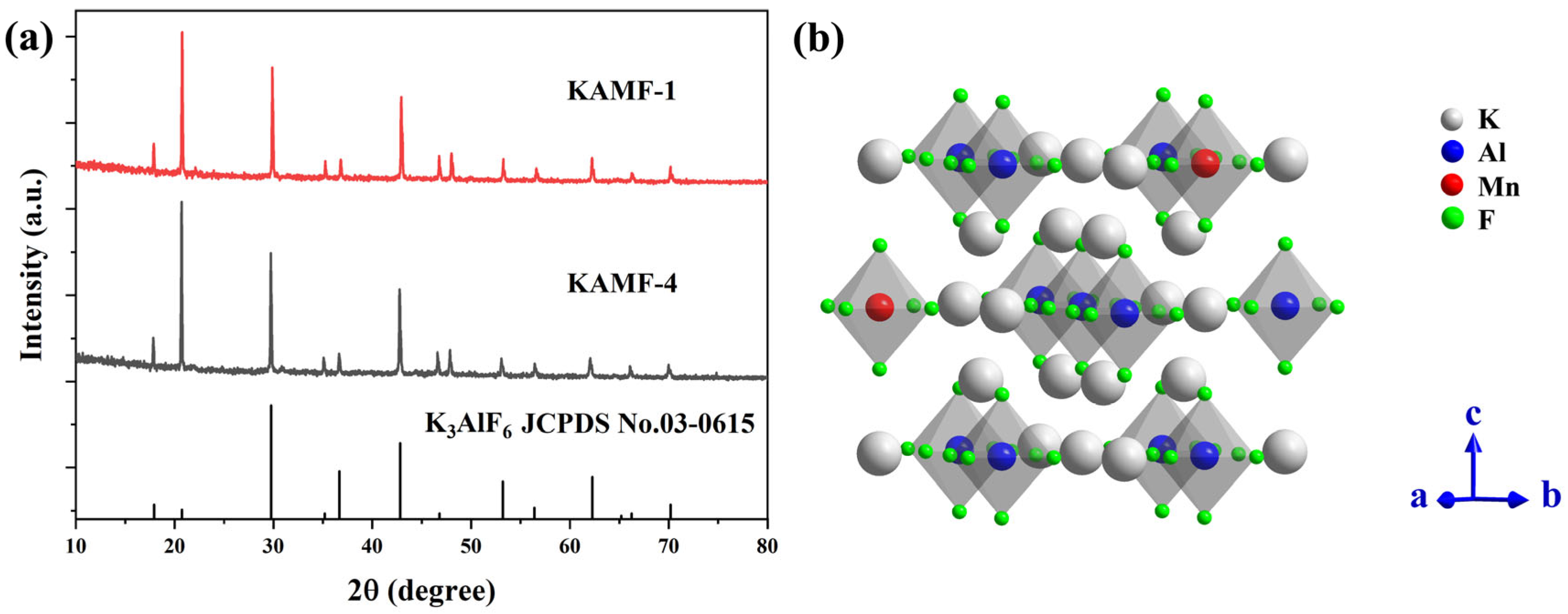
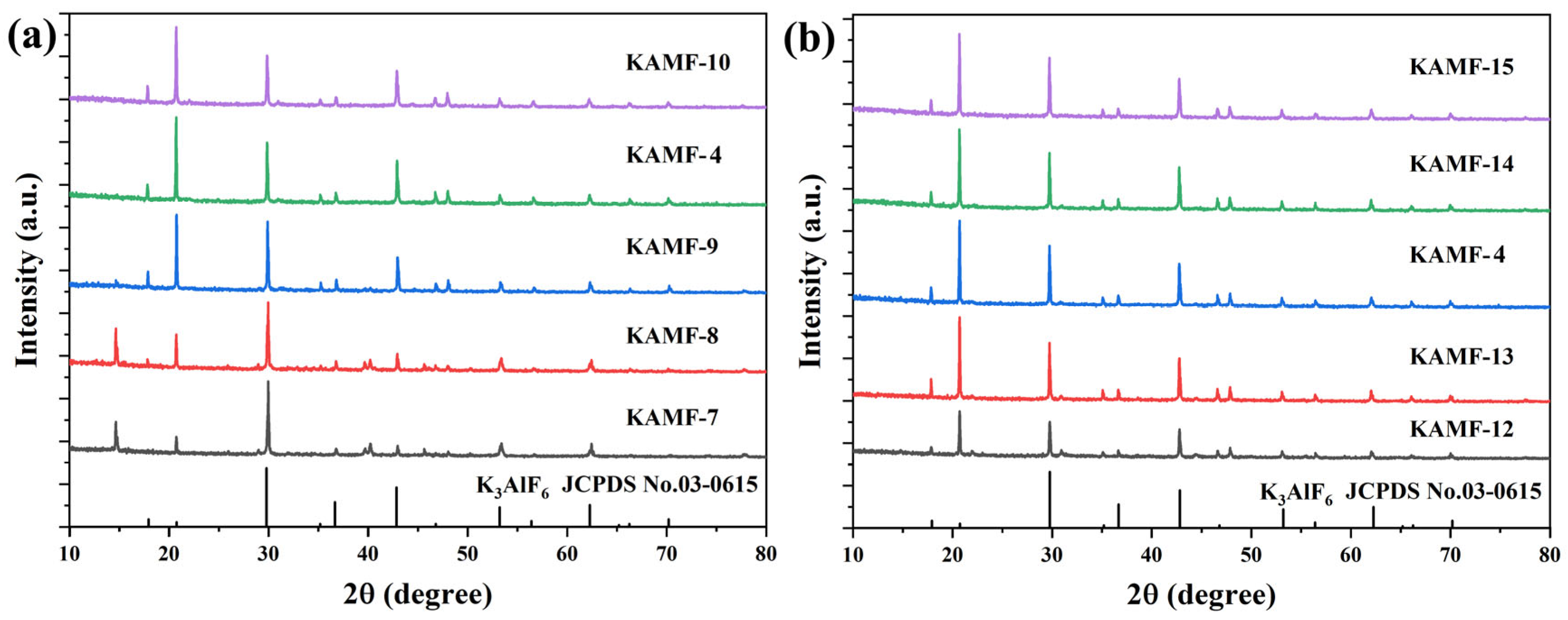
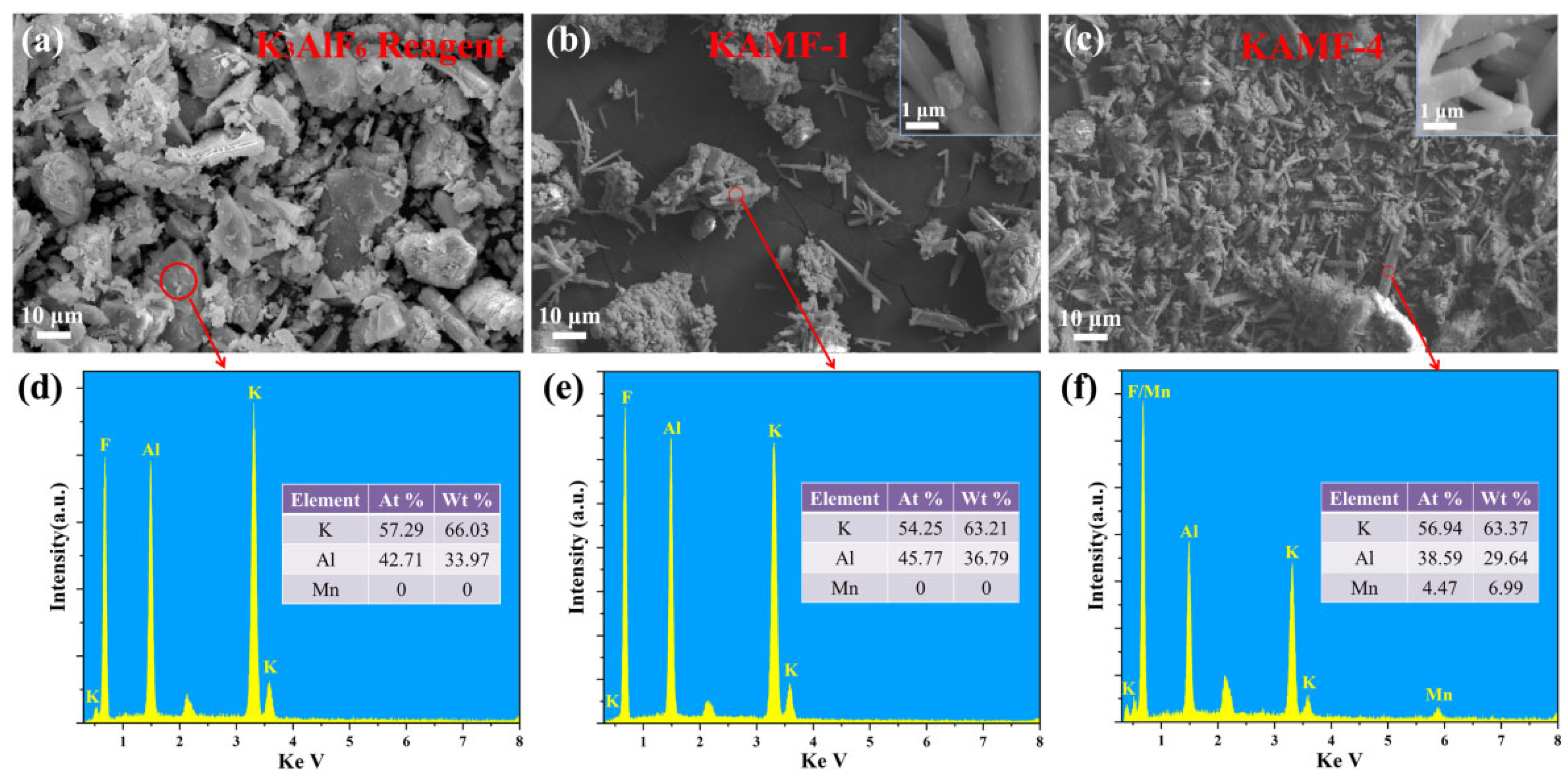
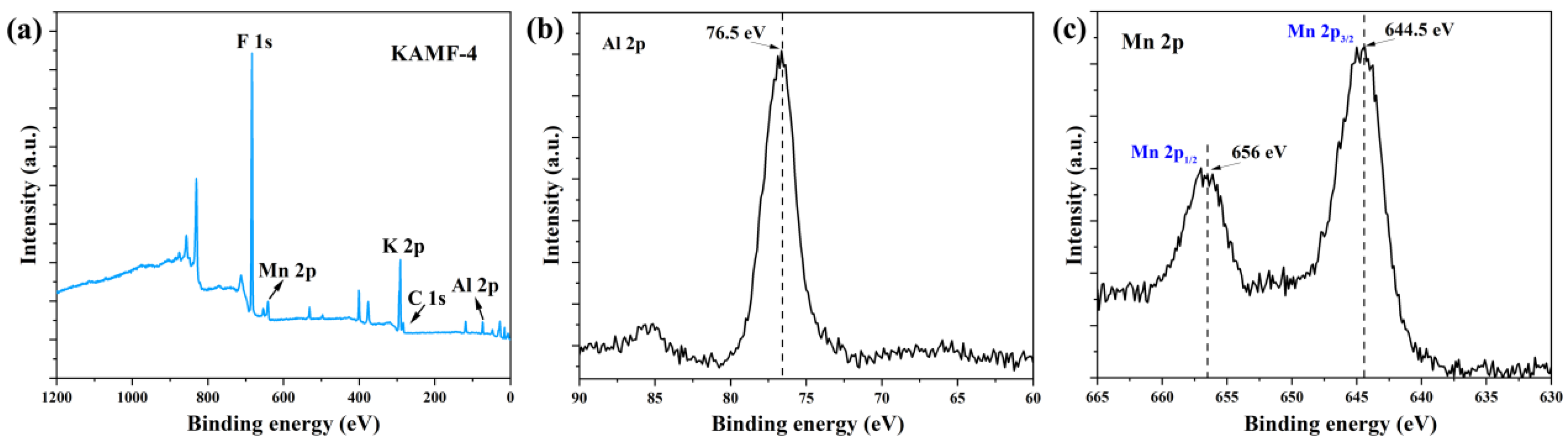
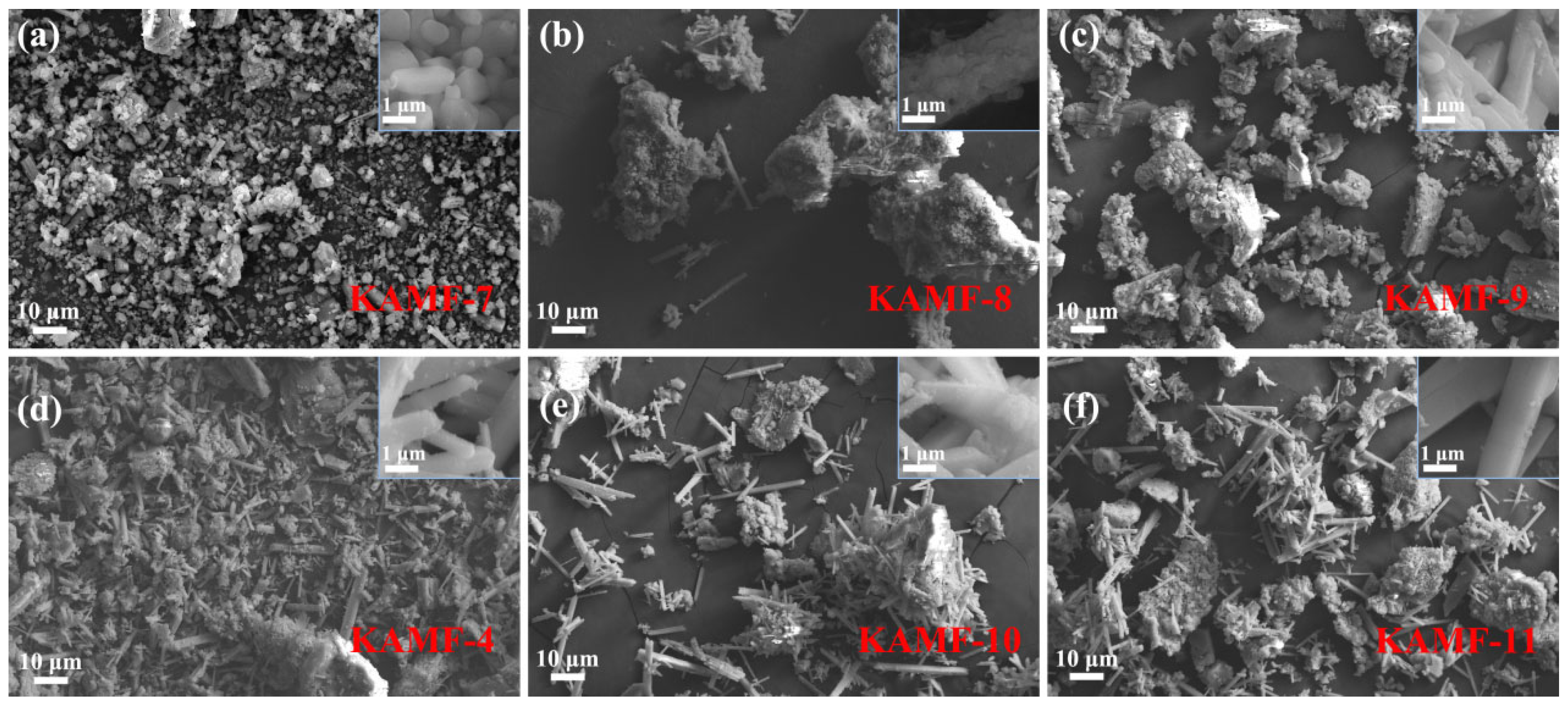
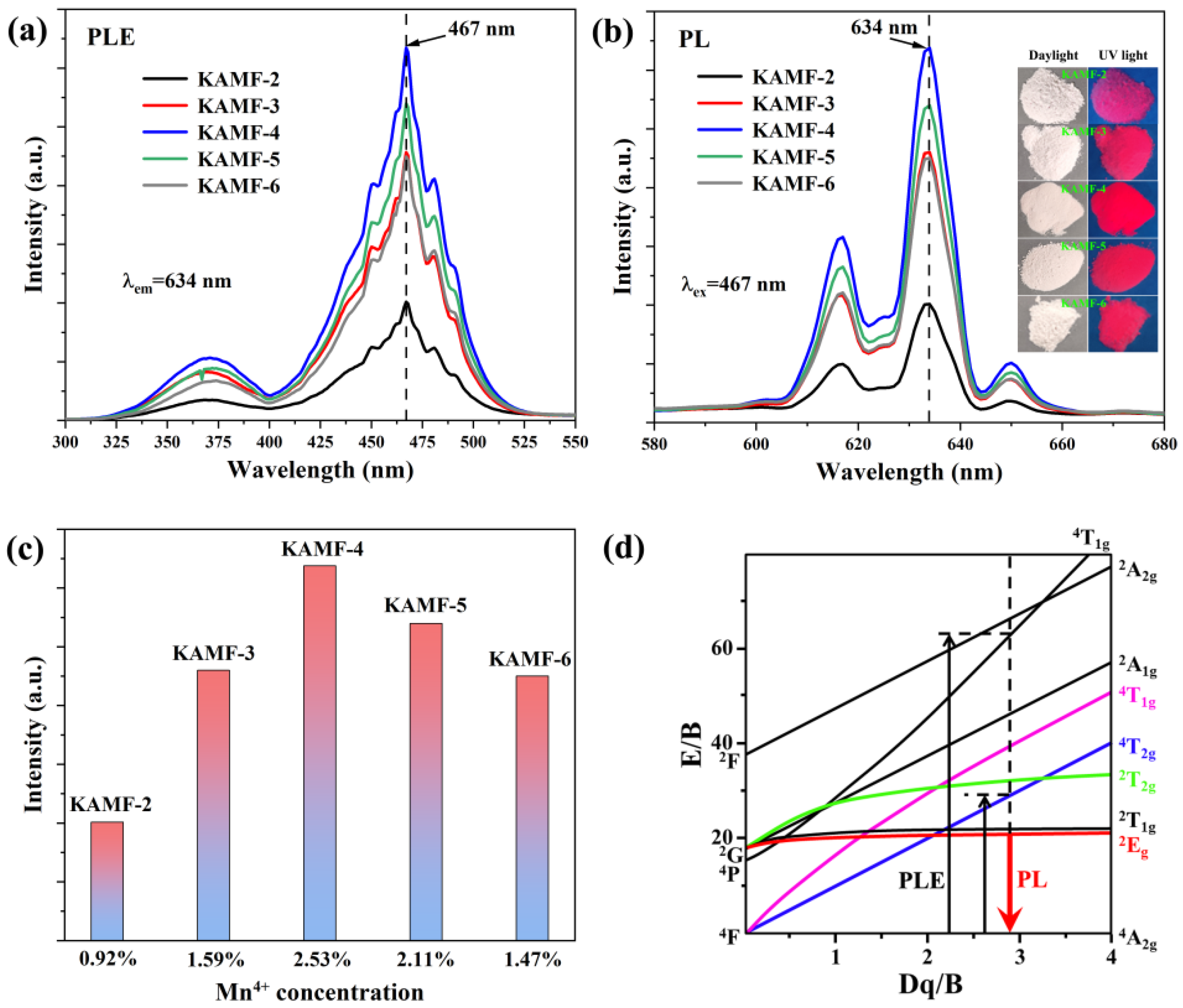
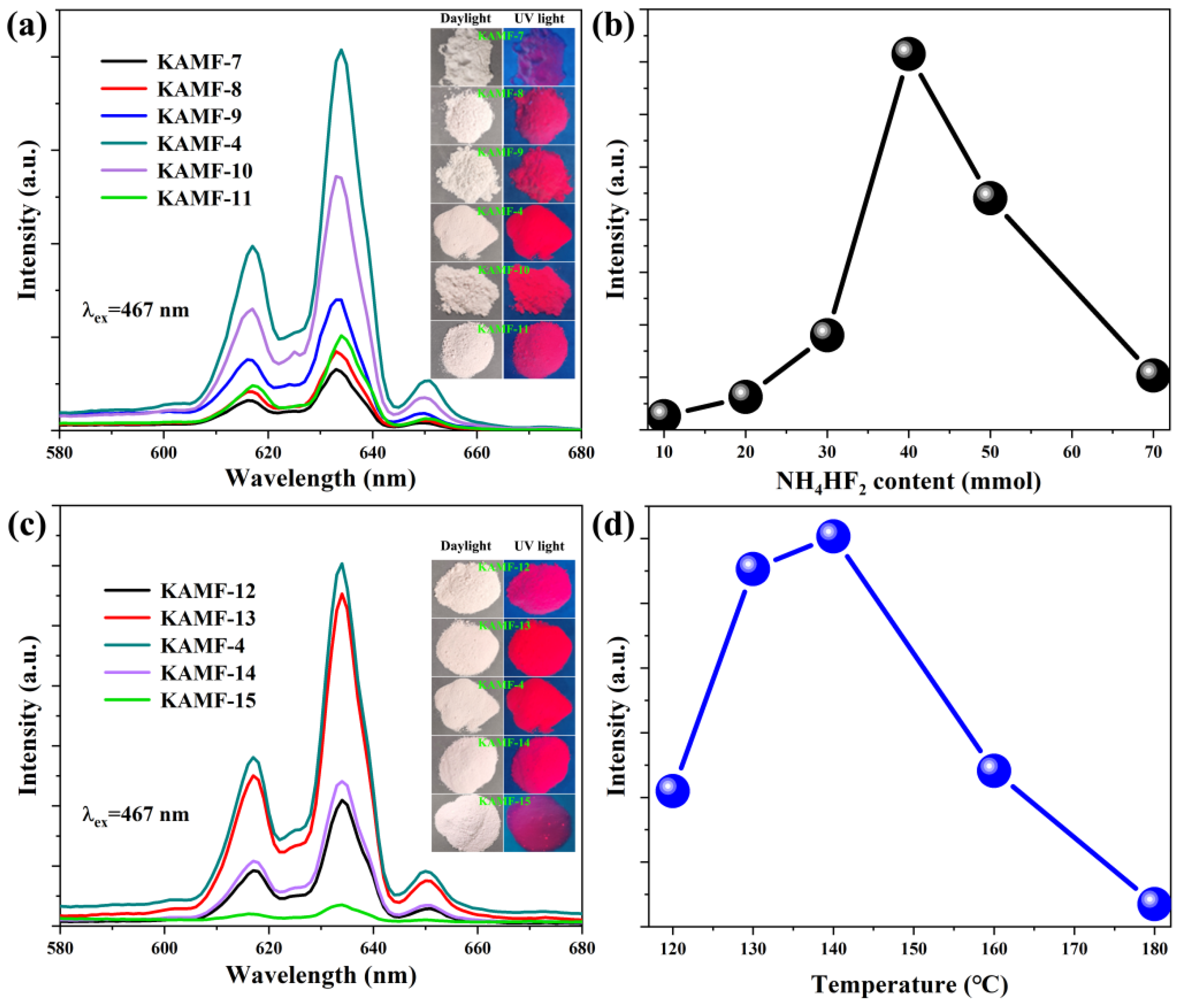
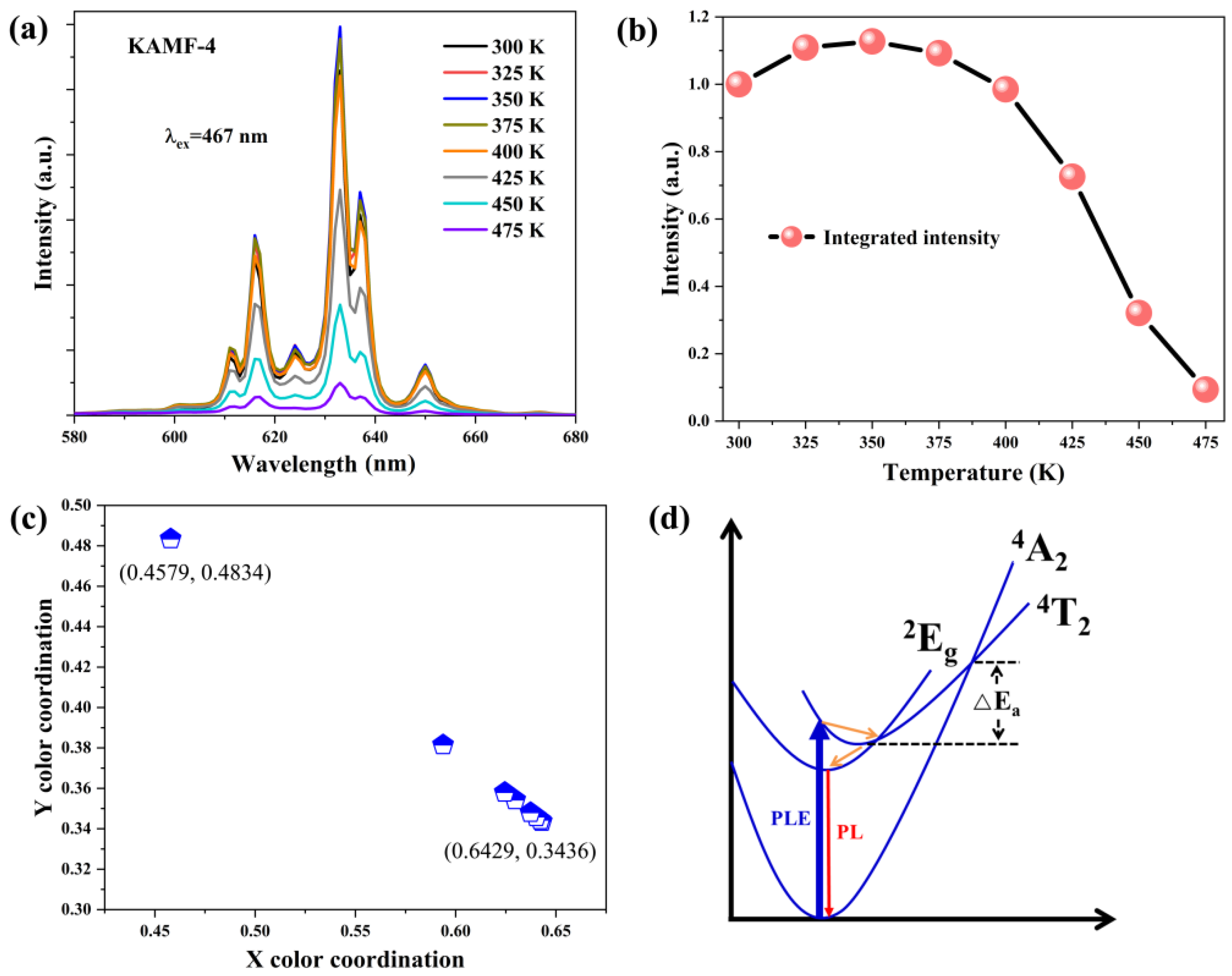
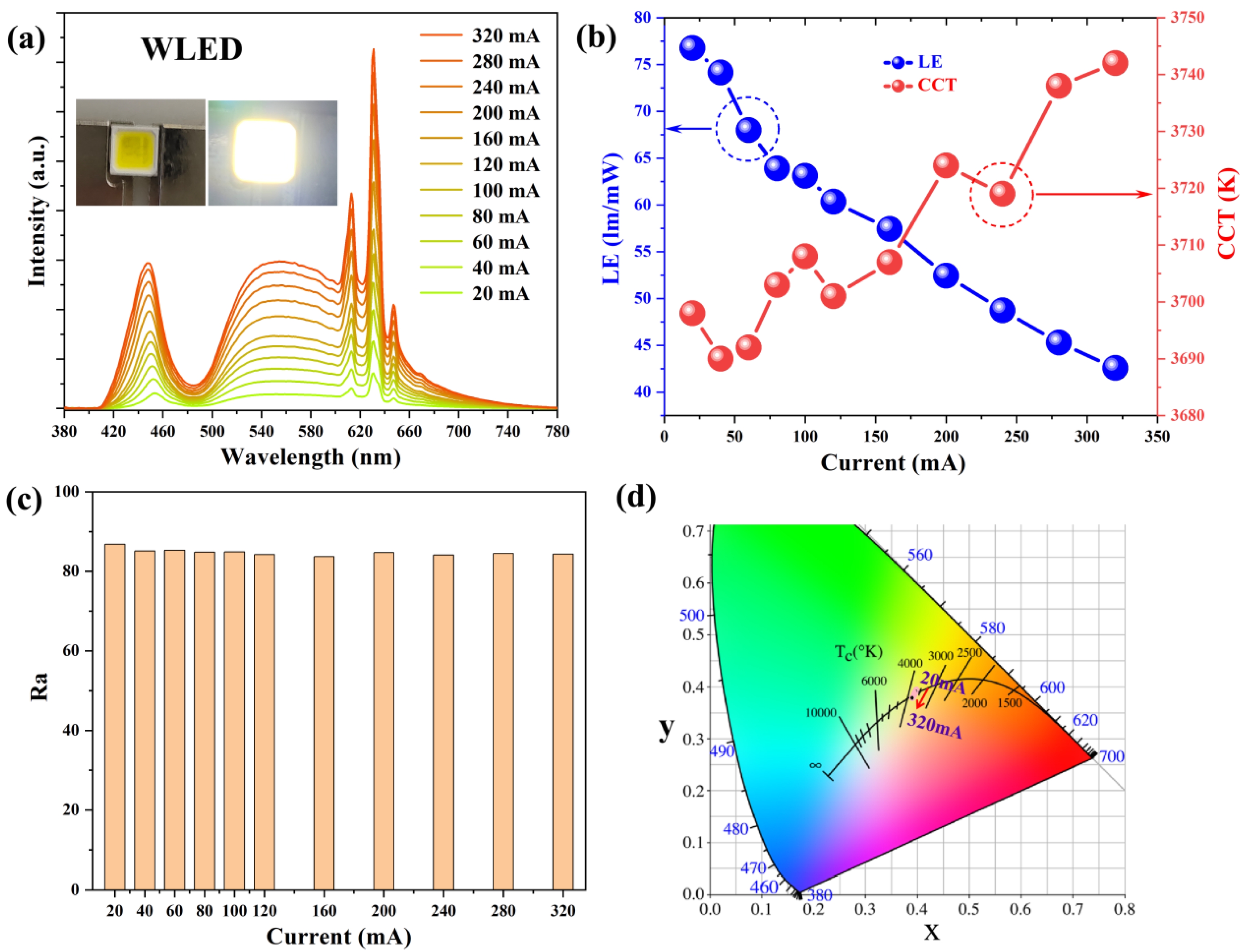
| No. | K3AlF6 (mmol) | KMnO4 (mmol) | MnF2 (mmol) | NH4HF2 (mmol) | Reaction Temperature (°C) |
|---|---|---|---|---|---|
| KAMF-1 | 10 | 0 | 0 | 40 | 140 |
| KAMF-2 | 10 | 0.25 | 0.375 | 40 | 140 |
| KAMF-3 | 10 | 0.5 | 0.75 | 40 | 140 |
| KAMF-4 | 10 | 1 | 1.5 | 40 | 140 |
| KAMF-5 | 10 | 2 | 3 | 40 | 140 |
| KAMF-6 | 10 | 3 | 4.5 | 40 | 140 |
| KAMF-7 | 10 | 1 | 1.5 | 10 | 140 |
| KAMF-8 | 10 | 1 | 1.5 | 20 | 140 |
| KAMF-9 | 10 | 1 | 1.5 | 30 | 140 |
| KAMF-10 | 10 | 1 | 1.5 | 50 | 140 |
| KAMF-11 | 10 | 1 | 1.5 | 70 | 140 |
| KAMF-12 | 10 | 1 | 1.5 | 40 | 120 |
| KAMF-13 | 10 | 1 | 1.5 | 40 | 130 |
| KAMF-14 | 10 | 1 | 1.5 | 40 | 160 |
| KAMF-15 | 10 | 1 | 1.5 | 40 | 180 |
| No. | Mole Ratio of KMnO4 to K3AlF6 | Mn4+ Concentration (at%) |
|---|---|---|
| KAMF-2 | 2.5:100 | 0.92 |
| KAMF-3 | 5:100 | 1.59 |
| KAMF-4 | 10:100 | 2.53 |
| KAMF-5 | 20:100 | 2.11 |
| KAMF-6 | 30:100 | 1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Zhou, F.; Xie, W.; Zhang, L. A HF-Free Synthesis Method for High-Luminescent Efficiency Narrow-Bandgap Red Phosphor K3AlF6: Mn4+ with NH4HF2 as the Molten Salt. Solids 2025, 6, 66. https://doi.org/10.3390/solids6040066
Liao C, Zhou F, Xie W, Zhang L. A HF-Free Synthesis Method for High-Luminescent Efficiency Narrow-Bandgap Red Phosphor K3AlF6: Mn4+ with NH4HF2 as the Molten Salt. Solids. 2025; 6(4):66. https://doi.org/10.3390/solids6040066
Chicago/Turabian StyleLiao, Chenxing, Feng Zhou, Wei Xie, and Liaolin Zhang. 2025. "A HF-Free Synthesis Method for High-Luminescent Efficiency Narrow-Bandgap Red Phosphor K3AlF6: Mn4+ with NH4HF2 as the Molten Salt" Solids 6, no. 4: 66. https://doi.org/10.3390/solids6040066
APA StyleLiao, C., Zhou, F., Xie, W., & Zhang, L. (2025). A HF-Free Synthesis Method for High-Luminescent Efficiency Narrow-Bandgap Red Phosphor K3AlF6: Mn4+ with NH4HF2 as the Molten Salt. Solids, 6(4), 66. https://doi.org/10.3390/solids6040066







