- Review
Marine-Derived Defenses Against HIV: Emerging Bioactive Molecules from the Seas
- Tiago Santos,
- Ana Pintão and
- Pedro Brandão
- + 1 author
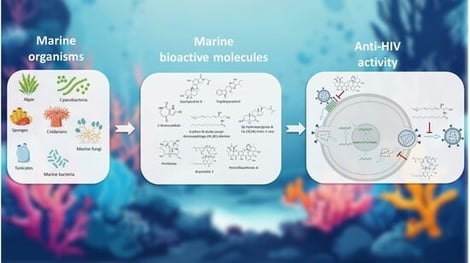
Marine ecosystems have yielded a remarkable diversity of bioactive metabolites with relevance for antiviral drug discovery. This article reviews recent advances in marine-derived compounds investigated as anti-HIV agents. Metabolites, such as sulfated polysaccharides, lectins, alkaloids, and terpenoids, display inhibitory activity across multiple stages of the HIV life cycle, including viral entry, reverse transcription, integration, and maturation. From sponge-inspired development of AZT to the application of Griffithin in clinical trials for the prophylaxis of the HIV infection, recent discoveries showcase the chemical diversity of marine ecosystems and validate their utility as hit and compound sources in drug discovery. We highlight possible mechanisms of action, as well as translational hurdles from research to clinical trials. Overall, marine biodiversity represents a valuable and underexploited reservoir for the development of novel HIV therapeutics.
7 February 2026




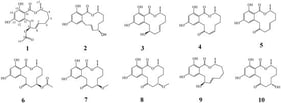
![Structures of brevetoxin A (A) and B (B) backbones [7].](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/marinedrugs/marinedrugs-24-00067/article_deploy/html/images/marinedrugs-24-00067-g001-550.jpg)

