The Pathogenesis of Neurological Disorders
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Neuroscience".
Viewed by 52662Editor
Interests: Alzheimer’s disease; Parkinson’s disease; stroke; DNA damage; neuroinflammation; oxidative stress and antioxidants; regulation of motor function; gut microbiota in neurological diseases; long noncoding RNAs and CNS in regulation of muscle weakness
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
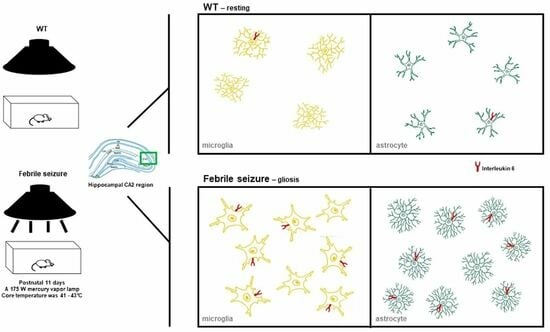
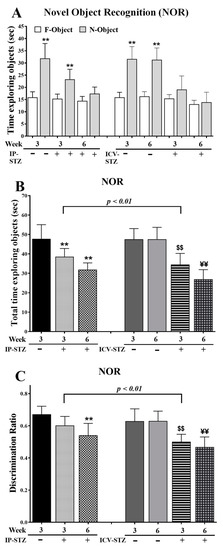
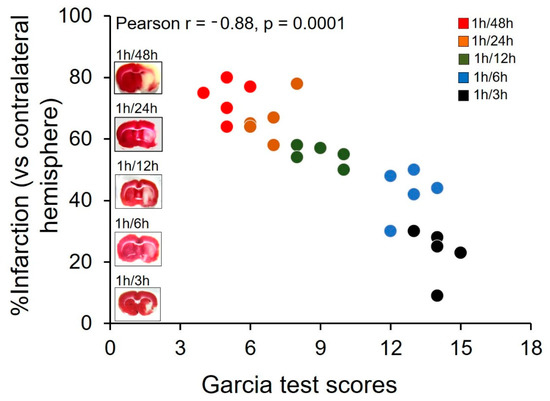
Neurological disorders are the leading cause of disability and second major cause of death worldwide. Currently, there are no therapeutic interventions that prevent or slow the progression of neurological disorders. The onset of neurological diseases mainly occurs in mid- to late life, so the prevalence is expected to increase as the population ages. Knowledge of the precise molecular and cellular mechanisms underlying neurodegeneration remains incomplete, and these gaps in our knowledge of fundamental neurobiology are a major barrier to therapeutic discovery. Thus, there is an unmet need to develop new and more effective therapeutic strategies that provide a higher quality of life for individuals affected with neurological disorders, which will only be achieved with a better understanding of the disease mechanisms.
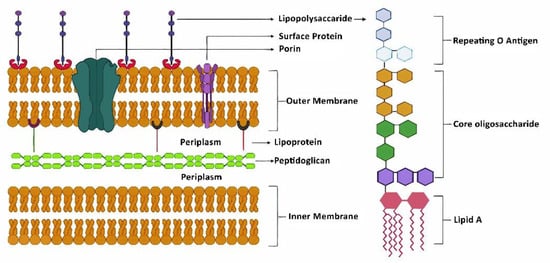
During the past several decades, a growing body of research on several aspects has increased our understanding of neurological disorders. The aggregation and deposition of specific proteins is hypothesized to underlie several neurological disorders. However, the mechanistic connection between the process of protein aggregation and neuronal loss is not yet fully understood. Our knowledge of the role of oxidative stress, neuroinflammation, and neuro-immune interactions in the pathogenesis of neurological disorders has increased over the past several decades, but therapeutic and diagnostic interventions using this knowledge have not been widely implemented. For decades, the pathogenesis of neurlogical disorders has mostly been studied in the context of the brain; however, the contribution of peripheral influences in the development and function is largely unexplored. Alteration in gut microbiome composition, often referred to as gut dysbiosis, has recently been suggested to contribute to the pathogenesis of several neurological disorders. Similarly, alterations in the expressions of noncoding, RNAs especially microRNAs, have been extensively studied in neurological disorder; however, the functional role of lncRNAs in neurological disorders is still not understood well.
This Topical Collection shall highlight the global research efforts aimed at exploring causes, identifying disease biomarkers, and strategies/therapies that can attenuate disease symptoms and reduce the social and economic impact of neurological disorders. For this Topical Collection, we invite articles from researchers addressing their findings on novel mechanisms involving neuroinflammation, oxidative stress, gut–brain interaction, and lncRNAs in neurological disorders. Additionally, we hope to attract studies aimed at discovering disease biomarkers for early diagnosis and novel therapeutic approaches or strategies to prevent or strop the progression of neurological disorders.
Dr. Mohammad Moshahid Khan
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- neurodegeneration
- neuroinflammation
- oxidative stress
- lncRNAs
- brain damage
- gut–brain axis
- neuroprotection