- Article
Fibroblast-Derived ECM as a Donor-Specific Pro-Osteogenic Coating Surpassing ASC- and Osteoblast-Derived ECM
- Kevin Arnke,
- Hans-Christoph Pape and
- Paolo Cinelli
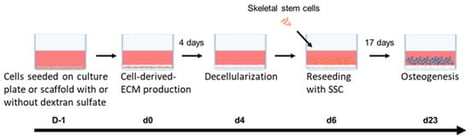
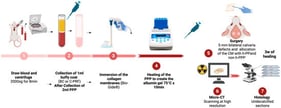
Large bone defects remain a major clinical challenge, as current treatments primarily provide mechanical stability while often insufficiently addressing the biological microenvironment. The cell-deposited extracellular matrix (CD-ECM) represents a promising strategy to improve implant bioactivity by mimicking key features of the native tissue. In this study, we compared CD-ECMs from adipose tissue-derived mesenchymal stromal cells (ASCs), ASC-derived osteoprogenitor cells, and dermal fibroblasts. ECM composition was analyzed, and its ability to support the osteogenesis of reseeded skeletal stem cells (SSCs) was assessed. Subsequently, the best performing cells were used to produce CD-ECM on a 3D scaffold. Furthermore, we improved the ECM by treating the ECM-producing cells with dextran sulfate (Dx-S). Fibroblast-derived ECM showed higher collagen and glycosaminoglycan contents compared to ASC-ECM or osteoprogenitor-ECM. Furthermore, only the fibroblast-derived ECM (Fibro-ECM) exerted a supportive effect on the osteogenesis of SSCs. SSCs seeded on ECM showed a higher proliferation rate and enhanced osteogenesis. Supplementation with dextran sulfate further increased ECM deposition and osteogenic potential. We showed that fibroblasts produced substantially more ECM with a stronger pro-osteogenic effect than ASCs or osteoprogenitor cells. The ECM and its pro-osteogenic effect could further be increased when fibroblasts were treated with Dx-S. Together, these results highlight Fibro-ECM as a promising and easily accessible cell-derived ECM deposition strategy to improve the biological performance of implants in bone regeneration.
14 February 2026