Ischemic Stroke Disrupts Sleep Homeostasis in Middle-Aged Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Statistics
2.3. Experiment 1
2.3.1. Assessment of Sensorimotor Deficit
2.3.2. Histology
2.3.3. Statistics
2.4. Experiment 2
Statistics
2.5. Experiment 3
Statistics
3. Results
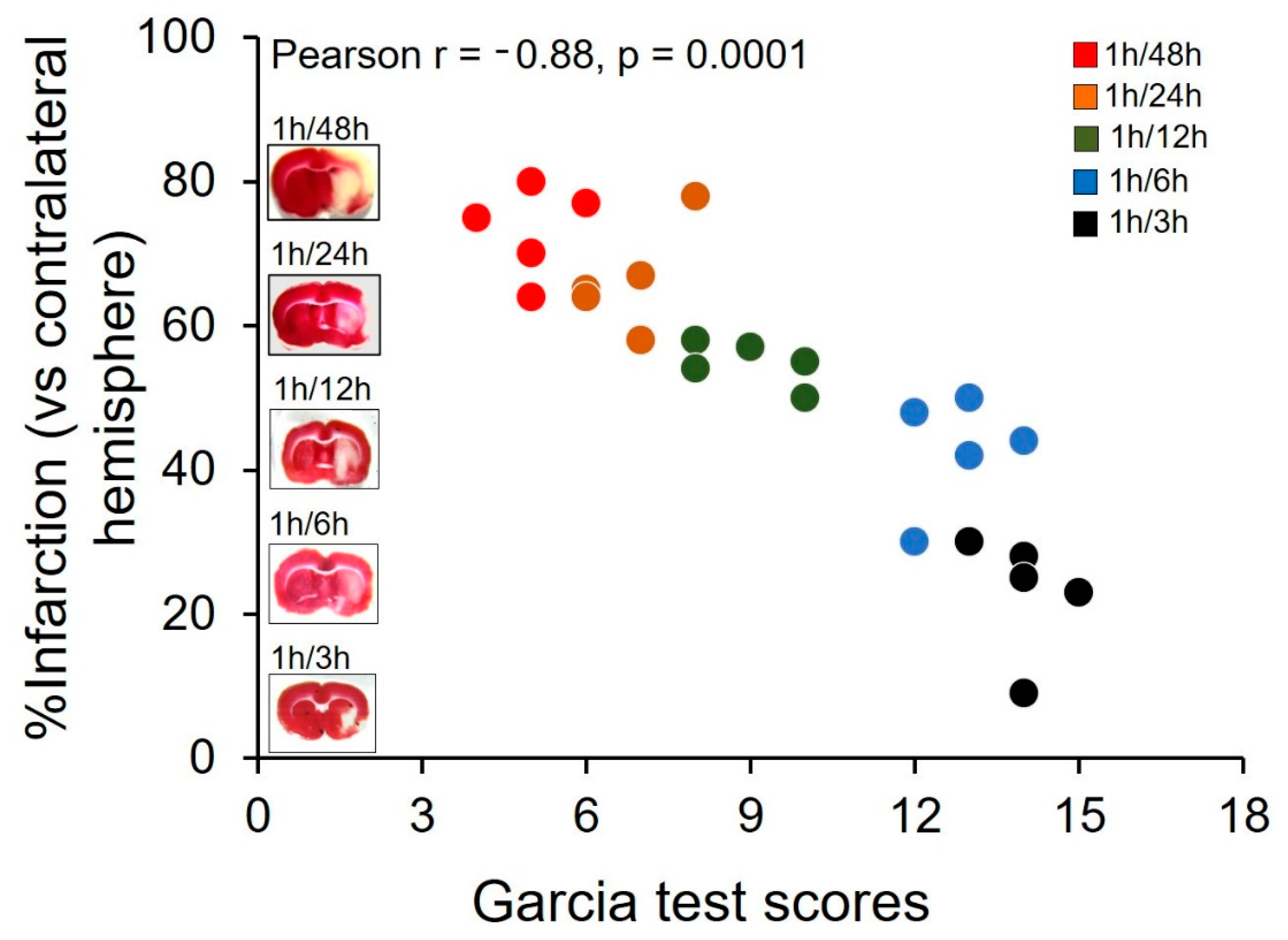
3.1. Validation of MCAO Model
3.2. IS Causes Insomnia-like Symptoms in Middle-Aged Mice
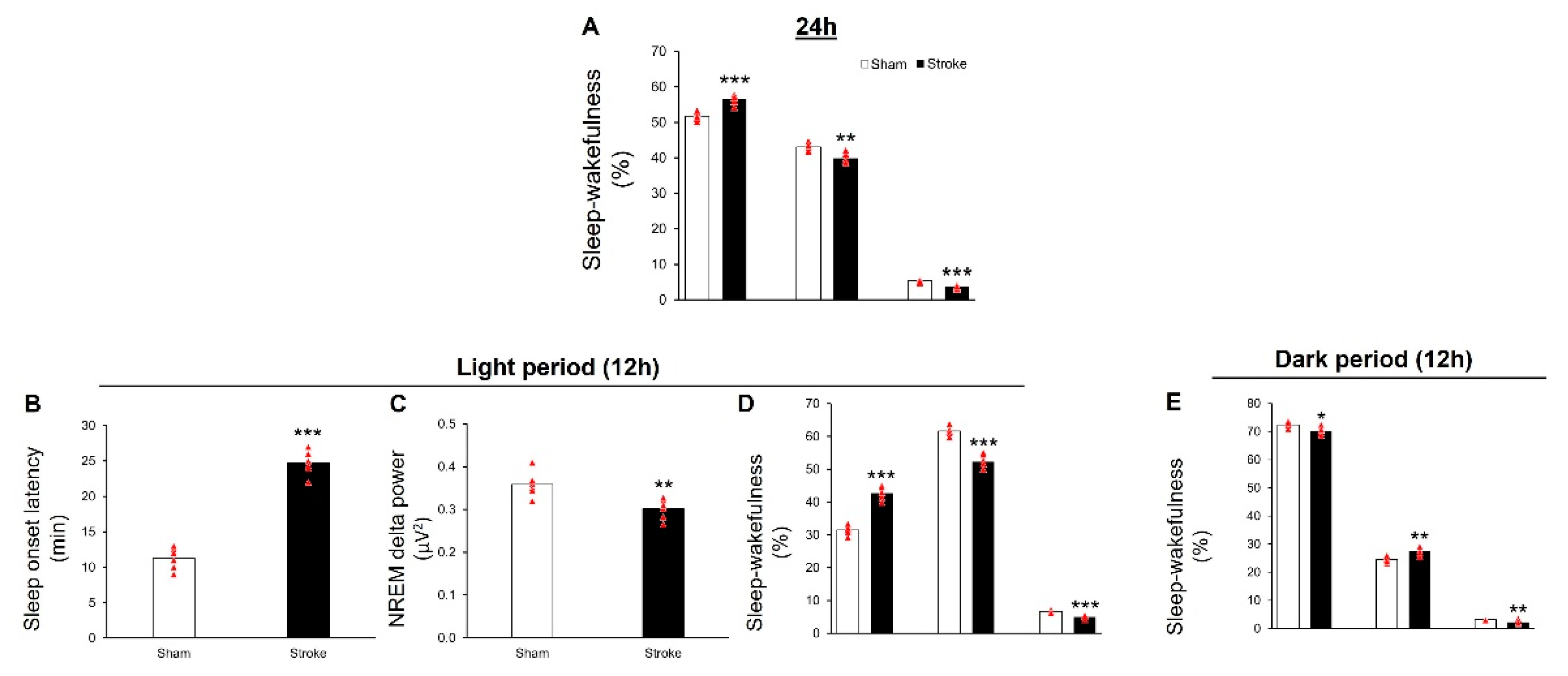
3.2.1. During 24 h
3.2.2. During Light Period
3.2.3. During Dark Period
3.3. IS Disrupts Sleep Homeostasis
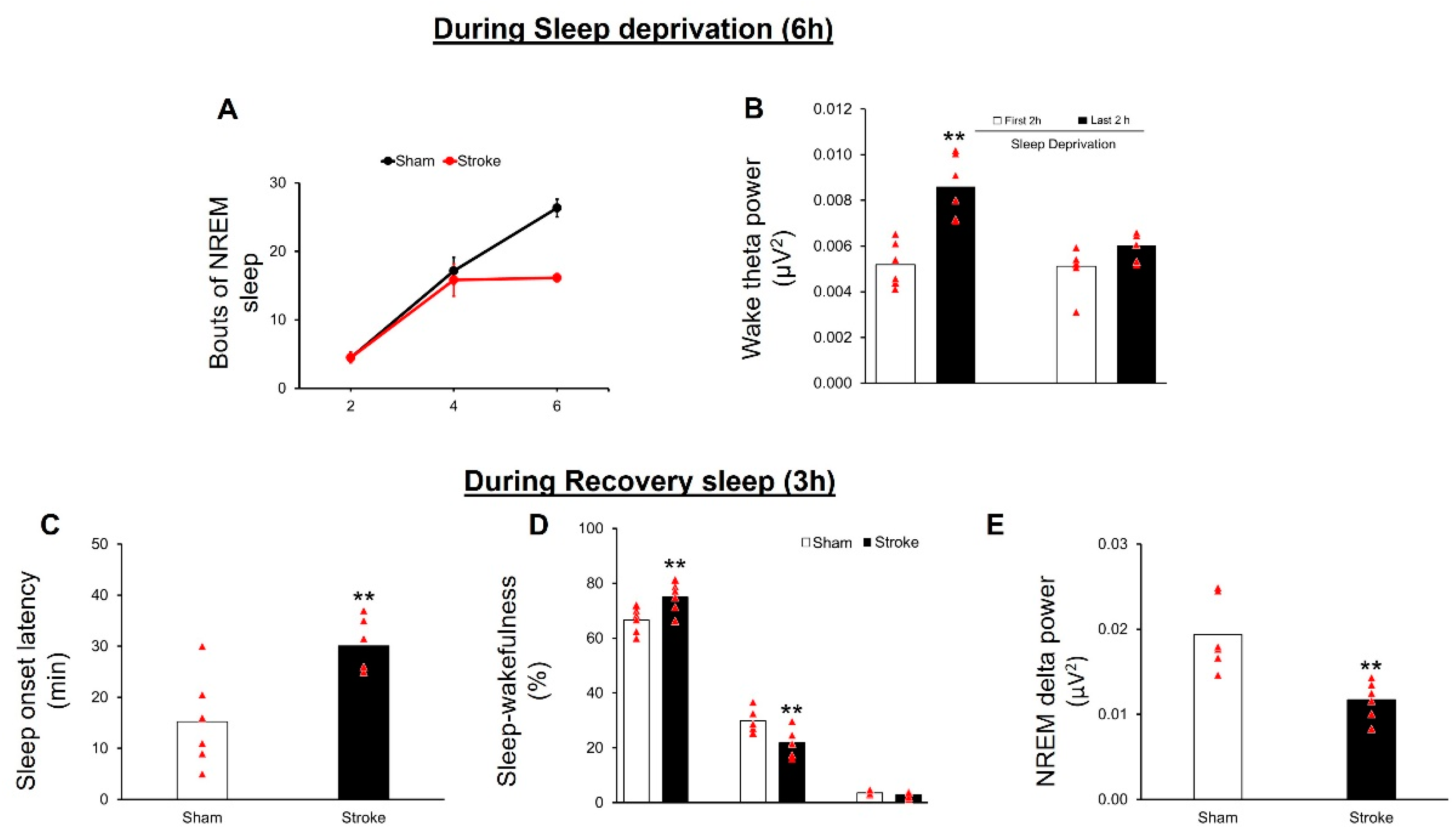
3.3.1. Sleep Deprivation
3.3.2. Recovery Sleep
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadi, P.; Lui, F. Acute Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Shakir, R. The struggle for stroke reclassification. Nat. Rev. Neurol. 2018, 14, 447–448. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Nogles, T.E.; Galuska, M.A. Middle Cerebral Artery Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lisabeth, L.D.; Baek, J.; Morgenstern, L.B.; Zahuranec, D.B.; Case, E.; Skolarus, L.E. Prognosis of Midlife Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 1153–1159. [Google Scholar] [CrossRef]
- Pajediene, E.; Pajeda, A.; Urnieziute, G.; Paulekas, E.; Liesiene, V.; Bileviciute-Ljungar, I.; Jurkeviciene, G.; Rastenyte, D.; Petrikonis, K. Subjective and objective features of sleep disorders in patients with acute ischemic or haemorrhagic stroke: It is not only sleep apnoea which is important. Med. Hypotheses 2020, 136, 109512. [Google Scholar] [CrossRef]
- Hasan, F.; Gordon, C.; Wu, D.; Huang, H.C.; Yuliana, L.T.; Susatia, B.; Marta, O.F.D.; Chiu, H.Y. Dynamic Prevalence of Sleep Disorders Following Stroke or Transient Ischemic Attack: Systematic Review and Meta-Analysis. Stroke 2021, 52, 655–663. [Google Scholar] [CrossRef]
- Fleming, M.K.; Smejka, T.; Henderson Slater, D.; van Gils, V.; Garratt, E.; Yilmaz Kara, E.; Johansen-Berg, H. Sleep Disruption after Brain Injury Is Associated with Worse Motor Outcomes and Slower Functional Recovery. Neurorehabil. Neural Repair. 2020, 34, 661–671. [Google Scholar] [CrossRef]
- Byun, E.; Kohen, R.; Becker, K.J.; Kirkness, C.J.; Khot, S.; Mitchell, P.H. Stroke impact symptoms are associated with sleep-related impairment. Heart Lung 2020, 49, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Siengsukon, C.F.; Boyd, L.A. Sleep enhances implicit motor skill learning in individuals poststroke. Top Stroke Rehabil. 2008, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Siengsukon, C.F.; Boyd, L.A. Sleep to learn after stroke: Implicit and explicit off-line motor learning. Neurosci. Lett. 2009, 451, 1–5. [Google Scholar] [CrossRef]
- Villablanca, J.; Marcus, R. Sleep-wakefulness, EEG and behavioral studies of chronic cats without neocortex and striatum: The ‘diencephalic’ cat. Arch. Ital. Biol. 1972, 110, 348–382. [Google Scholar]
- Gao, B.; Kilic, E.; Baumann, C.R.; Hermann, D.M.; Bassetti, C.L. Gamma-hydroxybutyrate accelerates functional recovery after focal cerebral ischemia. Cerebrovasc. Dis. 2008, 26, 413–419. [Google Scholar] [CrossRef]
- Hodor, A.; Palchykova, S.; Baracchi, F.; Noain, D.; Bassetti, C.L. Baclofen facilitates sleep, neuroplasticity, and recovery after stroke in rats. Ann. Clin. Transl. Neurol. 2014, 1, 765–777. [Google Scholar] [CrossRef]
- Pace, M.; Adamantidis, A.; Facchin, L.; Bassetti, C. Role of REM Sleep, Melanin Concentrating Hormone and Orexin/Hypocretin Systems in the Sleep Deprivation Pre-Ischemia. PLoS ONE 2017, 12, e0168430. [Google Scholar] [CrossRef]
- Hermann, D.M.; Bassetti, C.L. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology 2016, 87, 1407–1416. [Google Scholar] [CrossRef]
- Cai, H.; Wang, X.P.; Yang, G.Y. Sleep Disorders in Stroke: An Update on Management. Aging Dis. 2021, 12, 570–585. [Google Scholar] [CrossRef]
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- R Foundation for Statistical Computing. R-CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Wright, A.L.; Tan, R.P.; Gladbach, A.; Ittner, L.M.; Vissel, B. A Comparative Study of Variables Influencing Ischemic Injury in the Longa and Koizumi Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Mice. PLoS ONE 2016, 11, e0148503. [Google Scholar] [CrossRef] [Green Version]
- Llovera, G.; Simats, A.; Liesz, A. Modeling Stroke in Mice: Transient Middle Cerebral Artery Occlusion via the External Carotid Artery. J. Vis. Exp. 2021, e62573. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–634. [Google Scholar] [CrossRef]
- Yamauchi, K.; Nakano, Y.; Imai, T.; Takagi, T.; Tsuruma, K.; Shimazawa, M.; Iwama, T.; Hara, H. A novel nuclear factor erythroid 2-related factor 2 (Nrf2) activator RS9 attenuates brain injury after ischemia reperfusion in mice. Neuroscience 2016, 333, 302–310. [Google Scholar] [CrossRef]
- Joshi, C.N.; Jain, S.K.; Murthy, P.S. An optimized triphenyltetrazolium chloride method for identification of cerebral infarcts. Brain Res. Brain Res. Protoc. 2004, 13, 11–17. [Google Scholar] [CrossRef]
- Franklin, K.B.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Bieber, M.; Gronewold, J.; Scharf, A.C.; Schuhmann, M.K.; Langhauser, F.; Hopp, S.; Mencl, S.; Geuss, E.; Leinweber, J.; Guthmann, J.; et al. Validity and Reliability of Neurological Scores in Mice Exposed to Middle Cerebral Artery Occlusion. Stroke 2019, 50, 2875–2882. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Severe and protracted sleep disruptions in mouse model of post-traumatic stress disorder. Sleep 2018, 41, zsy003C. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. J. Pineal Res. 2018, 65, e12498. [Google Scholar] [CrossRef]
- Thakkar, M.M.; Engemann, S.C.; Walsh, K.M.; Sahota, P.K. Adenosine and the homeostatic control of sleep: Effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience 2008, 153, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Engemann, S.; Sahota, P.; Thakkar, M.M. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J. Neurochem. 2010, 115, 782–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Sahota, P.; Thakkar, M.M. A single episode of binge alcohol drinking causes sleep disturbance, disrupts sleep homeostasis, and down-regulates equilibrative nucleoside transporter 1. J. Neurochem. 2018, 146, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.K.; Grace Cherian, S.; Babu Phaniti, P.; Babu Chidambaram, S.; Rachel Vasanthi, A.H.; Arumugam, M. Preclinical animal studies in ischemic stroke: Challenges and some solutions. Anim. Model. Exp. Med. 2021, 4, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.-I.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Nosotchu 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Crupi, R.; Di Paola, R.; Esposito, E.; Cuzzocrea, S. Middle Cerebral Artery Occlusion by an Intraluminal Suture Method. Methods Mol. Biol. 2018, 1727, 393–401. [Google Scholar] [CrossRef]
- Elliott, W.J. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke 1998, 29, 992–996. [Google Scholar] [CrossRef]
- Esposito, E.; Li, W.; Mandeville, E.T.; Park, J.-H.; Şencan, I.; Guo, S.; Shi, J.; Lan, J.; Lee, J.; Hayakawa, K.; et al. Potential circadian effects on translational failure for neuroprotection. Nature 2020, 582, 395–398. [Google Scholar] [CrossRef]
- Kamat, P.K.; Khan, M.B.; Wood, K.; Siddiqui, S.; Rudic, D.R.; Dhandapani, K.; Waller, J.; Hess, D.C. Preclinical evaluation of circadian rhythm in ischemic stroke outcomes. Cond. Med. 2021, 4, 280–284. [Google Scholar]
- Ma, J.; Zhao, L.; Nowak, T.S., Jr. Selective, reversible occlusion of the middle cerebral artery in rats by an intraluminal approach. Optimized filament design and methodology. J. Neurosci. Methods 2006, 156, 76–83. [Google Scholar] [CrossRef]
- Vital, S.A.; Gavins, F.N. Surgical Approach for Middle Cerebral Artery Occlusion and Reperfusion Induced Stroke in Mice. J. Vis. Exp. 2016, e54302. [Google Scholar] [CrossRef] [PubMed]
- Wahid, D.; Rabbani, H.; Inam, A.; Akhtar, Z. A hemispheric comparison of cognitive dysfunction and sleep quality impairment in Middle Cerebral Artery infarction. Pak. J. Med. Sci. 2020, 36, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef] [PubMed]
- Pasic, Z.; Smajlovic, D.; Dostovic, Z.; Kojic, B.; Selmanovic, S. Incidence and types of sleep disorders in patients with stroke. Med. Arch. 2011, 65, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Sterr, A.; Kuhn, M.; Nissen, C.; Ettine, D.; Funk, S.; Feige, B.; Umarova, R.; Urbach, H.; Weiller, C.; Riemann, D. Post-stroke insomnia in community-dwelling patients with chronic motor stroke: Physiological evidence and implications for stroke care. Sci. Rep. 2018, 8, 8409. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef]
- Sterr, A.; Herron, K.; Dijk, D.J.; Ellis, J. Time to wake-up: Sleep problems and daytime sleepiness in long-term stroke survivors. Brain Inj. 2008, 22, 575–579. [Google Scholar] [CrossRef]
- Herron, K.; Dijk, D.J.; Dean, P.; Seiss, E.; Sterr, A. Quantitative electroencephalography and behavioural correlates of daytime sleepiness in chronic stroke. Biomed Res. Int. 2014, 2014, 794086. [Google Scholar] [CrossRef]
- Duss, S.B.; Seiler, A.; Schmidt, M.H.; Pace, M.; Adamantidis, A.; Muri, R.M.; Bassetti, C.L. The role of sleep in recovery following ischemic stroke: A review of human and animal data. Neurobiol. Sleep Circadian Rhythm. 2017, 2, 94–105. [Google Scholar] [CrossRef]
- Miano, S.; Fanfulla, F.; Nobili, L.; Heinzer, R.; Haba-Rubio, J.; Berger, M.; Cereda, C.W.; Schmidt, M.H.; Manconi, M.; Bassetti, C.L.A. SAS CARE 1: Sleep architecture changes in a cohort of patients with Ischemic Stroke/TIA. Sleep Med. 2022, 98, 106–113. [Google Scholar] [CrossRef]
- Bassetti, C.L.; Aldrich, M.S. Sleep electroencephalogram changes in acute hemispheric stroke. Sleep Med. 2001, 2, 185–194. [Google Scholar] [CrossRef]
- Qiu, M.H.; Vetrivelan, R.; Fuller, P.M.; Lu, J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur. J. Neurosci. 2010, 31, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Meng, H.; Liu, T.; Sutton, B.C.; Opp, M.R.; Borjigin, J.; Wang, M.M. Ischemic stroke selectively inhibits REM sleep of rats. Exp. Neurol. 2011, 232, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Baumann, C.R.; Kilic, E.; Petit, B.; Werth, E.; Hermann, D.M.; Tafti, M.; Bassetti, C.L. Sleep EEG changes after middle cerebral artery infarcts in mice: Different effects of striatal and cortical lesions. Sleep 2006, 29, 1339–1344. [Google Scholar] [CrossRef]
- Cavalcanti, P.R.; Campos, T.F.; Araujo, J.F. Circadian and homeostatic changes of sleep-wake and quality of life in stroke: Implications for neurorehabilitation. NeuroRehabilitation 2013, 32, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.M.; Sharma, R.; Sahota, P. Alcohol disrupts sleep homeostasis. Alcohol 2015, 49, 299–310. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T. Sleep homeostasis. Curr. Opin. Neurobiol. 2013, 23, 799–805. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef]
- Cui, C.; Noronha, A.; Warren, K.R.; Koob, G.F.; Sinha, R.; Thakkar, M.; Matochik, J.; Crews, F.T.; Chandler, L.J.; Pfefferbaum, A.; et al. Brain pathways to recovery from alcohol dependence. Alcohol 2015, 49, 435–452. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Tobler, I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005, 1050, 64–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Chischolm, A.; Parikh, M.; Qureshi, A.I.; Sahota, P.; Thakkar, M.M. Ischemic Stroke Disrupts Sleep Homeostasis in Middle-Aged Mice. Cells 2022, 11, 2818. https://doi.org/10.3390/cells11182818
Sharma R, Chischolm A, Parikh M, Qureshi AI, Sahota P, Thakkar MM. Ischemic Stroke Disrupts Sleep Homeostasis in Middle-Aged Mice. Cells. 2022; 11(18):2818. https://doi.org/10.3390/cells11182818
Chicago/Turabian StyleSharma, Rishi, Abigail Chischolm, Meet Parikh, Adnan I. Qureshi, Pradeep Sahota, and Mahesh M. Thakkar. 2022. "Ischemic Stroke Disrupts Sleep Homeostasis in Middle-Aged Mice" Cells 11, no. 18: 2818. https://doi.org/10.3390/cells11182818
APA StyleSharma, R., Chischolm, A., Parikh, M., Qureshi, A. I., Sahota, P., & Thakkar, M. M. (2022). Ischemic Stroke Disrupts Sleep Homeostasis in Middle-Aged Mice. Cells, 11(18), 2818. https://doi.org/10.3390/cells11182818



