Assessment of Bone Mineral Density, Total Body Composition and Joint Integrity in Long COVID: A 12-Month Longitudinal Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Study Population
- Participants who had hospitalisation due to COVID-19 requiring intubation, ICU admission, or ventilatory support (to exclude post-intensive care syndrome);
- Individuals with pre-existing osteoporosis or metabolic bone diseases (e.g., primary hyperparathyroidism, osteogenesis imperfecta);
- Those undergoing long-term corticosteroid therapy (≥5 mg prednisolone daily) or taking bisphosphonates, denosumab, or teriparatide;
- Pregnant or breastfeeding women, due to the use of ionising radiation in DXA scans;
- Participants with recent fractures (<12 months) or conditions affecting joint health, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).
2.3. Data Collection and Assessments
2.3.1. DXA Assessment
2.3.2. Ultrasound Assessment
- Synovial hypertrophy: Abnormal hypoechoic intra-articular tissue that is non-displaceable and poorly compressible, and which may exhibit a Doppler signal.
- Synovial effusion: Abnormal hypoechoic or anechoic intraarticular material that is displaceable and compressible but does not exhibit a Doppler signal.
- PD signal intensity: Area of colour signal within the joint capsule in the absence of background noise. Only when there is hypoechoic synovial hypertrophy.
2.4. Statistical Analysis
Power Calculation
3. Results
3.1. Demographics and Characteristics
3.2. Comparison of BMD Between LC and WR Groups
3.3. Total Body Composition
3.3.1. Gynoid Region
3.3.2. Android Region
3.3.3. Leg Region and Total Lean Mass
3.4. Intra-Articular Changes
4. Discussion
4.1. Long COVID Associated with Increased Total Body Composition in Both Android and Gynoid Areas
4.2. No Association of Bone Mineral Density in Long COVID
4.3. Long COVID Linked to Persistent Joint Pain with 12-Month Reduction in Hand Synovial Hypertrophy
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Bone Mineral Density |
| DXA | Dual-Energy X-Ray Absorptiometry |
| LC | Long COVID |
| MSK | Musculoskeletal |
| PD | Power Doppler |
| TBC | Total Body Composition |
| WR | Well Recovered |
References
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- National Health Service (NIH). COVID Recovery (Long COVID). Available online: https://www.nhshighland.scot.nhs.uk/your-services/all-services-a-z/covid-recovery-long-covid/ (accessed on 8 January 2025).
- Mahase, E. COVID-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef]
- Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 syndrome in patients with asymptomatic/mild form. Pathogens 2021, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Long-Term Effects of Coronavirus (Long COVID): What Is It? Available online: https://cks.nice.org.uk/topics/long-term-effects-of-coronavirus-long-covid/background-information/definition/ (accessed on 9 September 2025).
- World Health Organization (WHO). Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 31 May 2025).
- Nabavi, N. Long covid: How to define it and how to manage it. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef]
- Bakılan, F.; Gökmen, İ.G.; Ortanca, B.; Uçan, A.; Eker Güvenç, Ş.; Şahin Mutlu, F.; Gökmen, H.M.; Ekim, A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14734. [Google Scholar] [CrossRef]
- Karaarslan, F.; Demircioğlu Güneri, F.; Kardeş, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41, 1263–1271. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, F.; Guneri, F.D.; Kardes, S. Long COVID: Rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 2022, 41, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef]
- Ghosn, J.; Piroth, L.; Epaulard, O.; Le Turnier, P.; Mentré, F.; Bachelet, D.; Laouénan, C. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: Results from a large prospective cohort. Clin. Microbiol. Infect. 2021, 27, 1041.e1–1041.e4. [Google Scholar] [CrossRef]
- Vaishya, R.; Jain, V.K.; Iyengar, K.P. Musculoskeletal manifestations of COVID-19. J. Clin. Orthop. Trauma. 2021, 17, 280–281. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- Montes-Ibarra, M.; Orsso, C.E.; Limon-Miro, A.T.; Gonzalez, M.C.; Marzetti, E.; Landi, F.; Heymsfield, S.B.; Barazzoni, R.; Prado, C.M. Prevalence and clinical implications of abnormal body composition phenotypes in patients with COVID-19: A systematic review. Am. J. Clin. Nutr. 2023, 117, 1288–1305. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Dovjak, P.; Butylina, M.; Rainer, A.; Mayr, B.; Röggla, V.; Haslacher, H.; Weber, M.; Jordakieva, G.; Pietschmann, P. Moderate COVID-19 disease is associated with reduced bone turnover. J. Bone Miner. Res. 2023, 38, 943–950. [Google Scholar] [CrossRef]
- Al-Azzawi, I.S.; Mohammed, N.S.; Saad, I. The impact of angiotensin converting enzyme-2 (ACE-2) on bone remodeling marker osteoprotegerin (OPG) in post-COVID-19 Iraqi patients. Cureus 2022, 14, e29926. [Google Scholar] [CrossRef]
- Atieh, O.; Durieux, J.C.; Baissary, J.; Mouchati, C.; Labbato, D.; Thomas, A.; Merheb, A.; Ailstock, K.; Funderburg, N.; McComsey, G.A. The Long-Term Effect of COVID-19 Infection on Body Composition. Nutrients 2024, 16, 1364. [Google Scholar] [CrossRef] [PubMed]
- Fedorchenko, Y.; Zimba, O. Long COVID in autoimmune rheumatic diseases. Rheumatol. Int. 2023, 43, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Danda, D.; Kavadichanda, C.; Das, S.; Adarsh, M.B.; Negi, V.S. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol. Int. 2020, 40, 1539–1554. [Google Scholar] [CrossRef]
- Jaladhar, P.; S, C.; Salanke, M.; Kori, D. The Pattern of Post-viral Arthritis in COVID Pandemic State: An Experience of Tertiary Care Centre. J. Assoc. Physicians India 2021, 69, 11–12. [Google Scholar]
- Gulzar, R.; Rasheed, A.; Riaz, S.; Adnan, W.A.; Hafeez, U.; Malik, A.M. Musculoskeletal Symptoms in Patients Recovering from COVID-19. Muscles Ligaments Tendons J. (MLTJ) 2022, 12, 9–16. [Google Scholar] [CrossRef]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A. Post-COVID-19 syndrome: Involvement and interactions between respiratory, cardiovascular and nervous systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Bakalets, O.; Dzyha, S.; Behosh, N. Functional diagnostics of the respiratory system in patients with Long COVID. Bull. Med. Biol. Res. 2023, 16, 60–66. [Google Scholar] [CrossRef]
- Jutant, E.-M.; Meyrignac, O.; Beurnier, A.; Jaïs, X.; Pham, T.; Morin, L.; Boucly, A.; Bulifon, S.; Figueiredo, S.; Harrois, A. Respiratory symptoms and radiological findings in post-acute COVID-19 syndrome. ERJ Open Res. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Mokbel, K.; Meertens, R.; Obotiba, A.D.; Alharbi, M.; Knapp, K.M.; Strain, W.D. Bone Mineral Density, Bone Biomarkers, and Joints in Acute, Post, and Long COVID-19: A Systematic Review. Viruses 2024, 16, 1694. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Meertens, R.; Obotiba, A.D.; Harries, L.W.; Appleby, S.; Mokbel, K.; Knapp, K.M.; Strain, W.D. Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort. J. Clin. Med. 2025, 14, 7931. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.-K.; Leslie, W.D. Best practices for dual-energy X-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef]
- Knapp, K.; GJ, B.G. Dual energy x-ray absorptiometry: Quality assurance and governance. Osteoporos. Rev. 2014, 22, 1–6. [Google Scholar]
- Centers for Disease Control Prevention (CDC). Dual Energy X-Ray Absorptiometry (DXA) Procedures Manual—National Health and Nutrition Examination Survey. 2007. Available online: https://wwwn.cdc.gov/nchs/nhanes//continuousnhanes/manuals.aspx?BeginYear=2007 (accessed on 20 May 2023).
- Shuhart, C.; Cheung, A.; Gill, R.; Gani, L.; Goel, H.; Szalat, A. Executive Summary of the 2023 Adult Position Development Conference of the International Society for Clinical Densitometry: DXA reporting, follow-up BMD testing and trabecular bone score application and reporting. J. Clin. Densitom. 2024, 27, 101435. [Google Scholar] [CrossRef]
- Sheffield, U.O. FRAX® Tool. Available online: https://frax.shef.ac.uk/FRAX/tool.aspx?country=9 (accessed on 1 May 2025).
- Hippisley-Cox, J.; Coupland, C. QFracture® Risk Calculator. Available online: https://www.qfracture.org/ (accessed on 1 May 2025).
- National Institute for Health and Care Excellence. Osteoporosis—Prevention of Fragility Fractures. Available online: https://cks.nice.org.uk/topics/osteoporosis-prevention-of-fragility-fractures/ (accessed on 15 March 2025).
- Blake, G.M.; Fogelman, I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad. Med. J. 2007, 83, 509–517. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.; Lewiecki, E.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Backhaus, M.; Burmester, G.; Gerber, T.; Grassi, W.; Machold, K.; Swen, W.; Wakefield, R.; Manger, B. Guidelines for musculoskeletal ultrasound in rheumatology. Ann. Rheum. Dis. 2001, 60, 641–649. [Google Scholar] [CrossRef]
- Filippucci, E.; Unlu, Z.; Farina, A.; Grassi, W. Sonographic training in rheumatology: A self teaching approach. Ann. Rheum. Dis. 2003, 62, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Filippucci, E.; Backhaus, M.; D’Agostino, M.-A.; Sanchez, E.N.; Iagnocco, A.; Schmidt, W.A.; Bruyn, G.A. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487. [Google Scholar]
- Bruyn, G.A.; Iagnocco, A.; Naredo, E.; Balint, P.V.; Gutierrez, M.; Hammer, H.B.; Collado, P.; Filippou, G.; Schmidt, W.A.; Jousse-Joulin, S. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J. Rheumatol. 2019, 46, 1388–1393. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 955–962. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.A.; Conaghan, P.; Le Bars, M.; Baron, G.; Grassi, W.; Martin-Mola, E.; Wakefield, R.; Brasseur, J.-L.; So, A.; Backhaus, M. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: Prevalence of inflammation in osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1703–1709. [Google Scholar] [CrossRef]
- Martinoli, C. Musculoskeletal ultrasound: Technical guidelines. Insights Into Imaging 2010, 1, 99. [Google Scholar] [CrossRef]
- Kanis, J.A.; Burlet, N.; Cooper, C.; Delmas, P.D.; Reginster, J.Y.; Borgstrom, F.; Rizzoli, R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2008, 19, 399–428. [Google Scholar] [CrossRef]
- Sebro, R.; Ashok, S.S. A Statistical Approach Regarding the Diagnosis of Osteoporosis and Osteopenia From DXA: Are We Underdiagnosing Osteoporosis? JBMR Plus 2021, 5, e10444. [Google Scholar] [CrossRef] [PubMed]
- International Society for Clinical Densitometry. 2019 ISCD Official Positions Adult. Available online: https://iscd.org/wp-content/uploads/2021/09/2019-Official-Positions-Adult-1.pdf (accessed on 15 April 2025).
- Leslie, W.; Moayyeri, A. Minimum sample size requirements for bone density precision assessment produce inconsistency in clinical monitoring. Osteoporos. Int. 2006, 17, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Moayyeri, A.; Sadatsafavi, M.; Leslie, W.D.; Program, M.B.D. Sample size requirements for bone density precision assessments and effect on patient categorization: A Monte Carlo simulation study. Bone 2007, 41, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Maas Genannt Bermpohl, F.; Kucharczyk-Bodenburg, A.C.; Martin, A. Efficacy and Acceptance of Cognitive Behavioral Therapy in Adults with Chronic Fatigue Syndrome: A Meta-analysis. Int. J. Behav. Med. 2024, 31, 895–910. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.-W.; Wu, Y.; Rong, L.-J.; Ye, L.; Franco, O.H.; Chien, C.-W.; Feng, X.-R.; Chen, J.-Y.; Tung, T.-H. Prevalence and prognosis of sarcopenia in acute COVID-19 and long COVID: A systematic review and meta-analysis. Ann. Med. 2025, 57, 2519678. [Google Scholar] [CrossRef]
- Vélez-Santamaría, R.; Fernández-Solana, J.; Méndez-López, F.; Domínguez-García, M.; González-Bernal, J.J.; Magallón-Botaya, R.; Oliván-Blázquez, B.; González-Santos, J.; Santamaría-Peláez, M. Functionality, physical activity, fatigue and quality of life in patients with acute COVID-19 and Long COVID infection. Sci. Rep. 2023, 13, 19907. [Google Scholar] [CrossRef]
- Colosio, M.; Brocca, L.; Gatti, M.F.; Neri, M.; Crea, E.; Cadile, F.; Canepari, M.; Pellegrino, M.A.; Polla, B.; Porcelli, S. Structural and functional impairments of skeletal muscle in patients with postacute sequelae of SARS-CoV-2 infection. J. Appl. Physiol. 2023, 135, 902–917. [Google Scholar] [CrossRef]
- Falbová, D.; Beňuš, R.; Sulis, S.; Vorobeľová, L. Effect of COVID-19 pandemic on bioimpedance health indicators in young adults. Am. J. Hum. Biol. 2024, 36, e24110. [Google Scholar] [CrossRef]
- Karatzi, K.; Poulia, K.-A.; Papakonstantinou, E.; Zampelas, A. The Impact of Nutritional and Lifestyle Changes on Body Weight, Body Composition and Cardiometabolic Risk Factors in Children and Adolescents during the Pandemic of COVID-19: A Systematic Review. Children 2021, 8, 1130. [Google Scholar] [CrossRef]
- López-Sampalo, A.; Cobos-Palacios, L.; Vilches-Pérez, A.; Sanz-Cánovas, J.; Vargas-Candela, A.; Mancebo-Sevilla, J.J.; Hernández-Negrín, H.; Gómez-Huelgas, R.; Bernal-López, M.R. COVID-19 in Older Patients: Assessment of Post-COVID-19 Sarcopenia. Biomedicines 2023, 11, 733. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Legarra-Gorgoñon, G.; Oscoz-Ochandorena, S.; García-Alonso, Y.; García-Alonso, N.; Oteiza, J.; Ernaga Lorea, A.; Correa-Rodríguez, M.; Izquierdo, M. Reduced muscle strength in patients with long-COVID-19 syndrome is mediated by limb muscle mass. J. Appl. Physiol. 2023, 134, 50–58. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal consequences of COVID-19. J. Bone Jt. surgery. Am. Vol. 2020, 102, 1197. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur. J. Neurol. 2022, 29, 2832–2841. [Google Scholar] [CrossRef]
- Agergaard, J.; Leth, S.; Pedersen, T.; Harbo, T.; Blicher, J.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Nurkovic, J. COVID-19 impact on bone mineral density. In Proceedings of the World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases, London, UK, 26–29 August 2021; p. S209. [Google Scholar]
- Falbová, D.; Kovalčíková, V.; Beňuš, R.; Sulis, S.; Vorobeľová, L. Effect of COVID-19 pandemic on lifestyle and bone mineral density in young adults. Am. J. Hum. Biol. 2024, 36, e24009. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. J. Orthop. Res.® 2023, 41, 1945–1952. [Google Scholar] [CrossRef]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.-M.; Chan, C.C.-S.; Chu, H.; Yuan, S.; Yuen, T.T.-T.; Chik, K.K.-H.; Tsang, J.O.-L. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat. Commun. 2022, 13, 2539. [Google Scholar] [CrossRef]
- Awosanya, O.D.; Dalloul, C.E.; Blosser, R.J.; Dadwal, U.C.; Carozza, M.; Boschen, K.; Klemsz, M.J.; Johnston, N.A.; Bruzzaniti, A.; Robinson, C.M. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone 2022, 154, 116227. [Google Scholar] [CrossRef]
- Queiroz-Junior, C.M.; Santos, A.C.; Gonçalves, M.R.; Brito, C.B.; Barrioni, B.; Almeida, P.J.; Gonçalves-Pereira, M.H.; Silva, T.; Oliveira, S.R.; Pereira, M.M. Acute coronavirus infection triggers a TNF-dependent osteoporotic phenotype in mice. Life Sci. 2023, 324, 121750. [Google Scholar] [CrossRef]
- Elmedany, S.H.; Badr, O.I.; Abu-Zaid, M.H.; Tabra, S.A.A. Bone mineral density changes in osteoporotic and osteopenic patients after COVID-19 infection. Egypt. Rheumatol. Rehabil. 2022, 49, 64. [Google Scholar] [CrossRef]
- Ingale, D.; Kulkarni, P.; Electricwala, A.; Moghe, A.; Kamyab, S.; Jagtap, S.; Martson, A.; Koks, S.; Harsulkar, A. Synovium-Synovial Fluid Axis in Osteoarthritis Pathology: A Key Regulator of the Cartilage Degradation Process. Genes 2021, 12, 989. [Google Scholar] [CrossRef]
- Gasparotto, M.; Framba, V.; Piovella, C.; Doria, A.; Iaccarino, L. Post-COVID-19 arthritis: A case report and literature review. Clin. Rheumatol. 2021, 40, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.S.; Ishaq Ghauri, M.; Sethar, S.; Afsar, N.; Riaz, A.; Ishaq, K. COVID-19: An emerging culprit of inflammatory arthritis. Case Rep. Rheumatol. 2021, 2021, 6610340. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Gershwin, M.E. Diagnosis and classification of reactive arthritis. Autoimmun. Rev. 2014, 13, 546–549. [Google Scholar] [CrossRef]
- Siva, C.; Velazquez, C.; Mody, A.; Brasington, R. Diagnosing acute monoarthritis in adults: A practical approach for the family physician. Am. Fam. Physician 2003, 68, 83–90. [Google Scholar]
- Parisi, S.; Borrelli, R.; Bianchi, S.; Fusaro, E. Viral arthritis and COVID-19. Lancet Rheumatol. 2020, 2, e655–e657. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, D.; Calabrese, L.H. Virally associated arthritis 2008: Clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res. Ther. 2008, 10, 215. [Google Scholar] [CrossRef]
- Baimukhamedov, C.; Barskova, T.; Matucci-Cerinic, M. Arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021, 3, e324–e325. [Google Scholar] [CrossRef]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. Semin. Musculoskelet. Radiol. 2011, 15, 554–560. [Google Scholar] [CrossRef]
- Grassi, M.; Giorgi, V.; Nebuloni, M.; Zerbi, P.; Gismondo, M.R.; Salaffi, F.; Sarzi-Puttini, P.; Rimoldi, S.G.; Manzotti, A. SARS-CoV-2 in the knee joint: A cadaver study. Clin. Exp. Rheumatol. 2022, 40, 34665699. [Google Scholar] [CrossRef]
- Kuschner, Z.; Ortega, A.; Mukherji, P. A case of SARS-CoV-2-associated arthritis with detection of viral RNA in synovial fluid. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12452. [Google Scholar] [CrossRef]
- Dombret, S.; Skapenko, A.; Schulze-Koops, H. Reactive arthritis after SARS-CoV-2 infection. RMD Open. 2022, 8, e002519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liew, I.Y.; Mak, T.M.; Cui, L.; Vasoo, S.; Lim, X.R. A Case of Reactive Arthritis Secondary to Coronavirus Disease 2019 Infection. JCR J. Clin. Rheumatol. 2020, 26, 233. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Minematsu, N.; Katano, H.; Suzuki, T. Case of acute arthritis following SARS-CoV-2 infection. Ann. Rheum. Dis. 2021, 80, e101. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Kishimoto, M.; Shimasaki, T.; Uchida, H.; Kurai, D.; Deshpande, G.A.; Komagata, Y.; Kaname, S. Reactive arthritis after COVID-19 infection. RMD Open 2020, 6, e001350. [Google Scholar] [CrossRef]
- Grozier, C.D.; Genoese, F.; Collins, K.; Parmar, A.; Tolzman, J.; Kuenze, C.; Harkey, M.S. Knee Effusion-Synovitis Is Not Associated with Self-Reported Knee Pain in Division I Female Athletes. Sports Health 2025, 17, 19417381251323902. [Google Scholar] [CrossRef]
- Kulkarni, P.; Harsulkar, A.; Märtson, A.G.; Suutre, S.; Märtson, A.; Koks, S. Mast Cells Differentiated in Synovial Fluid and Resident in Osteophytes Exalt the Inflammatory Pathology of Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, W.Y.-J.; Sun, F.-H.; Wong, M.C.-S.; Siu, P.M.-F.; Chen, X.-K.; Wong, S.H.-S. Association of sedentary lifestyle with risk of acute and post-acute COVID-19 sequelae: A retrospective cohort study. Am. J. Med. 2023, 138, 298–307. [Google Scholar] [CrossRef] [PubMed]
- De Zwart, A.H.; Dekker, J.; Lems, W.F.; Roorda, L.D.; Van Der Esch, M.; Van Der Leeden, M. Factors associated with upper leg muscle strength in knee osteoarthritis: A scoping review. J. Rehabil. Med. 2018, 50, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Wang, J.; Niu, Y.; Wang, F. Obesity and hyperlipidemia were associated with more severe synovitis and structural abnormalities as well as inferior functional outcomes in knee osteoarthritis: A retrospective comparative study. J. Orthop. Surg. Res. 2024, 19, 845. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | ||||||
|---|---|---|---|---|---|---|
| >Variables | Baseline | Follow-up | ||||
| WR (n = 40) | >LC (n = 45) | > p | >WR (n = 30) | >LC (n = 36) | > p | |
| Age (yr) (₽) | 51 ± 15.17 | 52.22 ± 9.94 | 0.658 | 52.83 ± 14.85 | 53.28 ± 10.08 | 0.885 |
| Sex (Female), n (%) (X) | 19 (47) | 38 (84.45) | <0.001 * | 15 (50) | 29 (80.56) | 0.009 * |
| BMI (kg/m2) (‡) | 26.6 (23.8; 30.65) | 27.9 (24.7; 33) | 0.214 | 25.55 (23.4; 29.3) | 28.8 (23.8; 34.45) | 0.087 |
| Ethnicity, n (%) (¥) | 0.459 | 1.00 | ||||
| White or not stated | 39 (97.5) | 41 (91.1) | 30 (100) | 33 (91.7) | ||
| Indian | 0 (0.0) | 2 (4.4) | 0 (0.0) | 1 (2.8) | ||
| Pakistani | 0 (0.0) | 1 (2.2) | 0 (0.0) | 1 (2.8) | ||
| Black African | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Chinese | 0 (0.0) | 1 (2.22) | 0 (0.0) | 1 (2.8) | ||
| Socio-economic, n (%) (X) | 0.266 | 0.449 | ||||
| Upper | 20 (50) | 23 (51.1) | 15 (50) | 18 (50) | ||
| Upper Middle | 13 (32.5) | 19 (42.2) | 12 (40) | 17 (47.2) | ||
| Lower Middle | 7 (17.5) | 3 (6.7) | 3 (10) | 1 (1.8) | ||
| Smoking status, n (%) (¥) | 0.298 | 0.742 | ||||
| Non-smoker | 26 (65) | 35 (77.8) | 21 (70) | 26 (72.2) | ||
| Ex-smoker | 8 (20) | 8 (17.8) | 4 (13.3) | 6 (16.7) | ||
| Light smoker (less than 10) | 2 (5) | 2 (4.44) | 2 (6.67) | 3 (8.3) | ||
| Moderate smoker (10 to 19) | 3 (7.5) | 0 (0.0) | 1 (3.33) | 1 (2.8) | ||
| Heavy smoker (20 or over) | 1 (2.5) | 0 (0.0) | 2 (6.67) | 0 (0.0) | ||
| Alcohol status, n (%) (¥) | 0.526 | 0.396 | ||||
| Non | 15 (37.5) | 22 (48.89) | 16 (53.33) | 21 (58.3) | ||
| <1 unit per day | 12 (11.3) | 10 (22.2) | 7 (23.33) | 9 (25) | ||
| 1–2 units per day | 9 (22.5) | 8 (17.8) | 4 (13.33) | 4 (11.1) | ||
| 3–6 units per day | 1 (2.5) | 4 (8.9) | 3 (10) | 0 (0.0) | ||
| 7–9 units per day | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (2.8) | ||
| >9 units per day | 2 (5) | 1 (2.22) | 0 (0.0) | 1 (2.8) | ||
| Hormonal Replacement Therapy, n (%) (X) | 3 (7.5) | 8 (17.8) | 0.159 | |||
| Supplementation of Vitamin D, n (%) (X) | 6 (20) | 14 (38.9) | 0.080 | |||
| Bone Health Medication, n (%) (X) | - | - | - | 5 (16.7) | 1 (2.8) | 0.084 |
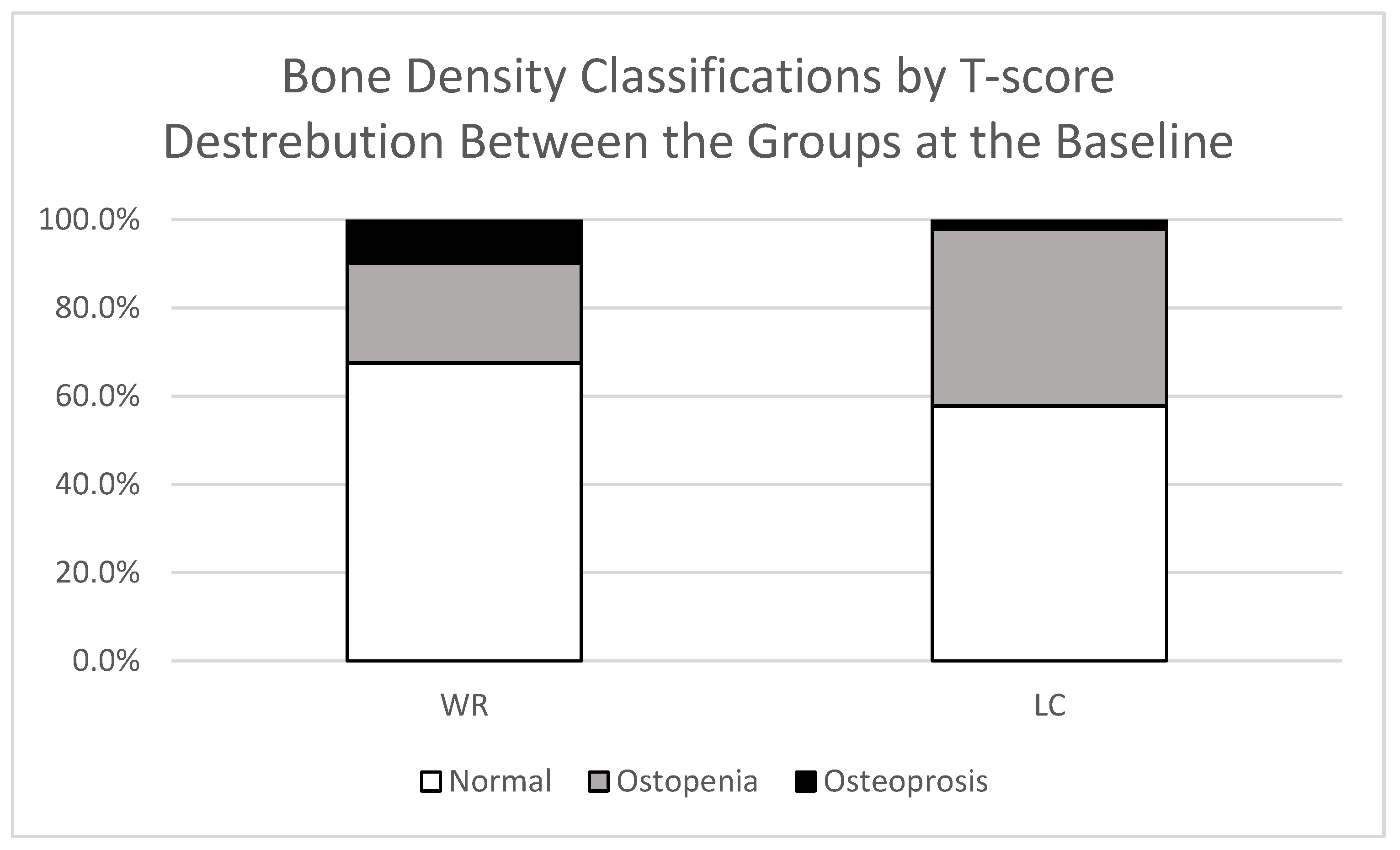
| DXA T-Score Baseline Results Between the Study Group WR and LC Participants. | |||||
|---|---|---|---|---|---|
| T-score | n = (WR/LC) | WR | LC | p | |
| Total Body (₽) | (39/45) | 0.534 ± 1.379 | 1.037 ± 1.161 | 0.073 | |
| L1–L4 (₽) | (32/33) | 0.244 ± 1.789 | 0.138 ± 1.184 | 0.780 | |
| Total Hip (₽) | RT | (36/37) | −0.247 ± 1.099 | 0.015 ± 1.036 | 0.263 |
| LT | (39/44) | −0.273 ± 1.174 | −0.033 ± 1.043 | 0.326 | |
| Fracture Risk (%) | |||||
| Major osteoporotic (‡) | (27/40) | 5.7 (3.3; 11.8) | 4.85 (2.85; 8.4) | 0.165 | |
| Hip (‡) | 0.5 (0.2; 2.5) | 0.45 (0.1; 0.9) | 0.221 | ||
| Musculoskeletal Imaging Results Comparing WR and LC Participants. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Baseline | Follow-Up | ||||||||
| Side | (n) | WR | LC | p | (n) | WR | LC | p | ||
| BMD | Total body (₽) | - | (39/45) | 1.219 ± 0.127 | 1.223 ± 0.099 | 0.876 | (30/36) | 1.215 ± 0.143 | 1.219 ± 0.104 | 0.909 |
| L1–L4 (₽) | - | (32/33) | 1.232 ± 0.221 | 1.204 ± 0.142 | 0.547 | (23/28) | 1.233 ± 0.258 | 1.195 ± 0.151 | 0.519 | |
| Femoral neck (₽) | Rt | (39/45) | 0.967 ± 0.144 | 0.974 ± 0.134 | 0.819 | (30/36) | 0.961 ± 0.144 | 0.98 ± 0.143 | 0.605 | |
| Lt | (39/45) | 0.969 ± 0.154 | 0.976 ± 0.152 | 0.843 | (30/36) | 0.965 ± 0.157 | 0.997 ± 0.223 | 0.519 | ||
| Total hip (₽) | Rt | (39/45) | 1.026 ± 0.160 | 1.024 ± 0.138 | 0.959 | (30/36) | 1.023 ± 0.176 | 1.029 ± 0.152 | 0.885 | |
| Lt | (39/44) | 1.023 ± 0.173 | 1.017 ± 0.135 | 0.876 | (30/34) | 1.021 ± 0.188 | 1.018 ± 0.149 | 0.938 | ||
| TBC | Gynoid Region Fat (%) (‡) | (39/45) | 0.402 ± 0.089 | 0.471 ± 0.084 | <0.001 * | (30/36) | 0.399 ± 0.087 | 0471 ± 0.077 | 0.001 * | |
| Gynoid Tissue Fat (%) (‡) | 0.411 ± 0.090 | 0.481 ± 0.085 | <0.001 * | 0.399 ± 0.0878 | 0.471 ± 0.077 | 0.001 * | ||||
| Gynoid Fat Mass (g) (‡) | 5171 ± 2040 | 6419 ± 2507 | 0.009 * | 5107 ± 2111 | 6355 ± 2221 | 0.008 * | ||||
| Gynoid Lean Mass (g) (‡) | 7175 ± 1691 | 6589 ± 1469 | 0.088 | 7199 ± 1824 | 6653 ± 1609 | 0.221 | ||||
| Android Region Fat (%) (‡),(₽) | 0.421±0.101 | 0.474 ± 0.112 | 0.006 * | 0.418 ± 0.094 | 0.483 ± 0.092 | 0.006 * | ||||
| Android Tissue Fat (%) (‡),(₽) | 0.426 ± 0.101 | 0.478 ± 0.112 | 0.006 * | 0.422 ± 0.094 | 0.487 ± 0.092 | 0.006 * | ||||
| Android Region Fat Mass (g) (‡) | 2809 ± 1439 | 3483 ± 1817 | 0.067 | 2731 ± 1481 | 3524 ± 1761 | 0.064 | ||||
| Legs Tissue Fat (%) (‡),(₽) | 0.363 ± 0.102 | 0.439 ± 0.101 | 0.001 * | 0.360 ± 0.106 | 0.437 ± 0.095 | 0.002 * | ||||
| Legs Lean Mass (g) (‡) | 16,156 ± 3655 | 14,526 ± 3464 | 0.026 | 15,863 ± 3585 | 14,629 ± 3686 | 0.132 | ||||
| Total Lean Mass (g) (‡) | 48,914 ± 10,060 | 45,326 ± 10,155 | 0.054 | 48,391 ± 10,804 | 45,763 ± 10,739 | 0.236 | ||||
| Intra-Articular | Hand Synovial Hypertrophy (‡) | (40/45) | 2 (1; 4) | 3 (1; 5) | 0.502 | (30/36) | 1.5 (0; 3) | 1 (0; 3) | 0.742 | |
| Hand Synovial Effusion (‡) | 0 (0; 1) | 0 (0; 1) | 0.684 | 0 (0; 1) | 0 (0; 0) | 0.212 | ||||
| Hand Power Doppler (‡) | 1 (0; 3) | 1 (0; 4) | 0.274 | 1 (0; 2) | 0.5 (0; 2) | 0.695 | ||||
| Knee Synovial Hypertrophy (X) | 13 (32.5) | 5 (11.1) | 0.016 | 7 (23.3) | 4 (11.1) | 0.185 | ||||
| Knee Synovial Effusion (X) | 18 (45) | 6 (13.3) | 0.001 * | 14 (46.7) | 13 (36.1) | 0.385 | ||||
| Knee Power Doppler (X) | (32/42) | 0 (0) | 0 (0) | 1.000 | (30/33) | 2 (6.67) | 2 (6.06) | 0.922 | ||
| Within the LC and WR Group, Changes in Musculoskeletal Imaging Results. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Side | (n) | WR | (n) | LC | |||||
| Baseline | Follow-up | p | Baseline | Follow-up | p | |||||
| BMD | Total body (P) | - | 30 | 1.216 ± 0.142 | 1.215 ± 0.143 | 0.837 | 36 | 1.224 ± 0.106 | 1.219 ± 0.104 | 0.068 |
| L1–L4 (P) | - | 23 | 1.235 ± 0.251 | 1.233 ± 0.258 | 0.812 | 24 | 1.197 ± 0.144 | 1.183 ± 0.148 | 0.173 | |
| Femoral neck (P) | Rt | 30 | 0.958 ± 0.148 | 0.961 ± 0.144 | 0.513 | 36 | 0.984 ± 0.144 | 0.98 ± 0.143 | 0.463 | |
| Lt | 0.97 ± 0.16 | 0.965 ± 0.157 | 0.310 | 0.976 ± 0.164 | 0.997 ± 0.223 | 0.158 | ||||
| Total hip (P) | Rt | 1.016 ± 0.171 | 1.023 ± 0.175 | 0.025 | 1.028 ± 0.146 | 1.029 ± 0.152 | 0.779 | |||
| Lt | 1.018 ± 0.185 | 1.021 ± 0.188 | 0.307 | 34 | 1.016 ± 0.146 | 1.018 ± 0.149 | 0.494 | |||
| TBC | Gynoid Region Fat (%) (ω) | 0.401 ± 0.083 | 0.399 ± 0.087 | 0.926 | 36 | 0.465 ± 0.085 | 0.471 ± 0.077 | 0.055 | ||
| Gynoid Tissue Fat (%) (ω) | 0.410 ± 0.084 | 0.399 ± 0.087 | 0.066 | 0.475 ± 0.085 | 0.471 ± 0.077 | 0.271 | ||||
| Gynoid Fat Mass (g) (ω) | 5187 ± 2110 | 5107 ± 2111 | 0.517 | 6259 ± 2319 | 6355 ± 2221 | 0.029 | ||||
| Gynoid Lean Mass (g) (ω) | 7236 ± 1858 | 7199 ± 1824 | 0.416 | 6654 ± 1539 | 6653 ± 1609 | 0.851 | ||||
| Android Region Fat (%) (ω) | 0.417 ± 0.097 | 0.417 ± 0.093 | 0.228 | 0.476 ± 0.106 | 0.482 ± 0.092 | 0.087 | ||||
| Android Region Fat Mass (g) (ω) | 2792 ± 1539 | 2731 ± 1481 | 0.428 | 3511 ± 1789 | 3524 ± 1761 | 0.307 | ||||
| Android Tissue Fat (%) (ω) | 0.421 ± 0.097 | 0.422 ± 0.094 | 0.229 | 0.480 ± 0.107 | 0.487 ± 0.092 | 0.102 | ||||
| Legs Tissue Fat (%) (ω) | 0.363 ± 0.099 | 0.360 ± 0.105 | 0.585 | 0.430 ± 0.101 | 0.437 ± 0.095 | 0.015 | ||||
| Legs Lean Mass (g) (ω) | 16,134 ± 4006 | 15,863 ± 3585 | 0.236 | 14,754 ± 3652 | 14,629 ± 3686 | 0.489 | ||||
| Total Lean Mass (g) (ω) | 48,759 ± 10,980 | 48,391 ± 10,804 | 0.089 | 45,991 ± 10,815 | 45,763 ± 10,739 | 0.441 | ||||
| Intra-Articular | Hand Synovial Hypertrophy (ω) | 2 (1; 4) | 1.5 (0; 3) | 0.123 | 2 (1; 5) | 1 (0; 3) | 0.012 | |||
| Hand Synovial Effusion (ω) | 0 (0; 1) | 0 (0; 1) | 0.702 | 0 (0; 0) | 0 (0; 0) | 0.139 | ||||
| Hand Power Doppler (ω) | 1.5 (0; 4) | 1 (0; 3) | 0.481 | 1 (0; 2) | 0.5 (0; 2) | 0.228 | ||||
| Knee Synovial Hypertrophy (₼) | 4 (66.7) | 2 (33.3) | 0.687 | 1 (33.3) | 2 (66.7) | 1.000 | ||||
| Knee Synovial Effusion (₼) | 4 (50) | 4 (50) | 1.000 | 2 (15.4) | 11 (84.6) | 0.023 | ||||
| Knee Power Doppler (₼) | 26 | 0 (0) | 2 (100) | 0.500 | 31 | 0 (0) | 2 (100) | 0.500 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, F.; Obotiba, A.D.; Meertens, R.; Alshalawi, O.; Mokbel, K.; Strain, W.D.; Knapp, K.M. Assessment of Bone Mineral Density, Total Body Composition and Joint Integrity in Long COVID: A 12-Month Longitudinal Feasibility Study. J. Clin. Med. 2025, 14, 8558. https://doi.org/10.3390/jcm14238558
Alghamdi F, Obotiba AD, Meertens R, Alshalawi O, Mokbel K, Strain WD, Knapp KM. Assessment of Bone Mineral Density, Total Body Composition and Joint Integrity in Long COVID: A 12-Month Longitudinal Feasibility Study. Journal of Clinical Medicine. 2025; 14(23):8558. https://doi.org/10.3390/jcm14238558
Chicago/Turabian StyleAlghamdi, Fahad, Abasiama Dick Obotiba, Robert Meertens, Omar Alshalawi, Kinan Mokbel, William David Strain, and Karen M. Knapp. 2025. "Assessment of Bone Mineral Density, Total Body Composition and Joint Integrity in Long COVID: A 12-Month Longitudinal Feasibility Study" Journal of Clinical Medicine 14, no. 23: 8558. https://doi.org/10.3390/jcm14238558
APA StyleAlghamdi, F., Obotiba, A. D., Meertens, R., Alshalawi, O., Mokbel, K., Strain, W. D., & Knapp, K. M. (2025). Assessment of Bone Mineral Density, Total Body Composition and Joint Integrity in Long COVID: A 12-Month Longitudinal Feasibility Study. Journal of Clinical Medicine, 14(23), 8558. https://doi.org/10.3390/jcm14238558






