Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Febrile Seizure Induction
2.3. Behavioral Tests
2.3.1. Social Recognition Test: Sociability and Social Novelty
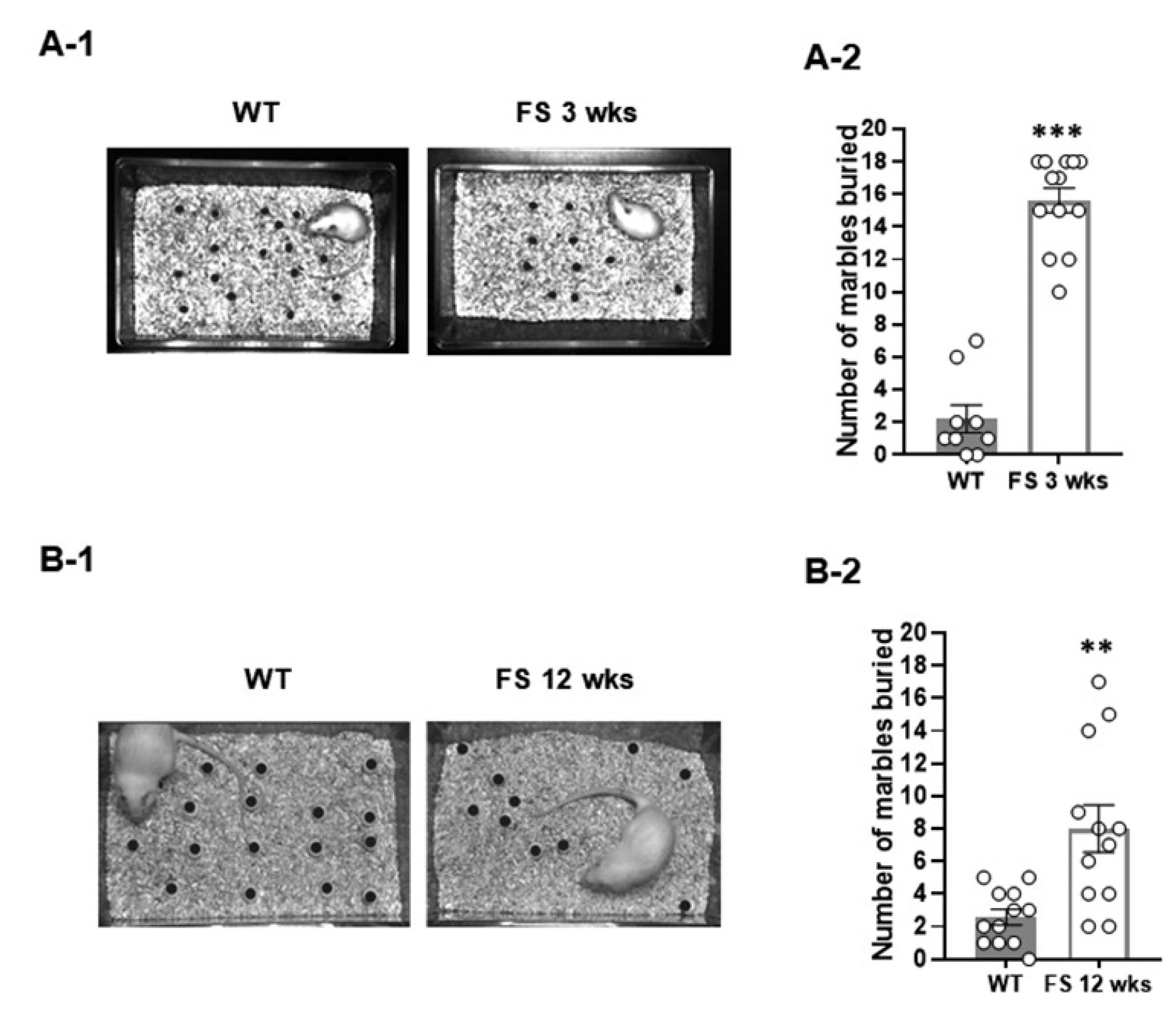
2.3.2. Marble-Burying Test
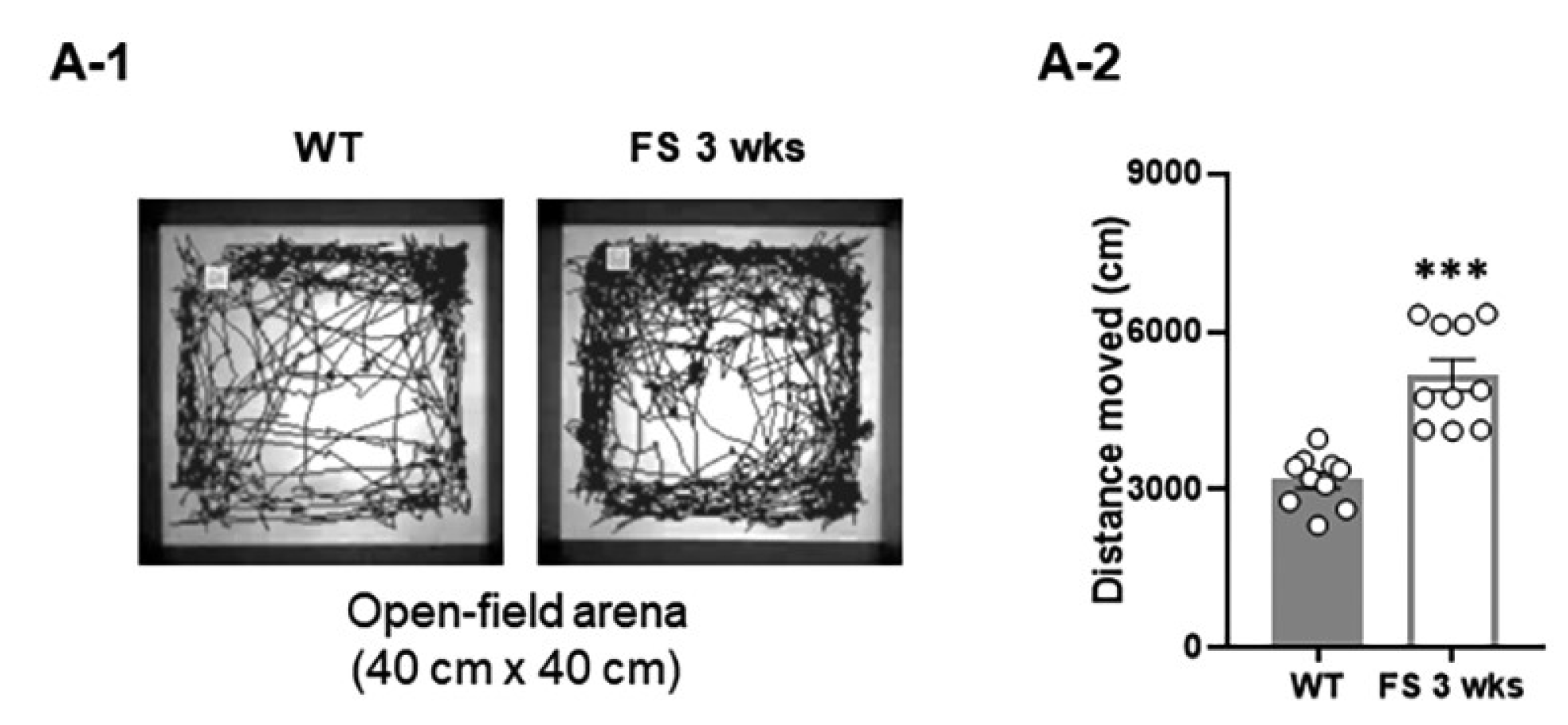
2.3.3. Open-Field Test
2.4. Tissue Processing
2.5. Immunohistochemistry and Immunofluorescence
2.6. Quantification of Data and Statistical Analysis
3. Results
3.1. FS Model Rats Exhibit Deficits of Social Novelty, Increased Repetitive Behaviors, and Hyperlocomotion
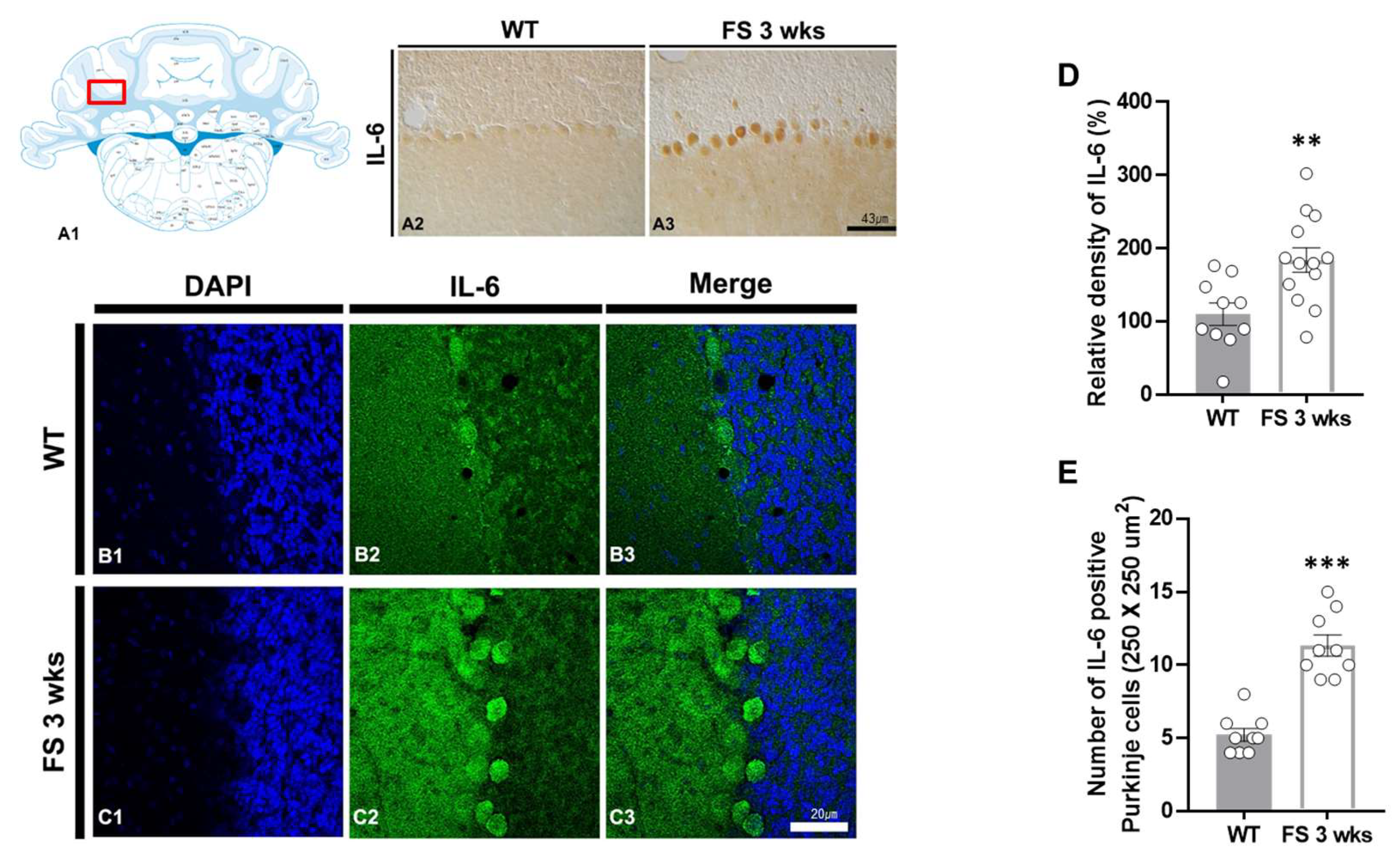
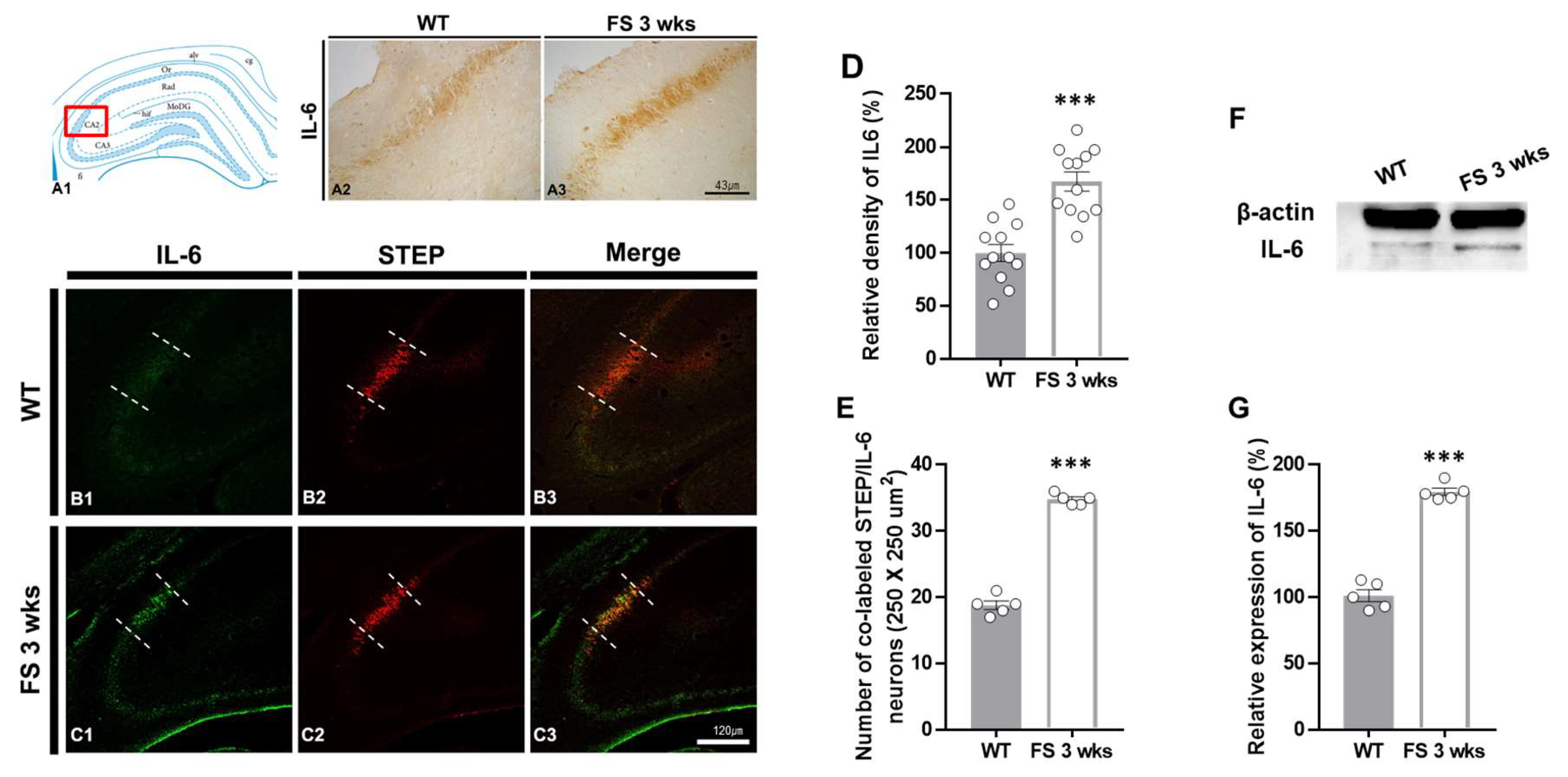
3.2. IL-6 Overexpression in the Hippocampal CA2 Region and Cerebellum of FS Model Rats
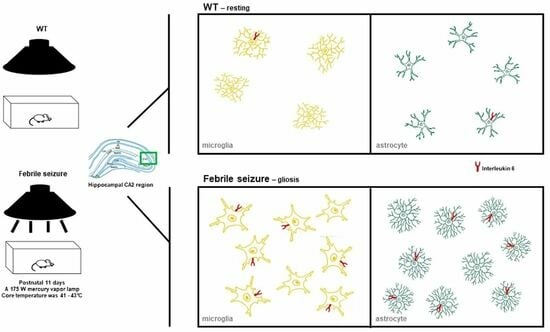
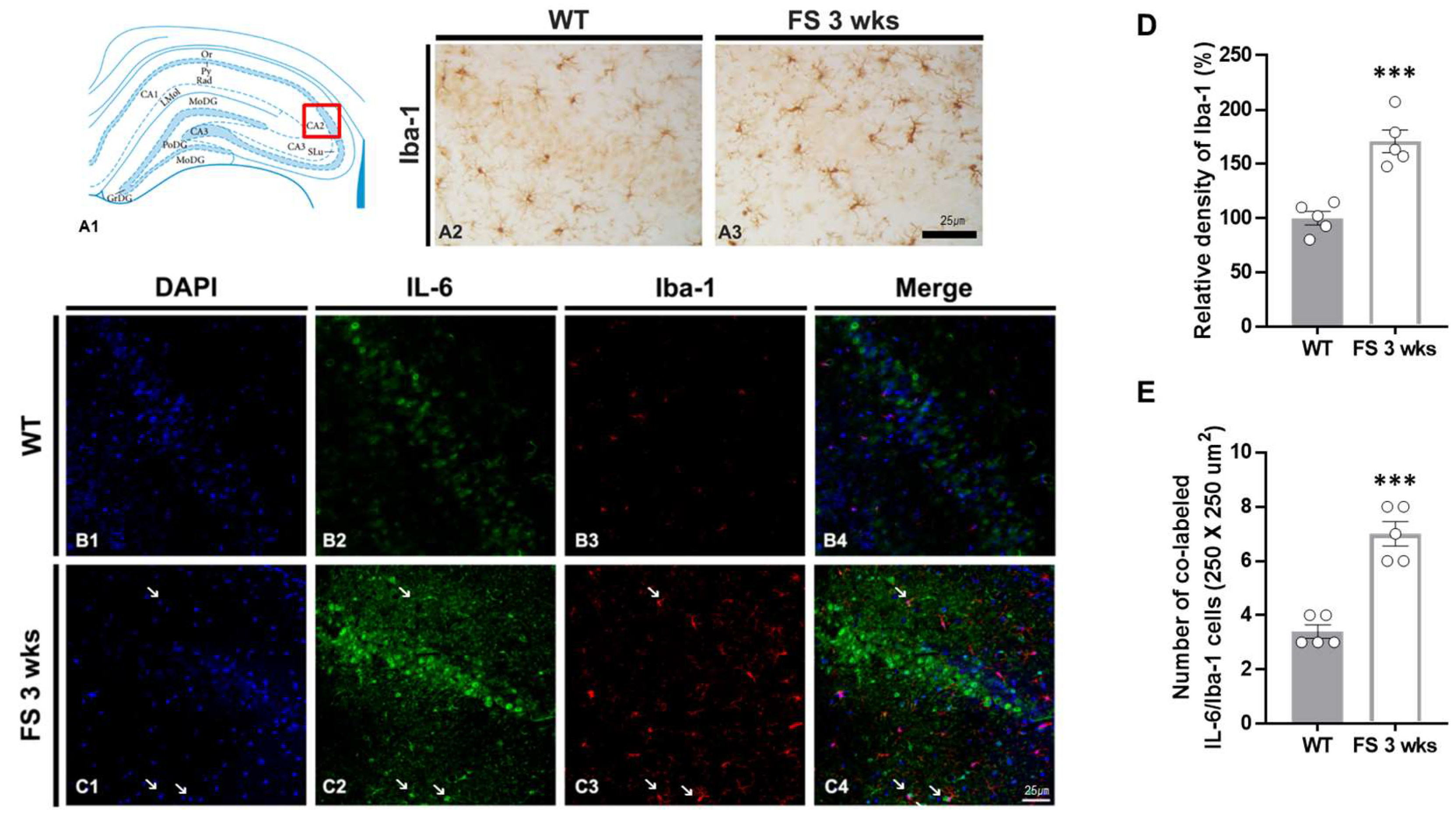
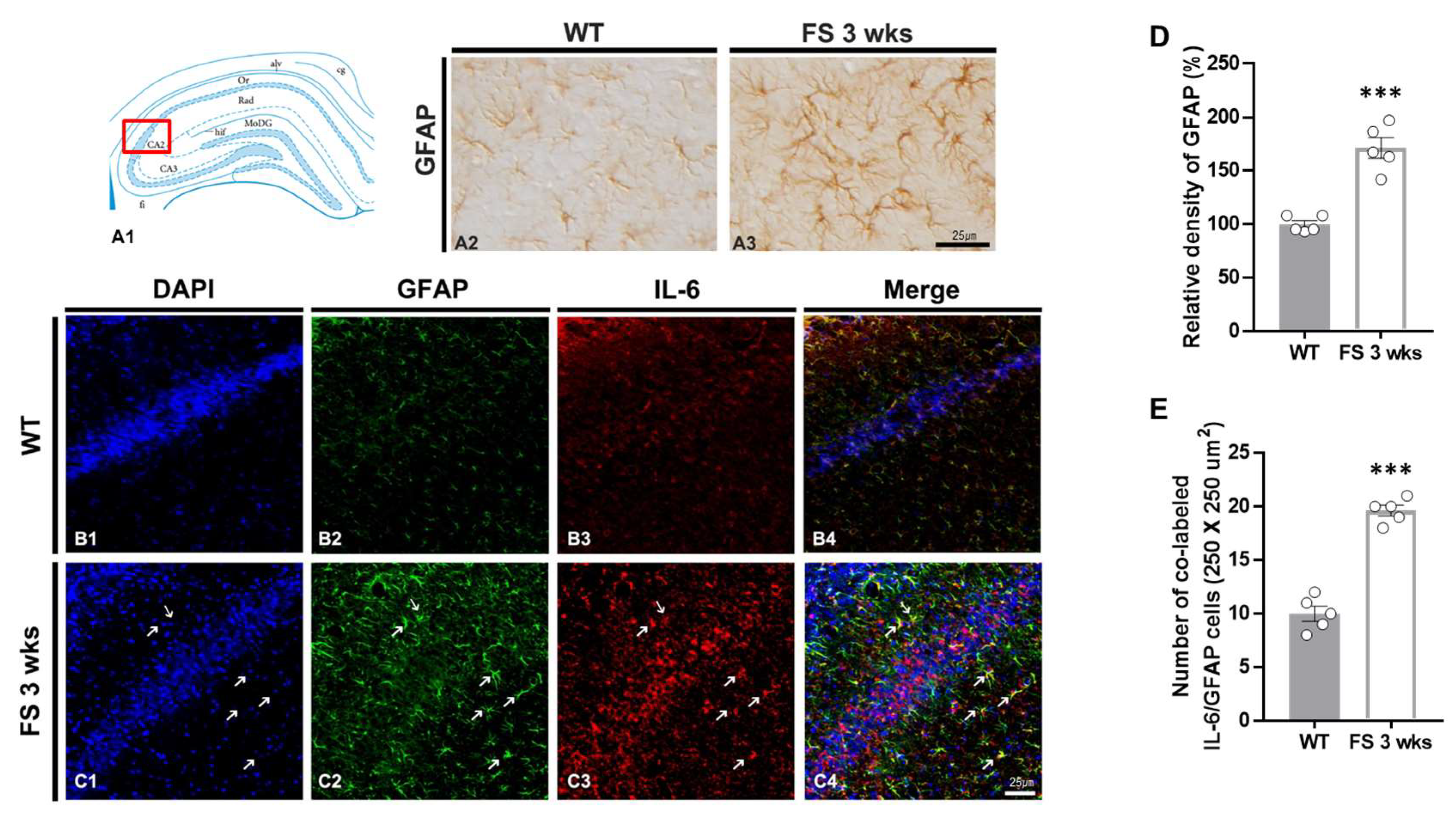
3.3. Gliosis in the Hippocampal CA2 Region Following Hyperthermic Convulsions
4. Discussion
4.1. Disordered Social Novelty and Increased Repetitive Behaviors after Hyperthermic Convulsions
4.2. Hyperkinesia-like Personality Induced by Locomotor Deficits following FS
4.3. Correlation between the Social Recognition Disorders and the Altered Functionalities of Hippocampal CA2 Neuronal Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postnikova, T.Y.; Griflyuk, A.V.; Amakhin, D.V.; Kovalenko, A.A.; Soboleva, E.B.; Zubareva, O.E.; Zaitsev, A.V. Early Life Febrile Seizures Impair Hippocampal Synaptic Plasticity in Young Rats. Int. J. Mol. Sci. 2021, 22, 8218. [Google Scholar] [CrossRef]
- Mosili, P.; Maikoo, S.; Mabandla, M.V.; Qulu, L. The Pathogenesis of Fever-Induced Febrile Seizures and Its Current State. Neurosci. Insights 2020, 15, 2633105520956973. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Lee, K.; Sin, D.S.; Park, K.H.; Park, D.K.; Kim, D.S. Altered Functional Efficacy of Hippocampal Interneuron during Epileptogenesis Following Febrile Seizures. Brain Res. Bull. 2017, 131, 25–38. [Google Scholar] [CrossRef]
- Holmes, G.L. Effects of Early Seizures on Later Behavior and Epileptogenicity. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Dutton, S.B.B.; Dutt, K.; Papale, L.A.; Helmers, S.; Goldin, A.L.; Escayg, A. Early-Life Febrile Seizures Worsen Adult Phenotypes in Scn1a Mutants. Exp. Neurol. 2017, 293, 159–171. [Google Scholar] [CrossRef]
- Holmes, G.L. Drug Treatment of Epilepsy Neuropsychiatric Comorbidities in Children. Pediatr. Drugs 2021, 23, 55–73. [Google Scholar] [CrossRef]
- Gillberg, C.; Lundström, S.; Fernell, E.; Nilsson, G.; Neville, B. Febrile Seizures and Epilepsy: Association With Autism and Other Neurodevelopmental Disorders in the Child and Adolescent Twin Study in Sweden. Pediatr. Neurol. 2017, 74, 80–86.e2. [Google Scholar] [CrossRef]
- Castelhano, A.S.S.; Cassane, G.d.S.T.; Scorza, F.A.; Cysneiros, R.M. Altered Anxiety-Related and Abnormal Social Behaviors in Rats Exposed to Early Life Seizures. Front. Behav. Neurosci. 2013, 7, 36. [Google Scholar] [CrossRef]
- Narita, M.; Oyabu, A.; Imura, Y.; Kamada, N.; Yokoyama, T.; Tano, K.; Uchida, A.; Narita, N. Nonexploratory Movement and Behavioral Alterations in a Thalidomide or Valproic Acid-Induced Autism Model Rat. Neurosci. Res. 2010, 66, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lin, W.D.; Chou, I.C.; Lee, I.C.; Hong, S.Y. Is Preterm Birth a Risk Factor for Subsequent Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder in Children with Febrile Seizure?—A Retrospective Study. Life 2021, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Rommelse, N.N.J.; Franke, B.; Geurts, H.M.; Hartman, C.A.; Buitelaar, J.K. Shared Heritability of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Eur. Child Adolesc. Psychiatry 2010, 19, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.W.; Im, H.; Song, Y.; Kim, S.J.; Lee, Y.R.; Kim, G.W.; Hwang, C.; Park, D.K.; Kim, D.S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells 2022, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a Mental Cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.M.; Jennische, E.; Hansson, H.A.; Holmäng, A. Prenatal Exposure to Interleukin-6 Results in Inflammatory Neurodegeneration in Hippocampus with NMDA/GABAA Dysregulation and Impaired Spatial Learning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1345–R1356. [Google Scholar] [CrossRef]
- Parker-Athill, E.C.; Tan, J. Maternal Immune Activation and Autism Spectrum Disorder: Interleukin-6 Signaling as a Key Mechanistic Pathway. NeuroSignals 2011, 18, 113–128. [Google Scholar] [CrossRef]
- Heida, J.G.; Pittman, Q.J. Causal Links between Brain Cytokines and Experimental Febrile Convulsions in the Rat. Epilepsia 2005, 46, 1906–1913. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Breyer, R.M.; Chen, C. Altered Hippocampal Long-Term Synaptic Plasticity in Mice Deficient in the PGE2 EP2 Receptor. J. Neurochem. 2009, 108, 295–304. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The Hippocampal CA2 Region Is Essential for Social Memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Tzakis, N.; Holahan, M.R. Social Memory and the Role of the Hippocampal CA2 Region. Front. Behav. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Smith, A.S.; Williams Avram, S.K.; Cymerblit-Sabba, A.; Song, J.; Young, W.S. Targeted Activation of the Hippocampal CA2 Area Strongly Enhances Social Memory. Mol. Psychiatry 2016, 21, 1137–1144. [Google Scholar] [CrossRef]
- Maytal, J.; Steele, R.; Eviatar, L.; Novak, G. The Value of Early Postictal EEG in Children with Complex Febrile Seizures. Epilepsia 2000, 41, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Graves, R.C.; Oehler, K.; Tingle, L.E. Febrile Seizures: Risks, Evaluation and Prognosis. Am. Fam. Physician 2012, 85, 149–153. [Google Scholar] [PubMed]
- Racine, R.J. Modification of Seizure Activity by Electrical Stimulation: II. Motor Seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- DeVito, L.M.; Konigsberg, R.; Lykken, C.; Sauvage, M.; Young, W.S.; Eichenbaum, H. Vasopressin 1b Receptor Knock-out Impairs Memory for Temporal Order. J. Neurosci. 2009, 29, 2676–2683. [Google Scholar] [CrossRef]
- Wong, C.T.; Bestard-Lorigados, I.; Crawford, D.A. Autism-Related Behaviors in the Cyclooxygenase-2-Deficient Mouse Model. Genes Brain Behav. 2019, 18, e12506. [Google Scholar] [CrossRef]
- Gangadharan, G.; Shin, J.; Kim, S.W.; Kim, A.; Paydar, A.; Kim, D.S.; Miyazaki, T.; Watanabe, M.; Yanagawa, Y.; Kim, J.; et al. Medial Septal GABAergic Projection Neurons Promote Object Exploration Behavior and Type 2 Theta Rhythm. Proc. Natl. Acad. Sci. USA 2016, 113, 6550–6555. [Google Scholar] [CrossRef]
- Tian, J.; Gao, X.; Yang, L. Repetitive Restricted Behaviors in Autism Spectrum Disorder: From Mechanism to Development of Therapeutics. Front. Neurosci. 2022, 16, 780407. [Google Scholar] [CrossRef]
- Virley, D.; Hernier, A.M.; Castagne, V. The Social Recognition Test of Memory in the Rat: A Reliable Primary in-Vivo Cognition Assay for Guiding Drug Discovery/Development Decision-Making Relevant to Neuropsychiatric Diseases. Alzheimer’s Dement. 2011, 7, S126. [Google Scholar] [CrossRef]
- Ives-Deliperi, V.L.; Jokeit, H. Impaired Social Cognition in Epilepsy: A Review of What We Have Learnt From Neuroimaging Studies. Front. Neurol. 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Meletti, S. Emotion Recognition in Temporal Lobe Epilepsy: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 55, 280–293. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wu, D.C.; Feng, B.; Chen, B.; Tang, Y.S.; Jin, M.M.; Zhao, H.W.; Dai, H.B.; Wang, Y.; Chen, Z. Prolonged Febrile Seizures Induce Inheritable Memory Deficits in Rats through DNA Methylation. CNS Neurosci. Ther. 2019, 25, 601–611. [Google Scholar] [CrossRef]
- Evans, D.W.; Uljarević, M.; Lusk, L.G.; Loth, E.; Frazier, T. Development of Two Dimensional Measures of Restricted and Repetitive Behavior in Parents and Children. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 51–58. [Google Scholar] [CrossRef]
- Mulligan, S.; Healy, O.; Lydon, S.; Moran, L.; Foody, C. An Analysis of Treatment Efficacy for Stereotyped and Repetitive Behaviors in Autism. Rev. J. Autism Dev. Disord. 2014, 1, 143–164. [Google Scholar] [CrossRef]
- Joseph, L.; Thurm, A.; Farmer, C.; Shumway, S. Repetitive Behavior and Restricted Interests in Young Children with Autism: Comparisons with Controls and Stability over 2 Years. Autism Res. 2013, 6, 584–595. [Google Scholar] [CrossRef]
- Aman, M.G. Management of Hyperactivity and Other Acting-out Problems in Patients with Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2004, 11, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Koval-Zaytsev, A.; Simashkova, N.; Ivanov, M. Comorbidity of Autism with Hyperkinetic Disorder. Eur. Psychiatry 2021, 64, S221–S222. [Google Scholar] [CrossRef]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef]
- Parisi, P.; Verrotti, A.; Paolino, M.C.; Ferretti, A.; Raucci, U.; Moavero, R.; Villa, M.P.; Curatolo, P. Headache and Attention Deficit and Hyperactivity Disorder in Children: Common Condition with Complex Relation and Disabling Consequences. Epilepsy Behav. 2014, 32, 72–75. [Google Scholar] [CrossRef]
- Hagihara, H.; Takao, K.; Walton, N.M.; Matsumoto, M.; Miyakawa, T. Immature Dentate Gyrus: An Endophenotype of Neuropsychiatric Disorders. Neural Plast. 2013, 2013, 318596. [Google Scholar] [CrossRef]
- Wei, H.; Zou, H.; Sheikh, A.M.; Malik, M.; Dobkin, C.; Brown, W.T.; Li, X. IL-6 Is Increased in the Cerebellum of Autistic Brain and Alters Neural Cell Adhesion, Migration and Synaptic Formation. J. Neuroinflam. 2011, 8, 52. [Google Scholar] [CrossRef]
- Mosconi, M.W.; Mohanty, S.; Greene, R.K.; Cook, E.H.; Vaillancourt, D.E.; Sweeney, J.A. Feedforward and Feedback Motor Control Abnormalities Implicate Cerebellar Dysfunctions in Autism Spectrum Disorder. J. Neurosci. 2015, 35, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mejias, R.; Chiu, S.L.; Han, M.; Rose, R.; Gil-Infante, A.; Zhao, Y.; Huganir, R.L.; Wang, T. Purkinje Cell-Specific Grip1/2 Knockout Mice Show Increased Repetitive Self-Grooming and Enhanced MGluR5 Signaling in Cerebellum. Neurobiol. Dis. 2019, 132, 104602. [Google Scholar] [CrossRef]
- Wei, H.; Ma, Y.; Liu, J.; Ding, C.; Jin, G.; Wang, Y.; Hu, F.; Yu, L. Inhibition of IL-6 Trans-Signaling in the Brain Increases Sociability in the BTBR Mouse Model of Autism. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1918–1925. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.S. Astrogliosis in CNS Pathologies: Is There a Role for Microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef]
- Marcaggi, P.; Attwell, D. Role of Glial Amino Acid Transporters in Synaptic Transmission and Brain Energetics. Glia 2004, 47, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The Diversity and Disparity of the Glial Scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain. Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Abramson, I.; Semendeferi, K.; Courchesne, E.; Everall, I.P. Abnormal Microglial-Neuronal Spatial Organization in the Dorsolateral Prefrontal Cortex in Autism. Brain Res. 2012, 1456, 72–81. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.H.; Kim, S.-W.; Im, H.; Lee, Y.R.; Kim, G.W.; Ryu, S.; Park, D.-K.; Kim, D.-S. Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats. Cells 2023, 12, 2446. https://doi.org/10.3390/cells12202446
Yu YH, Kim S-W, Im H, Lee YR, Kim GW, Ryu S, Park D-K, Kim D-S. Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats. Cells. 2023; 12(20):2446. https://doi.org/10.3390/cells12202446
Chicago/Turabian StyleYu, Yeon Hee, Seong-Wook Kim, Hyuna Im, Yu Ran Lee, Gun Woo Kim, Seongho Ryu, Dae-Kyoon Park, and Duk-Soo Kim. 2023. "Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats" Cells 12, no. 20: 2446. https://doi.org/10.3390/cells12202446
APA StyleYu, Y. H., Kim, S.-W., Im, H., Lee, Y. R., Kim, G. W., Ryu, S., Park, D.-K., & Kim, D.-S. (2023). Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats. Cells, 12(20), 2446. https://doi.org/10.3390/cells12202446