1. Introduction
The ataxia and male sterility (AMS) mouse is a mutant strain that spontaneously arose from autoimmune-prone MRL/
lpr mouse [
1]. The putative disease-causing mutation is inherited in an autosomal recessive manner. Since the disease phenotype was existed in this mouse strain even in the absence of
lpr mutation, the disease phenotype was demonstrated to be independent of this mutation [
2,
3]. Ataxia is a major clinical feature of this mouse strain that starts to appear at approximately 3 weeks of age [
1]. Initially, mice show a subtle sway of the trunk and failure to maintain a still posture. Subsequently, a mild-to-moderate ataxic gait appears, which is maintained throughout life. Later, a missense mutation in the 17th exon of the
nervous system nuclear protein induced by axotomy protein 1 (
Nna1) gene was revealed, causing a substitution of amino acid 808 from arginine to proline [
4]. Another name for the
Nna1 gene is
ATP/GTP binding protein 1 (
Agtpbp-1). Previous studies reported that mutations in the
Nna1 gene can induce ataxia in mice starting from an early age [
5,
6,
7]. The main pathological feature of these mouse strains is the early degeneration of cerebellar Purkinje cells (PC); hence, these mice are called PC degeneration (PCD) mice. AMS mice also show early PC degeneration, which explains the clinical feature of ataxia [
1]. At 4.5 months of age, about a 25% decrease in total brain weight can be observed in AMS mice, and their cerebellum is smaller compared to wild-type littermates [
1]. A total loss of PC somata and dendrites can reduce the volume of the molecular layers. Subsequent examination demonstrated the degeneration and shrinkage of the granular layer as well [
3]. Hence, the combined degeneration of PCs and granule cells in this mouse strain leads to a reduced cerebellum and altered cerebellar control over motor functions.
Since
Nna1 is the only mutated gene in AMS mice and NNA1 expression is relatively high in the cerebellum [
8], alteration of the protein function could play a vital role in the degeneration process of cerebellar neurons [
9]. In previous studies, it was demonstrated that the NNA1 protein is localized in mitochondria, where it is essentially involved in bioenergetics. The loss-of-function of NNA1 causes mitochondrial dysfunction and induces a PCD phenotype [
10]. Indeed, hippocampal neurons of AMS mice showed increased vulnerability during hypoxia due to oxidative stress, suggesting that mitochondrial function might be affected here due to
Nna1 mutation [
11]. However, hippocampal neurons do not show signs of early degeneration like cerebellar PC. These findings suggest functional differences between these neuron types and distinct roles for NNA1 in their functions. NNA1 also shows carboxypeptidase activity [
8]. Importantly, NNA1 catalyzes the deglutamylation of polyglutamylated proteins [
12]. Polyglutamylation is a posttranslational protein modification where strings of polyglutamate are added onto the gamma carboxyl groups of any of several glutamine residues near the C-terminus of either α- or β-tubulin [
13]. Increased tubulin polyglutamylation was observed in PCD mice strains [
14], suggesting that this NNA1 function could be indispensable for the pathology. Indeed, NNA1 was found to regulate mitochondrial motility through deglutamylation of tubulin [
14]. Motility is important for mitochondrial fusion, and the loss of NNA1-mediated mitochondrial fusion accounts for the exquisite vulnerability of Purkinje neurons in PCD mice [
14]. However, if the alteration of mitochondrial functions was the sole cause of PC death, then neurons of other areas like the hippocampus, where NNA1 expression is high, should be affected similarly. Yet, hippocampal neurons do not show similar patterns of neurodegeneration like PCs in AMS mice despite a high expression of NNA1 [
8,
11]. This suggests the existence of additional mechanisms that are important for PC neurodegeneration in AMS mice.
Modulation of the cytoskeleton is involved in various cellular processes including cell division, homing, and polarity during the developmental stages of neurons [
15]. As a component of cytoskeleton, microtubules have the ability to regulate these cellular processes. The main components of microtubules are α- and β-tubulin [
16]. In microtubules, extensive posttranslational modifications of tubulins, such as phosphorylation, glutamylation, glycylation, tyrosinylation, and acetylation are required [
17,
18]. Such posttranslational modifications determine the characteristics of microtubules that perform different types of functions depending on the cellular processes. Hence, posttranslational modifications of tubulins are tightly controlled to regulate its function and dynamics required for particular cellular processes. In postmitotic neurons, microtubule dynamics play an important role in development and maturation. For example, during the migration of newly formed neurons, microtubules were found to be indispensable [
19]. Besides, during maturation, microtubules play an important role in neurite outgrowth [
20,
21]. Functioning as a deglutamylase, NNA1 could influence such microtubule dynamics and maturation of neurons, and alteration of its function might affect neurodevelopmental processes. A recent study showed that mutations in the
NNA1 gene are indeed linked to human infantile-onset neurodegeneration, where dysregulation of tubulin polyglutamylation is evident [
22]. Another report demonstrated that the lack of NNA1 affects microtubule dynamics and flexibility, defects that contribute to morphological alterations of PCs [
23] and lead to progressive cerebellar degeneration. Since PC degeneration occurs during early postnatal development of the cerebellum and microtubule dynamics are important for the postmitotic development of cerebellar neurons, we sought to investigate the role of NNA1 during the early development of neurons in AMS mice. One way to examine the influence of NNA1 on neuronal development and maturation is to use cultured neural stem cells (NSCs) and to evaluate the role of the protein in these processes.
Thus, to investigate the role of NNA1 on neuronal development and maturation, we generated NSC lines from AMS mice carrying homozygous Nna1 mutation, heterozygous mice, and their wild-type littermates and checked their characteristics and differentiation potential. We found that the characteristics and maturation potential of the AMS NSC line were altered along with increased β-tubulin levels and tubulin glutamylation. Such altered neuronal maturation could be important for neuronal degeneration in AMS mice.
2. Materials and Methods
2.1. Materials
Dulbecco’s modified Eagle medium (DMEM) and Hank’s Balanced Salt Solution were purchased from Wako Pure Chemicals (Richmond, VA, USA), and fetal bovine serum (FBS) was from Gibco (Invitrogen, Carlsbad, CA, USA). F12 ham was purchased from Sigma-Aldrich (St. Lois, MO, USA). Epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were from PeproTech (Rocky Hill, NJ, USA). Cell culture plates and dishes were from Falcon (Corning, Oneonta, NY, USA). Permanox chamber slides, N2 supplement, and B27 supplement were purchased from Thermo Fisher (Waltham, MA, USA). Pre-stained protein size marker for Western blotting was purchased from NIPPON Genetics EUROPE GmbH (Duren, Germany), and 4–20% Tris–glycine polyacrylamide gel was from Bio-Rad (Hercules, CA, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from Millipore (Billerica, MA, USA).
2.2. Genotyping
The genotyping of AMS mice was performed as described previously [
11]. In brief, DNA was isolated from the tail, the spleen, or cultured cells using DNeasy blood and tissue isolation kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction. Then, a DNA fragment of the
Nna1 gene was PCR amplified, and the genotype was determined by restriction enzyme digestion using BstB1 (New England BioLab, Ipswich, MA, USA) that digested non-mutated DNA.
2.3. Cell Culture
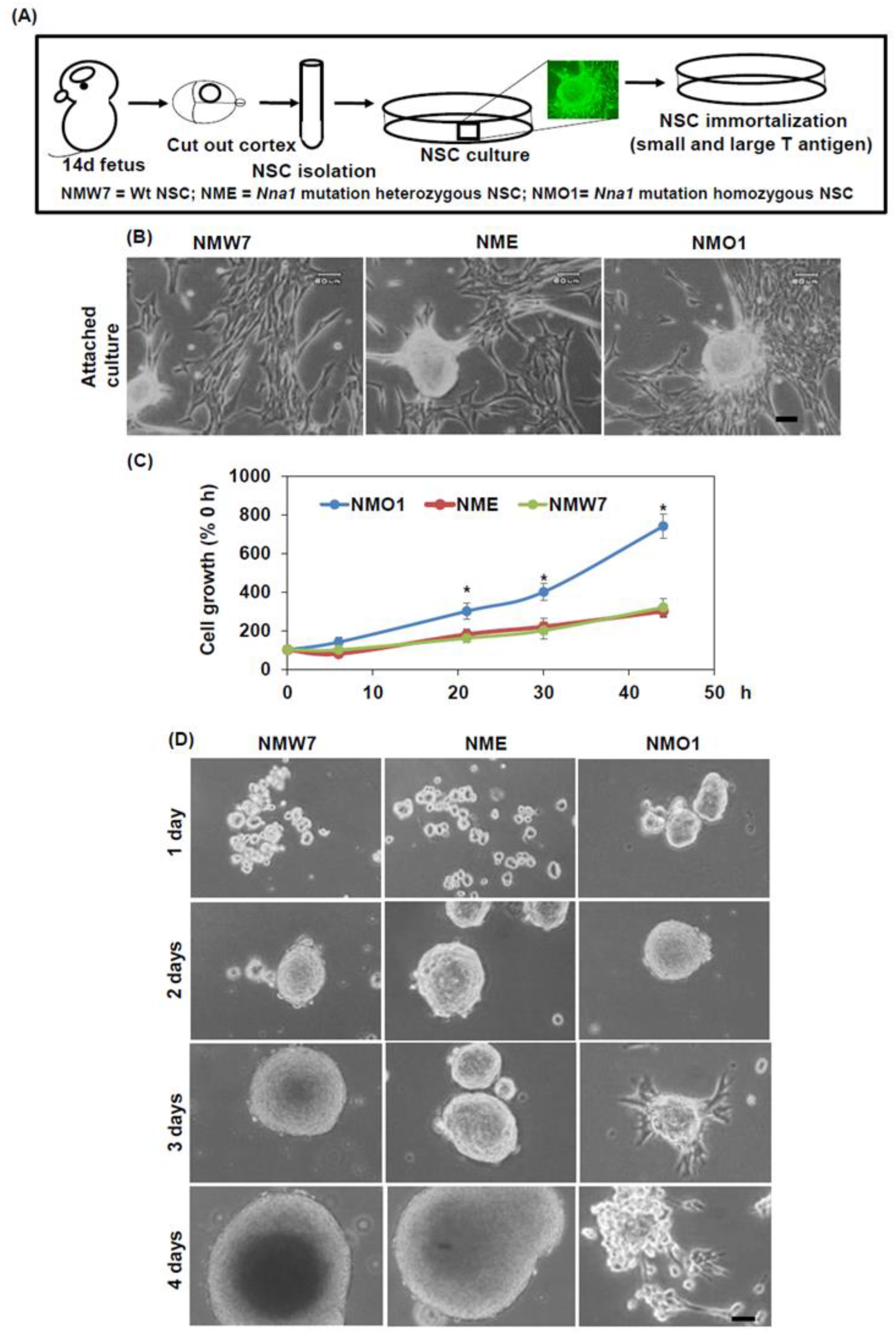
Isolation and Generation of Embryonic NSC Lines
All experimental protocols and procedures were approved by the ethics committee of the School of Medicine at Shimane University. All animal procedures were done according to the guidelines of the Animal Institute, Shimane University. Heterozygous mice carrying the Nna1 mutation were mated. At 14.5 days of gestation, a pregnant mouse was deeply anesthetized, and the fetuses were collected under aseptic conditions. Fetal brain cortical tissue was isolated under a dissection microscope in a cell culture bench. The spleen was collected to isolate DNA for genotyping. The tissue was cut into small pieces in DMEM using a scalpel. After removing DMEM by centrifugation, the tissue was re-suspended in 0.25% trypsin and DNAse1 and was incubated in a water bath at 37 °C for 10 min. To neutralize trypsin, FBS was added, and the suspension was centrifuged to collect the cells. After washing with DMEM, the cells were plated in a tissue culture dish in complete media (high glucose DMEM: F12 ham 1:1, 2% FBS, N2 supplement, bFGF 20 ng/mL, and EGF 20 ng/mL).
When the cultured cells showed features of NSCs, the cells were infected with a lentiviral vector expressing small and large T antigen (pLenti CMV/TO SV40 small + Large T). pLenti CMV/TO SV40 small + Large T (w612-1) was a gift from Dr. Eric Campeau (Addgene plasmid # 22298;
http://n2t.net/addgene:22298; RRID:Addgene_22298). Immortalization of NSCs was carried out according to the guidelines approved by the ethics committee of the School of Medicine at Shimane University. After about 10 subcultures, cells were plated into 100-mm cell culture dishes at low concentrations to generate colonies from single cells. Several colonies were picked up and expanded to get a homogenous culture. The cells were genotyped for
Nna1, and
small and large T antigens. Then, neurosphere assay was done for all colonies generated from a single fetus, and one colony among them that showed best neurosphere formation properties was selected, expanded, and further characterized.
2.4. Preparation of Neurosphere
To prepare the neurosphere, the cell lines were cultured in a non-tissue culture dish. The following medium was used to generate neurosphere, high glucose DMEM: F12 ham 1:1, bFGF 20 ng/mL, EGF 20 ng/mL, N2 supplement, and B27 supplement. For an experiment, neurospheres from all 3 NSC lines were grown simultaneously and then processed on the same day. Time-dependent changes in neurosphere morphologies were checked using a cell culture microscope. For immunostaining, neurospheres were fixed with 4% paraformaldehyde. Then, Tissue-Tek® O.T.C compound (Sakura Finetek, Tokyo, Japan) was added to the neurospheres, and frozen blocks were prepared. These blocks were sectioned using a microtome.
2.5. Differentiation of NSCs to Neurons
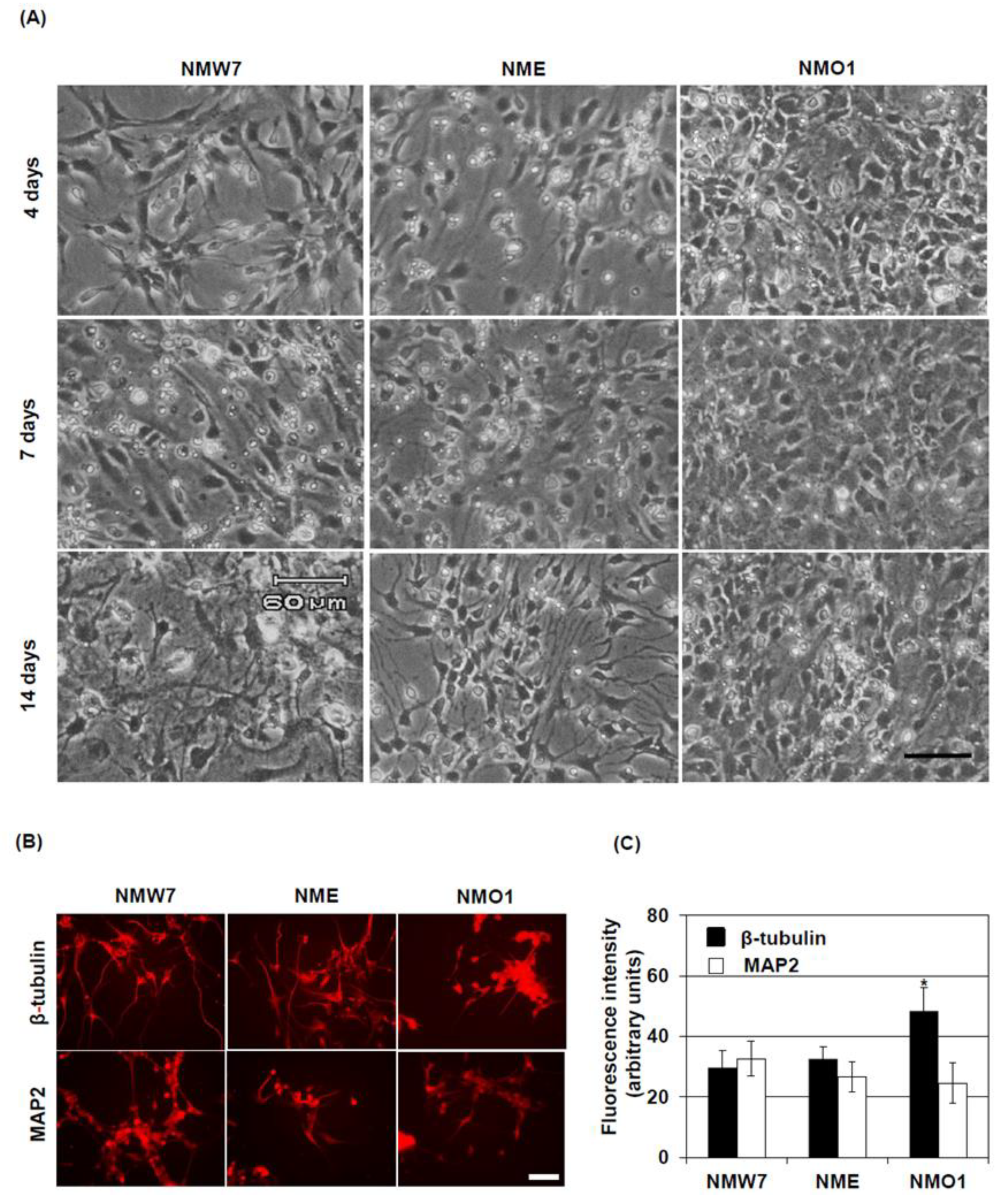
For neuronal differentiation, poly-L-lysine (PL)-coated dishes were used. After preparation, the neurospheres in neurosphere culture medium were directly transferred into PL-coated dishes, and the same amount of differentiation medium (DMEM: F12 ham 1:1, B27) was added. After 6 h, when the neurospheres were attached to the bottom of the dish, the medium was removed, fresh differentiation medium was added, and the cells were cultured for another 14 days with medium changes every 2 days. For the immunocytochemistry of mature neurons, cells were differentiated on PL-coated Permanox chamber slides.
2.6. Immunostaining
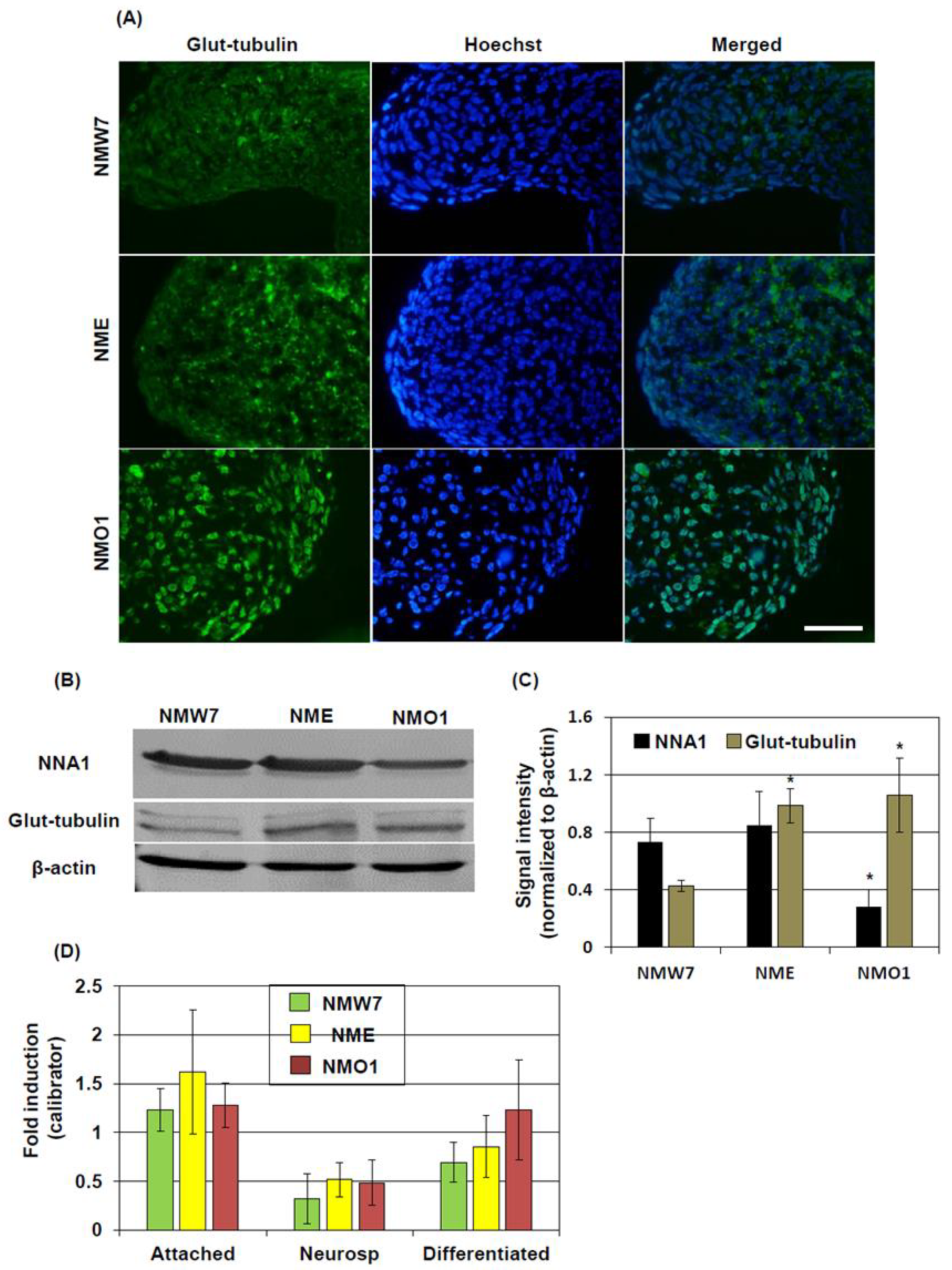
For immunostaining of the cerebellum, mice were deeply anesthetized with Isofluran, and the cerebellum was isolated, fixed in formalin, and embedded in paraffin. Then, 10-μm tissue slices were prepared using a microtome. After deparaffinization, antigen retrieval was performed by boiling the tissue section in 10 mM citrate buffer (pH 6.0). After quenching the endogenous peroxidase activity, the sections were incubated in a blocking solution containing 5% normal goat or horse serum and 0.1% Triton X-100 in phosphate-buffered saline (PBS). The sections were incubated with anti-NNA1 IgG (goat, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-β-tubulin IgG (rabbit, 1:1000, Abcam, Cambridge, UK), anti-glutamylated tubulin IgG (mouse, 1:1000, Adipogen, Liestal, Switzerland), or anti-microtubule-associated protein 2 (MAP2) IgG (rabbit, 1:1000, Abcam), followed by incubation with the appropriate secondary antibodies. In the cases of β-tubulin, glutamylated tubulin, or MAP2, the secondary antibodies used were conjugated with fluorescein isothiocyanate (FITC) or Texas Red (Santa Cruz). Immunofluorescence staining was done after protecting the tissues from light to minimize photobleaching. In the case of NNA1, the secondary antibody was biotin-conjugated (1:100, Vector, Ingold Road, CA, USA). Then, the section was incubated with an avidin–biotin–peroxidase complex (ABC, Vector) and the immune reaction products were visualized with 3, 30-diaminobenzidine (DAB, Sigma). Stained sections were examined under a fluorescent microscope (ECLIPSE E600, NIKON, Tokyo, Japan) in a blinded manner.
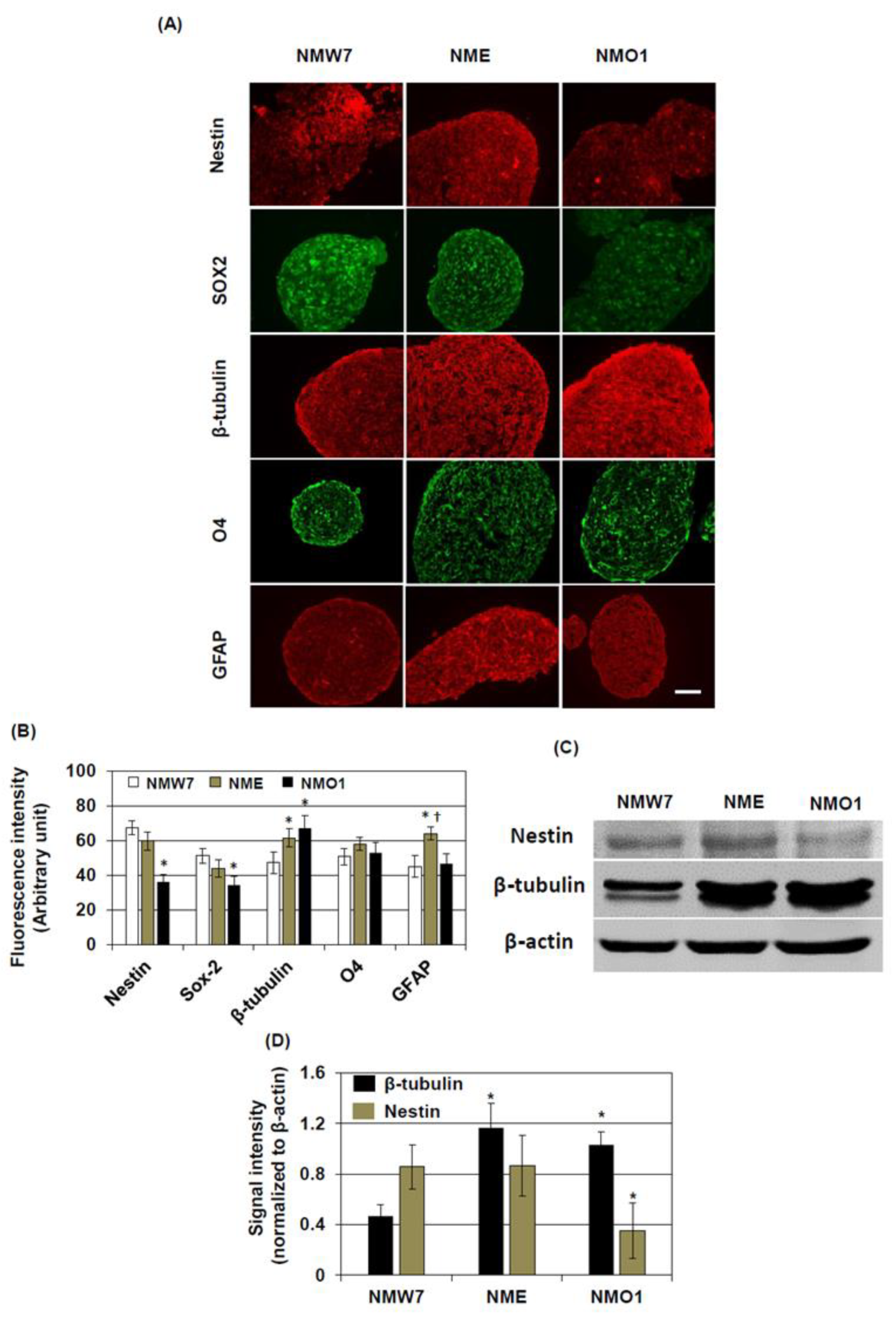
For immunostaining of neurospheres, 10-μm slices were prepared from the block and antigen retrieval was performed by boiling the slices in 10 mM citrate buffer (pH 6.0). The slices were incubated in a blocking solution containing 5% normal goat or horse serum and 0.1% Triton X-100 in PBS. The sections were incubated with anti-nestin IgG (rabbit, 1:200, Abcam), anti-β-tubulin IgG (rabbit, 1:1000, Abcam), anti-glial fibrillary acidic protein (GFAP, rabbit, 1:1000, Abcam), anti-O4 IgG (rabbit, 1:1000, Cell Signaling, Beverly, MA, USA), or anti-SOX2 IgG (rabbit, 1:1000, Cell Signaling). Then, the sections were incubated with appropriate secondary antibodies conjugated with FITC or Texas Red. Stained sections were examined under a fluorescent microscope (ECLIPSE E600, NIKON) in a blinded manner.
Fluorescent intensities of the stained photomicrographs were quantified using ImageJ (NIH, Bethesda, MD, USA). After opening the photomicrograph with ImageJ, the immunoreactive fluorescent area of interest was selected and the fluorescence intensity was measured. Then, fluorescence intensities of at least five background areas were measured, averaged, and subtracted from the fluorescence intensities of the area of interest. In an experiment, at least six photomicrographs per condition were processed, and background-normalized fluorescence intensities were averaged.
2.7. Total RNA Isolation, Reverse Transcription, and Real-Time PCR
Total RNA was isolated from attached NSC cultures after neuronal differentiation and from neurospheres using Trizol reagent (Invitrogen) following the manufacturer’s instructions. To prepare first-strand cDNA, 2 μg of total RNA was reverse transcribed with oligo dT primers and reverse transcriptase enzyme (RiverTraAce, Toyobo, Osaka, Japan) in a 20-μL reaction mixture. To analyze the mRNA level of β-tubulin, real-time PCR was performed with the SyBr green PCR system (Applied Biosystem, Warrington, UK) and gene specific primers, using an ABI Prism 7900 Sequence Detector system (Applied Biosystems). The mRNA level was normalized to the corresponding Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and quantified by a relative quantification method, where a sample of an attached neuronal stem cell line from wild-type (NMW7) culture was used as the calibrator.
2.8. Western Blot Analysis
Western blotting was done as described previously [
24]. Briefly, total protein was isolated from cultured cells or murine brain tissue using ice cold RIPA buffer (PBS, pH 7.4, 1% Nonidet p-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/mL PMSF, and 1 mg/mL aprotinin). To homogenize brain tissue, 20× wt/vol of RIPA buffer was used. Total protein (20–60 μg) was separated by 4–20% SDS polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Then, the membrane was incubated with anti-NNA1 IgG, anti-β-tubulin IgG, anti-glutamylated tubulin IgG, anti-MAP2 IgG, or anti-nestin IgG. NNA1, β-tubulin, MAP2, and nestin were detected using the appropriate infrared-fluorophore-conjugated secondary antibodies (Li-COR, Lincoln, NE, USA). Anti-glutamylated tubulin IgG was conjugated with biotin; hence, infrared-fluorophore-conjugated streptavidin was used for detection. Immunoblotting was done after protecting the membrane from light to minimize photobleaching. Immunoreactive proteins in the membrane were detected using an infrared scanner (Li-COR). After scanning, the bands of Immunoreactive proteins were selected and densitometrically analyzed using Odyssey V3.0 (Li-COR), or ImageJ. The signal intensities of background areas of the same size were subtracted from the signals of immunoreactive proteins. β-actin was used as a loading control, and its signal intensity was used to normalize the signals of target proteins. Necessary processing of the photographs of the blots was done using ImageJ (NIH).
2.9. Statistical Analysis
Numerical data are presented as mean ± standard deviation (SD). Statistical differences among groups were assessed using one-way ANOVA followed by Scheffe’s post hoc test or the paired t-test. A p value < 0.05 was considered as statistically significant.
4. Discussion
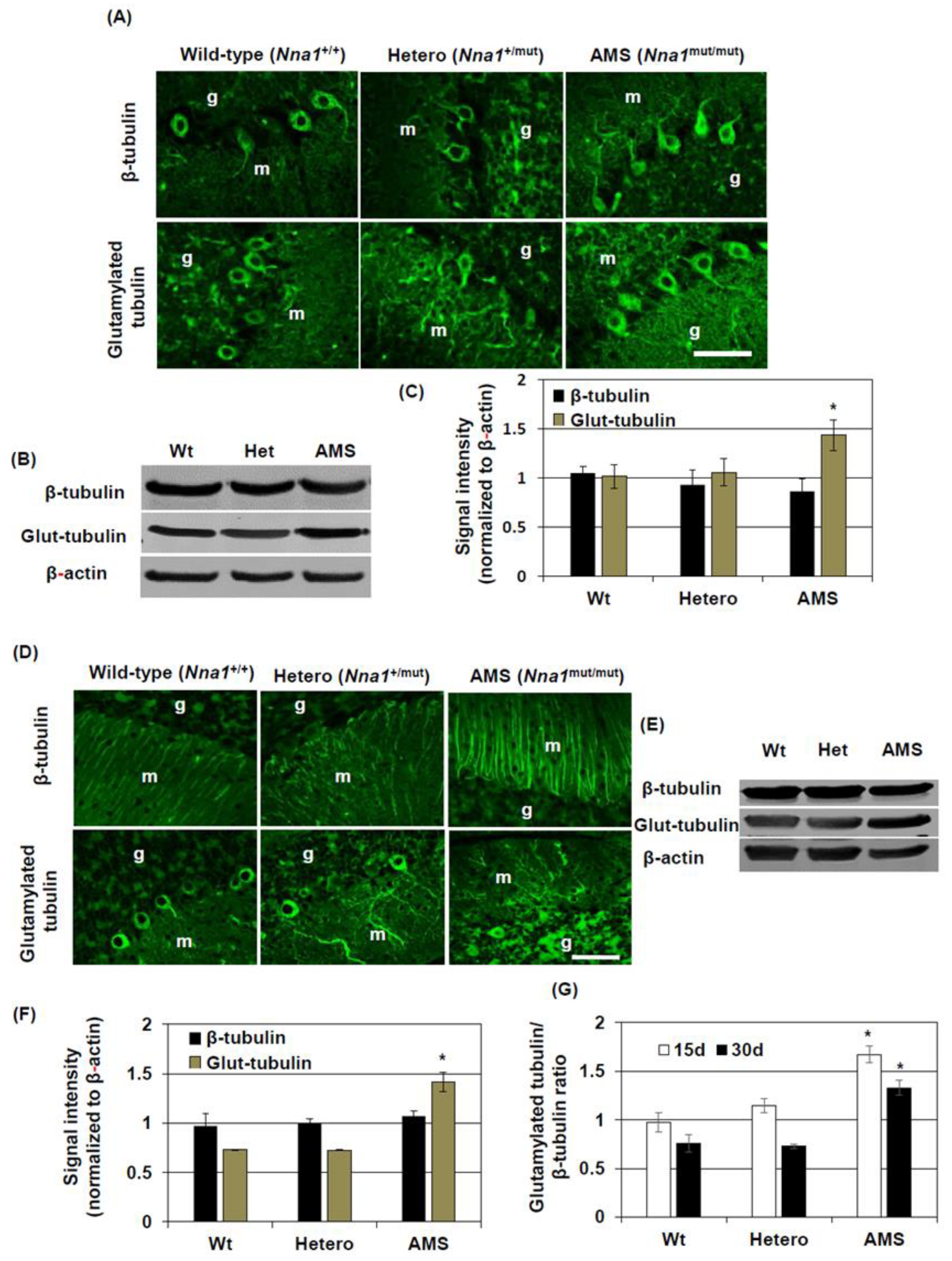
In our previous study, we found that dying PCs in AMS mice present some morphological features of apoptosis at 3 weeks of age [
3]. Since NNA1 has a deglytamylase activity and β-tubulin can be glutamylated posttranslationally [
12], we considered β-tubulin glutamylation as a target of further investigation to elucidate the mechanism of cell death in this mouse. In this study, we demonstrated that the glutamylated tubulin level was increased in the cerebellum of AMS mice. Cytoskeletal proteins like actins or tubulins can undergo several types of posttranslational modifications including acetylation, argenylation, oxidation, and phosphorylation [
17,
18,
26,
27]. Such posttranslational modifications are important because precise modifications are essential for proper functioning of the cytoskeleton. Among the posttranslational modifications of cytoskeletal proteins, glutamylation was only observed in tubulins. Other cytoskeletal proteins like β-actin did not undergo deglutamylation, suggesting that, among cytoskeletal proteins, tubulin could be the only target of NNA1 deglutamylase activity. Since a missense mutation was described in the
Nna1 gene [
4], increased glutamylated tubulin levels could result from the defective function of this deglutamylase in AMS mice. A similar finding was reported in PCD mice, where its loss-of-function due to mutation caused increased tubulin glutamylation [
14]. The increased glutamylation alters microtubule dynamics and functions, and this is deleterious for PCs and retinal photoreceptors [
14,
28]. As a mechanism, it was suggested that hyperglutamylation could lead to mitochondrial fragmentation resulting in PC degeneration [
14]. Moreover, in the retina, hyperglutamylation causes decreased glycylation of tubulin, and the subsequent imbalance of the glutamylation-glycylation equilibrium leads to retinal degeneration [
28]. In the present study, we demonstrated that increased glutamylation may lead to defective functions of neural stem cells in terms of their growth and differentiation. Such functional defects might lead to impaired maturation and ultimately PC degeneration. Nevertheless, it will be interesting to know the detailed mechanisms by which tubulin glutamylation affects cell death processes like apoptosis.
In AMS mice, homozygous mutation of the
Nna1 gene was found, causing an amino acid change in the protein sequence at position 808. Previous studies showed that amino acid changes in this region of the protein (686 Y to D, 843 T to M, and 910 R to W) crucially affect the deglutamylation activity [
22]. Moreover, in AMS mice, cerebellar total NNA1 protein levels were found to be decreased at the age of 15 days and older. At the age of 30 days, AMS mice did not present NNA1 stained PC due to their degeneration, although the staining patterns did not differ much among the strains at 15 days of age. The reason for reduction is not fully elucidated, but a probable cause could be the decreased translation or stability of the protein induced by the mutation [
4]. Nevertheless, this reduced expression might also contribute to decreased deglutamylation activity. Since,
Nna1 is highly expressed in developing neurons [
29], it is conceivable that it has a role in maturation of the neurons. Hence, due to decreased protein level and reduced deglutamylation activity caused by the mutation, the maturation of neurons could be affected in AMS mice.
As demonstrated in our previous report, the overall size of the cerebellum is decreased in 30-day-old mice, although at 15 days, the cerebellar size of AMS mice is similar to that of wild-type mice [
1]. During the first 2 weeks, profound morphological and functional changes occur in rodent PCs [
30]. PC degeneration starts in AMS mice just after these morphological changes. Migration, maturation, and synaptic contacts of cerebellar granule neurons occur during the first 3 weeks of a mouse pup’s life [
31]. Since the volume of granular layer is decreased just after this time point and the overall size of the cerebellum is decreased, the maturation process of cerebellar neurons could also be altered in AMS mice. Hence, the
Nna1 mutation may cause disturbances in the function of immature neurons or neural stem cells. Consistent with that hypothesis, our in vitro cell culture system demonstrated that the properties of an NSC line derived from AMS (NMO1) are different from those derived from wild-type or
Nna1 mutation heterozygous mice, such as decreased nestin and SOX2 levels in the neurosphere. Nestin and SOX2 are widely considered as neural stem cell markers. Several reports have demonstrated their essential role during self-renewal process of NSCs [
32,
33], and their expressions are decreased during differentiation into neurons or glia. Since nestin and SOX2 are considered to be involved in maintaining stem cell properties, decreased nestin and SOX2 in NMO1 neurospheres might imply that the stemness of NSCs in this mouse strain was decreased due to the
Nna1 mutation. On the other hand, the neuronal marker β-tubulin level was increased in NMO1 neurospheres, suggesting that the NSC might spontaneously differentiate to neurons in neurosphere culture condition. Attachment of these neurosphere to the bottom of the culture plates also suggests the same. However, neuron differentiation experiments do not support the idea that NMO1 has an improved differentiation ability. For maturation of neurons, proper and balanced posttranslational modifications of microtubule proteins are essential. Since glutamylation is increased in NSC generated from AMS, such a balanced could be disturbed. A previous study showed that tubulin hyperglutamylation increases the stability of microtubules in the cell body and decreases it in the axoneme [
34]. Since β-tubulin expression was not changed at the transcriptional level among the NSC lines, stabilization of microtubules in the cell body could be the reason for the increased β-tubulin level in AMS NSCs. In differentiated neurons, the β-tubulin level was found to be increased in the cell body. However, the processes seem smaller in length in neurons differentiated from the AMS-derived NSC line. Interestingly, The NSC line derived from
Nna1 mutation heterozygous mice also showed increased β-tubulin levels in neurosphere cultures, but in cultured mature neurons, the length of processes and β-tubulin levels seemed to be similar to those in neurons derived from wild-type NSCs. Since half of the protein should function normally in this cell line, these findings could highlight the importance of functional NNA1 levels in microtubule physiology during neuronal development.
In contrast to cell culture, the total β-tubulin level did not change in the cerebellum of AMS mice as shown by Western blotting. However, immunostaining results showed that β-tubulin expression was increased in the processes of the molecular layer. This result could indicate a differential β-tubulin regulation in the cerebellum in vivo compared to culture conditions. However, a strong β-tubulin immunostaining signal in the molecular layer of AMS mice may also suggests that, although overall β-tubulin level was not changed, it indeed increased in some types of cells where NNA1 expression is considered high. NNA1 is expressed highly in cerebellar PCs [
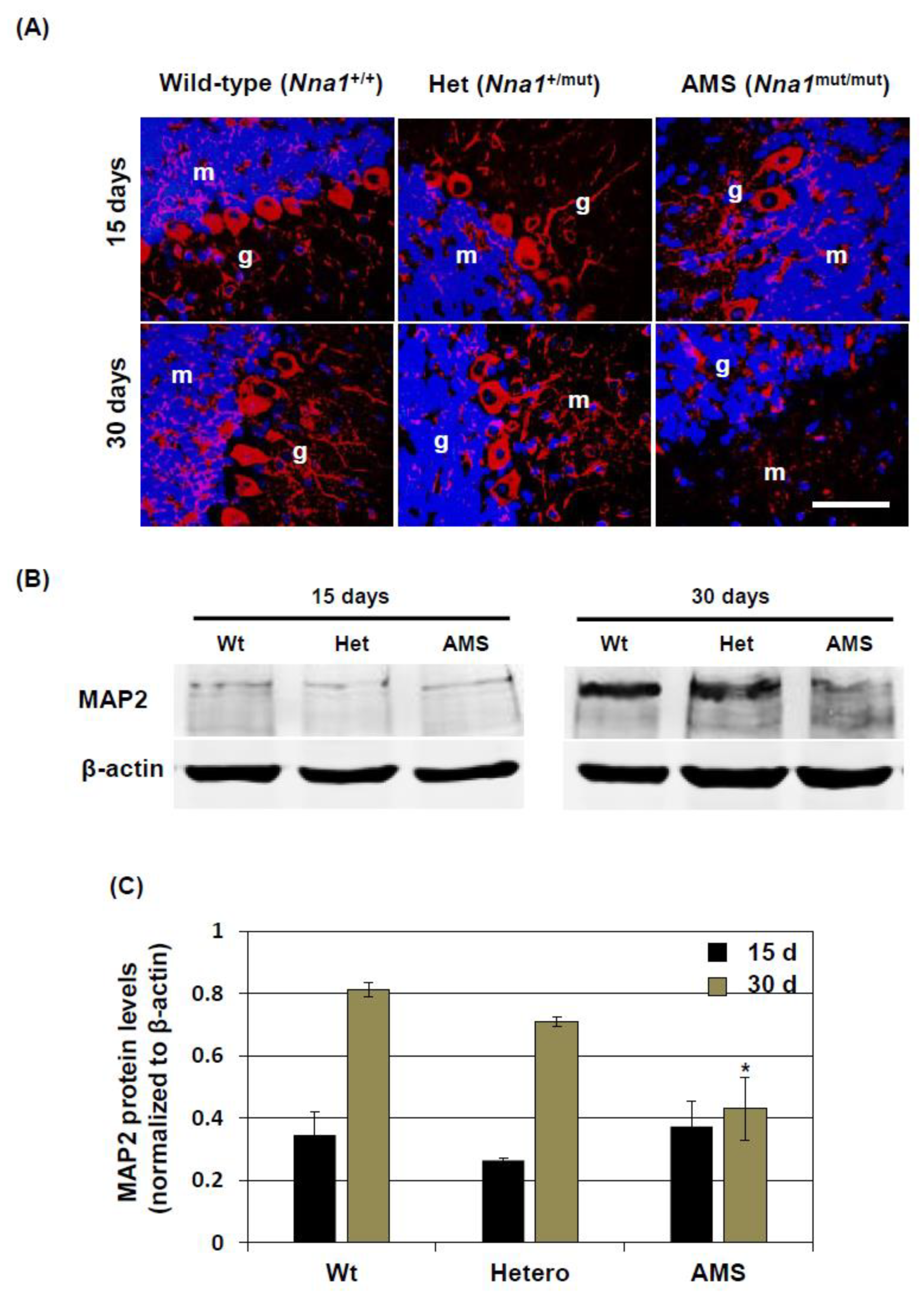
8]. Hence, a decreased function of NNA1, evidenced by increased tubulin glutamylation, might alter β-tubulin stability and microtubule regulation in the PCs. In other cell-types, due to decreased NNA1 expression, this regulation might not come into play at this stage, resulting in a similar overall β-tubulin turnover in the cerebellum of AMS mice. On the other hand, MAP2 expression is increased in mature neurons. In AMS mice, the MAP2 protein level was decreased, further confirming the deregulation of microtubule assembly in this mouse strain. This deregulation could induce PC degeneration. Since NNA1 is also expressed in granule cells, these cells as well are vulnerable to the deregulated microtubule assembly due to loss-of-function of NNA1.
In this study, we found that the NSC line generated from AMS mice grows faster than those of wild-type or
Nna1 mutation heterozygous mice. In the neuronal cells, microtubules can be found not only in the cell body and processes but also in centrioles and mitotic spindle. In a previous report, it was shown that, during the mitotic process, glutamylation of β-tubulin is increased in centrioles and mitotic spindles [
35]. Due to defective NNA1 functions, glutamylated β-tubulin levels might be increased in the cytoplasm and is therefore responsible for the increased growth of the AMS-derived NSC line. This accelerated growth might also disrupt the differentiation process, as seen in our neuronal differentiation culture.