Clinical Approach to Acute Recreational Drug Intoxication in the Emergency Setting: A Practical Guide Based on Swiss Experience
Abstract
1. Introduction
2. Materials and Methods
- Prevalence of use, based on national epidemiological indicators from MonAM (FOPH) and Addiction Suisse reports;
- Frequency of presentation to emergency departments, based on the authors’ clinical experience and local data; and
- Potential danger in the acute phase, defined as the risk of life-threatening complications or the need for specific treatment.
3. Results
3.1. Stimulants
3.1.1. Cocaine
3.1.2. Amphetamines (Including Methamphetamine and MDMA) and Synthetic Cathinones
3.2. Opioids
- Intravenous (IV): 0.4 mg every two to three minutes until a clinical response is obtained or a maximum cumulative dose of 10 mg is reached.
- Intramuscular (IM): 2 mg as a single dose, useful in pre-hospital settings or if venous access is difficult.
- Intranasal (IN): 4 mg per unilateral spray. Suitable for self-administration or by first responders.
- If a favourable response is observed, a continuous IV infusion of naloxone may be necessary, starting at 0.1–0.6 mg/h and adjusted according to the pharmacokinetics of the substance involved, the severity of intoxication, and the clinical course. It is important to note that highly potent synthetic opioids such as fentanyl and its analogues (e.g., carfentanil and sufentanil) have a much greater affinity for the µ receptor. This often justifies higher doses of the antagonist or prolonged treatment, sometimes for more than 12 h.
- If there is no clinical response to a 10 mg IV dose of naloxone, the diagnosis should be reassessed with particular attention to mixed intoxication (e.g., benzodiazepines, alcohol or other central nervous system depressants) or an alternative neurological cause.
- Cardiovascular complications should be managed in accordance with ACLS® protocols, particularly in the following cases:
- Torsade de pointes: administer IV magnesium sulphate.
- QTc prolongation or hemodynamic instability: short-acting, cardioselective beta-blockers (e.g., esmolol).
3.3. Hallucinogens/Psychedelics
3.3.1. LSD and Lysergamide-Type NPS
- Avoidance of any stimulation (keep them in a quiet room).
- Attempt to de-escalate in the event of psychomotor agitation.
- Sedation with benzodiazepines (e.g., midazolam, 1–2 mg IV) is the first-line treatment.
- Body temperature monitoring, IV rehydration, and external cooling if necessary.
- Avoid serotonergic antiemetics (e.g., ondansetron); prefer non-5-HT3 alternatives. Severe cases:
- Intensive care hospitalisation.
- Active cooling (e.g., cooling blankets or cold baths).
- Respiratory or hemodynamic support, if necessary.
- Cyproheptadine (a 5-HT2A antagonist) may be considered if serotonin syndrome is confirmed. Due to the lack of an IV formulation, its use is limited in the acute setting, but it can be administered via nasogastric tube in a sedated patient. The recommended initial dose is 12 mg orally, followed by 2 mg every two hours. The maximum dose is 32 mg per 24 h. This molecule is, however, not available in all countries.
3.3.2. Cannabinoids
Natural Cannabinoids (THC)
Synthetic Cannabinoids
4. Implications for Practice and Training
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Over 3 Million Annual Deaths Due to Alcohol and Drug Use, Majority Among Men. Available online: https://www.who.int/news/item/25-06-2024-over-3-million-annual-deaths-due-to-alcohol-and-drug-use-majority-among-men (accessed on 5 July 2025).
- Drug-Related Hospital Emergency Presentations in Europe: Update from the Euro-DEN Plus Expert Network. Office of the European Union: Luxembourg, 2020; Available online: https://www.drugsandalcohol.ie/31612/ (accessed on 5 July 2025).
- Office Fédéral de la Santé Publique OFSP. Décès Liés à la Consommation de Drogue en Suisse. Available online: https://www.bag.admin.ch/dam/fr/sd-web/KkcNTaUDsaCx/faktenblatt-drogentodesfaelle-2017.pdf (accessed on 1 April 2025).
- Office Fédéral de la Santé Publique. Plan de Mesures de la Stratégie Nationale Addictions 2017–2024; S. l. Office Fédéral de la Santé Publique: Köniz, Switzerland, 2016. [Google Scholar]
- Décès dus à la Drogue|MonAM|OFSP. Available online: https://ind.obsan.admin.ch/fr/indicator/monam/deces-dus-a-la-drogue (accessed on 2 April 2025).
- Addiction: Évolution des Admissions en Traitement|MonAM|OFSP. Available online: https://ind.obsan.admin.ch/fr/indicator/monam/addiction-evolution-des-admissions-en-traitement (accessed on 2 April 2025).
- Abuse NI on D. Drug Overdose Deaths: Facts and Figures|National Institute on Drug Abuse (NIDA). 2024. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 5 July 2025).
- Les Cathinones de Synthèse: Fiche Drogue. Available online: https://www.euda.europa.eu/publications/drug-profiles/synthetic-cathinones_fr (accessed on 2 April 2025).
- Kuropka, P.; Zawadzki, M.; Szpot, P. A review of synthetic cathinones emerging in recent years (2019–2022). Forensic Toxicol. 2023, 41, 25–46. [Google Scholar] [CrossRef]
- Alertes Substances—Infodrog.ch. Available online: https://www.infodrog.ch/fr/aide/alertes-substances.html (accessed on 1 April 2025).
- Guéniat, O. Histoire, Production et Trafic de la Cocaïne. Available online: https://www.grea.ch/sites/default/files/1_article_10.pdf (accessed on 5 July 2025).
- MacNeil, S.D.; Rotenberg, B.; Sowerby, L.; Allen, B.; Richard, L.; Shariff, S.Z. Medical use of cocaine and perioperative morbidity following sinonasal surgery—A population study. PLoS ONE 2020, 15, e0236356. [Google Scholar] [CrossRef] [PubMed]
- Monitorage Suisse des Addictions—Cocaïne. Available online: https://www.suchtmonitoring.ch/fr/5.html?cocaine (accessed on 2 April 2025).
- Roque Bravo, R.; Faria, A.C.; Brito-da-Costa, A.M.; Carmo, H.; Mladěnka, P.; Dias da Silva, D.; Remião, F. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- Irwin and Rippe’s Intensive Care Medicine: Print. Available online: https://shop.lww.com/Irwin-and-Rippe-s-Intensive-Care-Medicine--Print---eBook-with-Multimedia/p/9781975181444?srsltid=AfmBOoqTzl9p8NFM4vMbyHVbWympmgTVc6MYcGUw-oRnc1JGpudh6rR_ (accessed on 2 April 2025).
- Lucyk, S.N. Acute Cardiovascular Toxicity of Cocaine. Can. J. Cardiol. 2022, 38, 1384–1394. [Google Scholar] [CrossRef]
- Maraj, S.; Figueredo, V.M.; Lynn Morris, D. Cocaine and the heart. Clin. Cardiol. 2010, 33, 264–269. [Google Scholar] [CrossRef]
- Farooq, U.; Alcantar, D.; Ahmed, Z.; Abegunde, A.T. Outcomes of Vasoconstrictor-Induced Non-Occlusive Mesenteric Ischemia of the Colon: A Systematic Review. Clin. Med. Res. 2022, 20, 164–169. [Google Scholar] [CrossRef]
- Glauser, J.; Queen, J.R. An overview of non-cardiac cocaine toxicity. J. Emerg. Med. 2007, 32, 181–186. [Google Scholar] [CrossRef]
- Richards, J.R.; Garber, D.; Laurin, E.G.; Albertson, T.E.; Derlet, R.W.; Amsterdam, E.A.; Olson, K.R.; Ramoska, E.A.; Lange, R.A. Treatment of cocaine cardiovascular toxicity: A systematic review. Clin. Toxicol. 2016, 54, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.S.; Howland, M.A.; Lewin, N.A.; Smith, S.W.; Goldfrank, L.R.; Hoffman, R.S. Goldfrank’s Toxicologic Emergencies, 11th ed.; McGraw Hill/Medical: New York, NY, USA, 2019; p. 4. [Google Scholar]
- Edeleanu, L. XLII.—Some derivatives of phenylmethacrylic acid. J. Chem. Soc. Trans. 1888, 53, 558–561. [Google Scholar] [CrossRef]
- Deniker, P. Les agents pharmacodynamiques en psychopathologie et en psychiatrie. Bull. Psychol. 1964, 17, 700–708. [Google Scholar] [CrossRef]
- Drugs for ADHD. Med. Lett. Drugs Ther. 2020, 62, 9–15.
- Christophersen, A.S. Amphetamine designer drugs—An overview and epidemiology. Toxicol. Lett. 2000, 112–113, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Nations Unies: Office des Nations Unies Contre la Drogue et le Crime. Les Nouvelles Substances Psychoactives Posent de Lourds Défis aux Systèmes de Santé Publique, a Déclaré le Directeur de l’ONUDC lors du Lancement de Haut Niveau du Rapport Mondial sur les Drogues 2013. Available online: https://www.unodc.org/unodc/fr/frontpage/2013/June/new-psychoactive-substances-pose-severe-challenges-to-public-health-systems-says-unodc-chief-at-launch-of-world-drug-report-2013.html (accessed on 6 July 2025).
- Monitorage Suisse des Addictions—Amphétamines, Ecstasy et Stimulants Similaires. Available online: https://www.suchtmonitoring.ch/fr/8.html?amphetamines-ecstasy (accessed on 3 April 2025).
- Consommation de Substances Illégales (hors Cannabis; âge: 15–64)|MonAM|OFSP. Available online: https://ind.obsan.admin.ch/fr/indicator/monam/consommation-de-substances-illegales-hors-cannabis-age-15-64 (accessed on 2 April 2025).
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Reith, M.E.A.; Gnegy, M.E. Molecular Mechanisms of Amphetamines. Handb. Exp. Pharmacol. 2020, 258, 265–297. [Google Scholar]
- Waters, K. Pharmacologic Similarities and Differences Among Hallucinogens. J. Clin. Pharmacol. 2021, 61 (Suppl. S2), S100–S113. [Google Scholar] [CrossRef]
- Paratz, E.D.; Cunningham, N.J.; MacIsaac, A.I. The Cardiac Complications of Methamphetamines. Heart Lung Circ. 2016, 25, 325–332. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M.G. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010, 48, 675–694. [Google Scholar] [CrossRef]
- Carvalho, M.; Carmo, H.; Costa, V.M.; Capela, J.P.; Pontes, H.; Remião, F.; Carvalho, F.; Bastos, M.L. Toxicity of amphetamines: An update. Arch. Toxicol. 2012, 86, 1167–1231. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Chen, J.H.; Lee, M.H.; Deng, J.F. Fatal and nonfatal methamphetamine intoxication in the intensive care unit. J. Toxicol. Clin. Toxicol. 1994, 32, 147–155. [Google Scholar] [CrossRef]
- Banks, M.L.; Worst, T.J.; Rusyniak, D.E.; Sprague, J.E. Synthetic cathinones (‘bath salts’). J. Emerg. Med. 2014, 46, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Kiyatkin, E.A.; Sharma, H.S. Acute methamphetamine intoxication: Brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int. Rev. Neurobiol. 2009, 88, 65–100. [Google Scholar]
- Ghosh, S.M.; Kung, J.Y.; Crockford, D.; Harpur, L.; Tanguay, R.; Dyer, D.; Lang, E.; Ayas, T.; Feng, G.; VandenBerg, S.D. Systematic review protocol to determine the most effective pharmacological and non-pharmacological interventions for the management of acute methamphetamine toxicity. BMJ Open 2024, 14, e083089. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, N.; Krampe-Scheidler, A.; Christ, M.; Fleischmann, H.; Looser, W.; Schoett, K.; Vilsmeier, F.; Bothe, L.; Schaefer, C.; Gouzoulis-Mayfrank, E. Evidence-Based Guidelines for the Pharmacological Management of Acute Methamphetamine-Related Disorders and Toxicity. Pharmacopsychiatry 2017, 50, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Senon, J.L.; Valleur, M.; Collectif; Kahn, A. Dictionnaire des Drogues et des Dépendances; Larousse: Paris, France, 2009; p. 768. [Google Scholar]
- Le Marec, C. Histoire de l’opium médicinal: Du pavot aux alcaloïdes de l’opium. Douleurs Eval. Diagn. Trait. 2004, 5, 83–98. [Google Scholar]
- Boysen, P.G.; Patel, J.H.; King, A.N. Brief History of Opioids in Perioperative and Periprocedural Medicine to Inform the Future. Ochsner J. 2023, 23, 43–49. [Google Scholar] [CrossRef]
- Narcotics and Psychotropic Substances. Available online: https://www.bfs.admin.ch/content/bfs/en/home/statistics/crime-criminal-justice/police/narcotic-drugs-substances.html (accessed on 3 April 2025).
- Traitements par Agonistes Opioïdes|MonAM|OFSP. Available online: https://ind.obsan.admin.ch/fr/indicator/monam/traitements-par-agonistes-opiodes (accessed on 3 April 2025).
- Hooijman, M.F.; la Torre, A.M.D.; Weiler, S.; Burden, A.M. Opioid sales and opioid-related poisonings in Switzerland: A descriptive population-based time-series analysis. Lancet Reg. Health Eur. 2022, 20, 100437. Available online: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762%2822%2900131-4/fulltext (accessed on 3 April 2025). [CrossRef] [PubMed]
- Daoust, R.; Paquet, J.; Moore, L.; Cournoyer, A.; Émond, M.; Gosselin, S.; Lavigne, G.; Boulanger, A.; Mac-Thiong, J.-M.; Chauny, J.M. Opioid Poisoning and Opioid Use Disorder in Older Trauma Patients. Clin. Interv. Aging 2020, 15, 763–770. [Google Scholar] [CrossRef]
- Fiches d’Information—Infodrog.ch. Available online: https://www.infodrog.ch/fr/publications/fiches-d-information.html (accessed on 3 April 2025).
- Masson, E. EM-Consulte. Pharmacologie des Opioïdes. Available online: https://www.em-consulte.com/article/1191442/pharmacologie-des-opioides (accessed on 3 April 2025).
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Doshi, R.; Majmundar, M.; Kansara, T.; Desai, R.; Shah, J.; Kumar, A.; Patel, K. Frequency of Cardiovascular Events and In-hospital Mortality with Opioid Overdose Hospitalizations. Am. J. Cardiol. 2019, 124, 1528–1533. [Google Scholar] [CrossRef]
- Krantz, M.J.; Palmer, R.B.; Haigney, M.C.P. Cardiovascular Complications of Opioid Use: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 205–223. [Google Scholar] [CrossRef]
- Opioid-Associated Out-of-Hospital Cardiac Arrest: Distinctive Clinical Features and Implications for Health Care and Public Responses: A Scientific Statement from the American Heart Association. Available online: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000958?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org (accessed on 12 November 2025).
- 2023 American Heart Association Focused Update on the Management of Patients with Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care|Circulation. Available online: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001161?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org (accessed on 12 November 2025).
- Saari, T.I.; Strang, J.; Dale, O. Clinical Pharmacokinetics and Pharmacodynamics of Naloxone. Clin. Pharmacokinet. 2024, 63, 397–422. [Google Scholar] [CrossRef]
- Boyer, E.W. Management of Opioid Analgesic Overdose. N. Engl. J. Med. 2012, 367, 146–155. [Google Scholar] [CrossRef]
- Rivera, J.V.; Vance, E.G.; Rushton, W.F.; Arnold, J.K. Novel Psychoactive Substances and Trends of Abuse. Crit. Care Nurs. Q. 2017, 40, 374–382. [Google Scholar] [CrossRef]
- Beck, F.; Bonnet, N. The substance ou l’histoire mouvementée du LSD. Med. Sci. 2013, 29, 430–433. [Google Scholar]
- Hofmann, A. LSD, Mon Enfant Terrible; Lezard: Paris, France, 1997; p. 255. [Google Scholar]
- Hallucinogènes—Consommation. Addiction Suisse. Available online: https://www.addictionsuisse.ch/faits-et-chiffres/autres-substances-illegales/hallucinogenes/hallucinogenes-consommation/ (accessed on 7 July 2025).
- The Polypharmacology of Psychedelics Reveals Multiple Targets for Potential Therapeutics: Neuron. Available online: https://www.cell.com/neuron/abstract/S0896-6273(25)00470-2?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627325004702%3Fshowall%3Dtrue (accessed on 12 November 2025).
- Kozell, L.B.; Eshleman, A.J.; Swanson, T.L.; Bloom, S.H.; Wolfrum, K.M.; Schmachtenberg, J.L.; Olson, R.J.; Janowsky, A.J.; Abbas, A.I. Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter. J. Pharmacol. Exp. Ther. 2023, 385, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Gumpper, R.H.; Nichols, D.E. Chemistry/structural biology of psychedelic drugs and their receptor(s). Br. J. Pharmacol. 2024. ahead of print. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/bph.17361 (accessed on 12 November 2025).
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Johnson, R.A.; Janowsky, A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors. Biochem. Pharmacol. 2018, 158, 27–34. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Comai, S.; Posa, L.; Gobbi, G. d-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. Int. J. Mol. Sci. 2016, 17, 1953. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Duflou, J.; Peacock, A.; Farrell, M.; Hall, W.; Lappin, J. A retrospective study of the characteristics and toxicology of cases of lysergic acid diethylamide (LSD)- and psilocybin-related death in Australia. Addiction 2024, 119, 1564–1571. [Google Scholar] [CrossRef]
- Blaho, K.; Merigian, K.; Winbery, S.; Geraci, S.A.; Smartt, C. Clinical pharmacology of lysergic acid diethylamide: Case reports and review of the treatment of intoxication. Am. J. Ther. 1997, 4, 211–221. [Google Scholar] [CrossRef]
- Adamowicz, P. Blood concentrations of synthetic cannabinoids. Clin. Toxicol. 2021, 59, 246–251. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, Metabolism, and Toxicology of Cannabis: Clinical Implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Foster, B.C.; Abramovici, H.; Harris, C.S. Cannabis and Cannabinoids: Kinetics and Interactions. Am. J. Med. 2019, 132, 1266–1270. [Google Scholar] [CrossRef]
- Moutaouakkil, Y.; Mounir, R.; Cadi, M.A.E.; Lamsaouri, J.; Bousliman, Y.; ElJaoudi, R. Toxicologie des cannabinoïdes de synthèse. Ann. Biol. Clin. 2024, 82, 151–173. [Google Scholar]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Massoure, P.L.; Fourcade, L. Exercise-induced acute coronary syndrome in a 24-year-old man with massive cannabis consumption. Acta Cardiol. 2013, 68, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Caldicott, D.G.E.; Holmes, J.; Roberts-Thomson, K.C.; Mahar, L. Keep off the grass: Marijuana use and acute cardiovascular events. Eur. J. Emerg. Med. 2005, 12, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Bisconti, M.; Marulli, G.; Pacifici, R.; Sollitto, F.; Nex, G.; Trabucco, X.; Ardò, N.P.; Rotolo, M.C.; De Iaco, G.; Panza, T.; et al. Cannabinoids Identification in Lung Tissues of Young Cannabis Smokers Operated for Primary Spontaneous Pneumothorax and Correlation with Pathologic Findings. Respiration 2019, 98, 503–511. [Google Scholar] [CrossRef]
- Bisconti, M.; De Palma, A.; Pacifici, R.; Rotolo, M.C.; Pichini, S.; Brascia, D.; Trabucco, X.; Pellegrini, M.; Carrozzi, L.; Pistelli, F.; et al. Spontaneous Pneumothorax Secondary to Bullous Lung Emphysema Positive for Cannabinoids upon Toxicological Examination. J. Clin. Med. 2023, 12, 4956. [Google Scholar] [CrossRef]
- Moritz, E.; Austin, C.; Wahl, M.; DesLauriers, C.; Navon, L.; Walblay, K.; Hendrickson, M.; Phillips, A.; Kerins, J.; Pennington, A.F.; et al. Notes from the Field: Outbreak of Severe Illness Linked to the Vitamin K Antagonist Brodifacoum and Use of Synthetic Cannabinoids—Illinois, March–April 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 607–608. [Google Scholar] [CrossRef]
- Lurie, Y.; Nadir, Y.; Hoffman, R.; Miller, A.; Efrati, E.; Ring, G.; Sonenfeld, D.; Bar, N.; Zaidani, H.; Strizevsky, A.; et al. An outbreak of severe coagulopathy in northern Israel among users of illicit synthetic cannabinoids adulterated with brodifacoum. Clin. Toxicol. 2023, 61, 429–435. [Google Scholar] [CrossRef]
- Kelkar, A.H.; Smith, N.A.; Martial, A.; Moole, H.; Tarantino, M.D.; Roberts, J.C. An Outbreak of Synthetic Cannabinoid-Associated Coagulopathy in Illinois. N. Engl. J. Med. 2018, 379, 1216–1223. [Google Scholar] [CrossRef]
- Thornton, S.L.; Wood, C.; Friesen, M.W.; Gerona, R.R. Synthetic cannabinoid use associated with acute kidney injury. Clin. Toxicol. 2013, 51, 189–190. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Acute kidney injury associated with synthetic cannabinoid use—Multiple states, 2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 93–98. [Google Scholar]
- Buser, G.L.; Gerona, R.R.; Horowitz, B.Z.; Vian, K.P.; Troxell, M.L.; Hendrickson, R.G.; Houghton, D.C.; Rozansky, D.; Su, S.W.; Leman, R.F. Acute kidney injury associated with smoking synthetic cannabinoid. Clin. Toxicol. 2014, 52, 664–673. [Google Scholar] [CrossRef]
- Bhanushali, G.K.; Jain, G.; Fatima, H.; Leisch, L.J.; Thornley-Brown, D. AKI associated with synthetic cannabinoids: A case series. Clin. J. Am. Soc. Nephrol. 2013, 8, 523–526. [Google Scholar] [CrossRef]
- Rose, D.Z.; Guerrero, W.R.; Mokin, M.V.; Gooch, C.L.; Bozeman, A.C.; Pearson, J.M.; Burgin, W.S. Hemorrhagic stroke following use of the synthetic marijuana ‘spice’. Neurology 2015, 85, 1177–1179. [Google Scholar] [CrossRef]
- Freeman, M.J.; Rose, D.Z.; Myers, M.A.; Gooch, C.L.; Bozeman, A.C.; Burgin, W.S. Ischemic stroke after use of the synthetic marijuana ‘spice’. Neurology 2013, 81, 2090–2093. [Google Scholar] [CrossRef]
- Coble, N.; Mulay, P.; Funk, A.; Arnold, J.; Wiese, M. Notes from the Field: Coagulopathy Associated with Brodifacoum Poisoning—Florida, December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M. Hemorrhagic Highs from Synthetic Cannabinoids—A New Epidemic. N. Engl. J. Med. 2018, 379, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
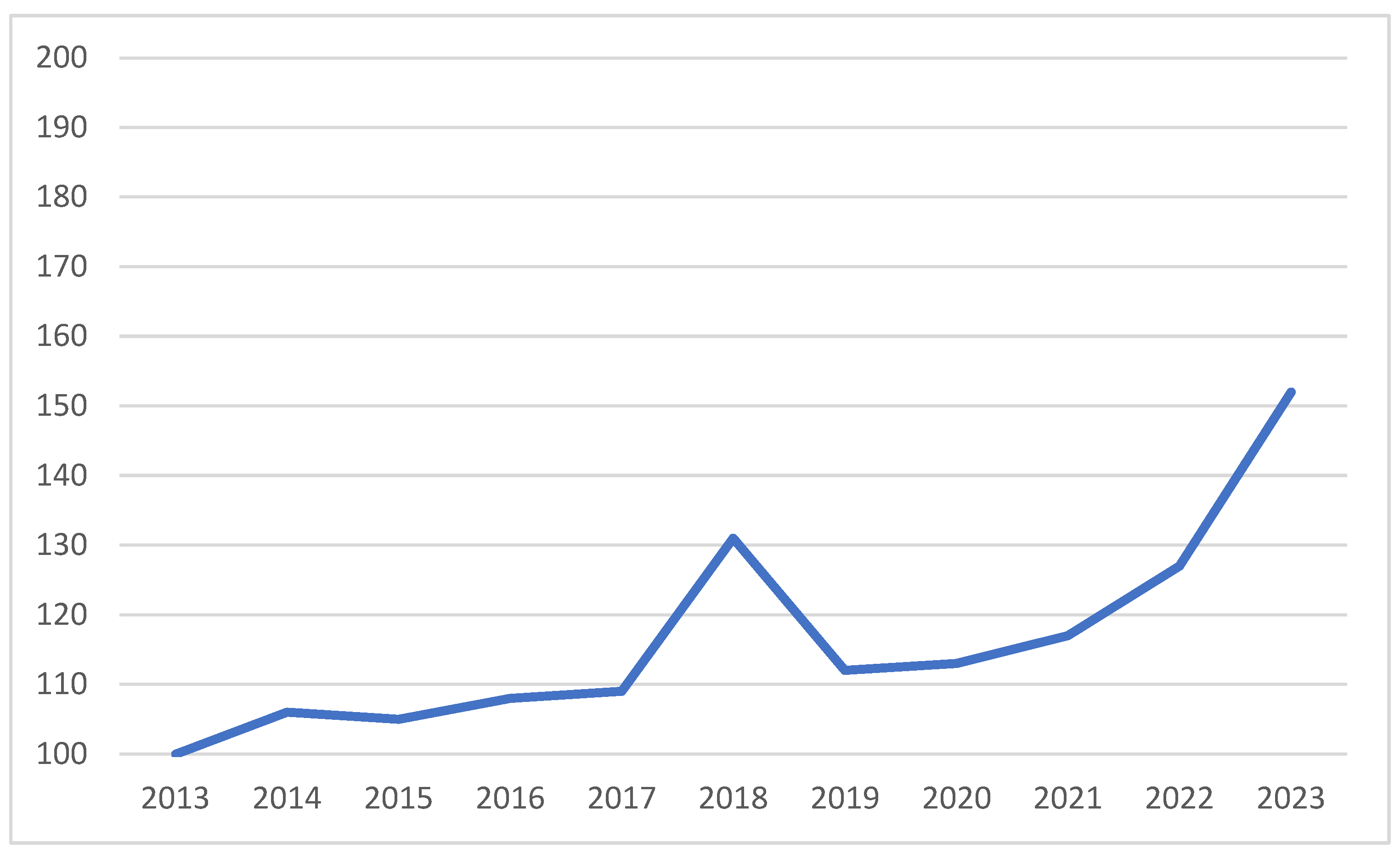
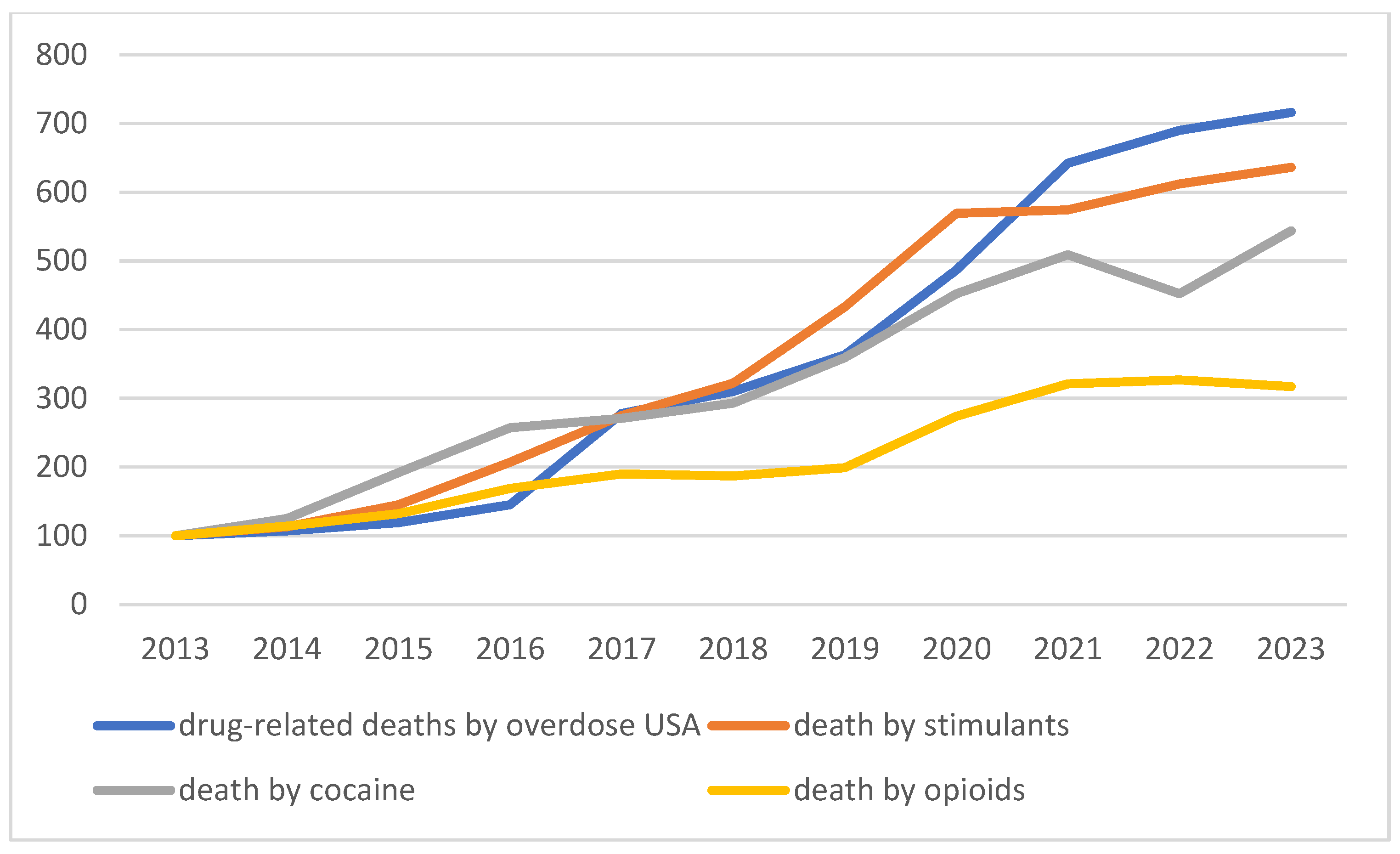
| Drug | Typical Clinical Features | Pitfalls | Treatment |
|---|---|---|---|
| Cocaine | Tachycardia, hypertension, arrhythmias, chest pain, myocardial infarction, hyperthermia (rhabdomyolysis, DIC), mydriasis, ischemic strokes, intracranial haemorrhages, seizures, agitation, psychotic episodes. |
|
|
| Amphetamines and cathinones | Sympathomimetic toxidrome: tachycardia, hypertension, myocardial ischemia, mydriasis, agitation, hallucinations, psychosis, hyperthermia (rhabdomyolysis, DIC), seizures, ischemic stroke. |
|
|
| Opioids (heroin, morphine, fentanyl, tramadol, oxycodone, loperamide) | Respiratory depression, bradypnea, coma, miosis, bradycardia, hypotension, constipation, seizures (tramadol), pseudoallergic reactions. |
|
|
| Hallucinogens (LSD, psilocybin, lysergamide-type NPS) | Visual/auditory hallucinations, agitation, delirium, panic attacks, serotonin syndrome (HTN, hyperthermia, seizures), hyponatraemic cerebral oedema, metabolic acidosis and acute renal failure. |
|
|
| Cannabinoids (THC, synthetics) | Asthma exacerbation, tachycardia, thoracic pain, seizures (synthetics), hallucinations, seizures (synthetics), hyperthermia (synthetics), hyperemesis, acute anxiety, panic attacks, agitation. |
|
|
| Complication | Management | Additional Measures/Remarks |
|---|---|---|
| Hyperthermia |
|
|
| Seizures |
|
|
| Status epilepticus |
|
|
| Cerebral oedema |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bless, P.; Blaser, D.; Castelain, T.; Pugnale, S.; Ribordy, V.; Guechi, Y. Clinical Approach to Acute Recreational Drug Intoxication in the Emergency Setting: A Practical Guide Based on Swiss Experience. Toxics 2025, 13, 1034. https://doi.org/10.3390/toxics13121034
Bless P, Blaser D, Castelain T, Pugnale S, Ribordy V, Guechi Y. Clinical Approach to Acute Recreational Drug Intoxication in the Emergency Setting: A Practical Guide Based on Swiss Experience. Toxics. 2025; 13(12):1034. https://doi.org/10.3390/toxics13121034
Chicago/Turabian StyleBless, Patrick, Diane Blaser, Thomas Castelain, Sébastien Pugnale, Vincent Ribordy, and Youcef Guechi. 2025. "Clinical Approach to Acute Recreational Drug Intoxication in the Emergency Setting: A Practical Guide Based on Swiss Experience" Toxics 13, no. 12: 1034. https://doi.org/10.3390/toxics13121034
APA StyleBless, P., Blaser, D., Castelain, T., Pugnale, S., Ribordy, V., & Guechi, Y. (2025). Clinical Approach to Acute Recreational Drug Intoxication in the Emergency Setting: A Practical Guide Based on Swiss Experience. Toxics, 13(12), 1034. https://doi.org/10.3390/toxics13121034






