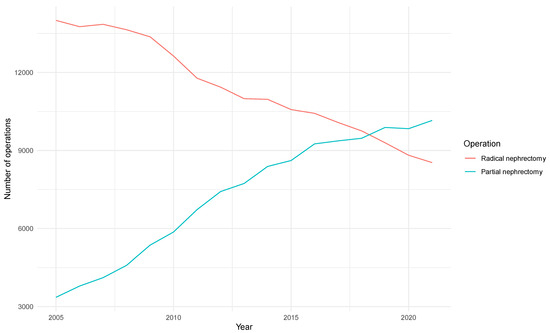
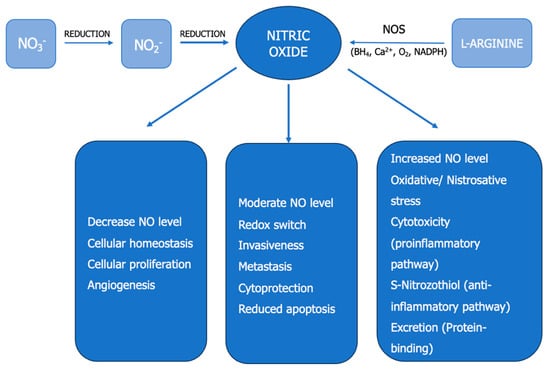
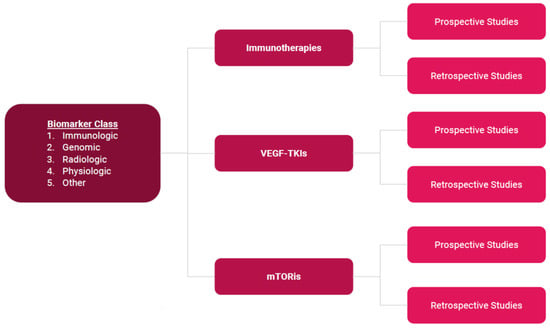
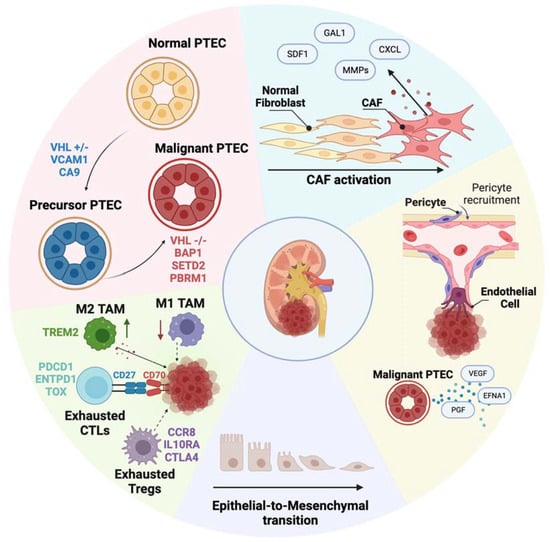
Clear Cell Renal Cell Carcinoma 2022–2023
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Pathophysiology".
Submission Status: Closed | Viewed by 53823Editors
Interests: translational uropathology; pathological diagnosis; basic fundamentals of tumor biology
Special Issues, Collections and Topics in MDPI journals
Interests: urogenital and neurosurgical pathology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Clear cell renal cell carcinoma is one of the most interesting areas of study in oncology right now. Despite the advances obtained, this tumor continues to be a health problem of major concern in Western societies, affecting very seriously their public health services. Several characteristics of this tumor make it an exciting meeting point for translational collaboration between clinicians and basic researchers.
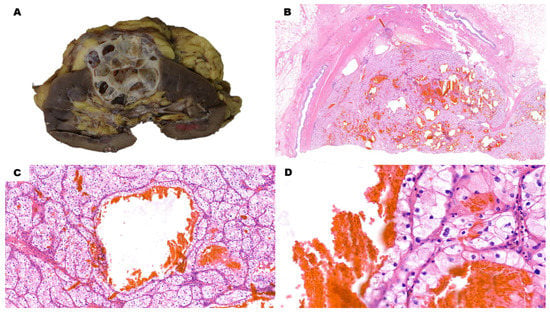
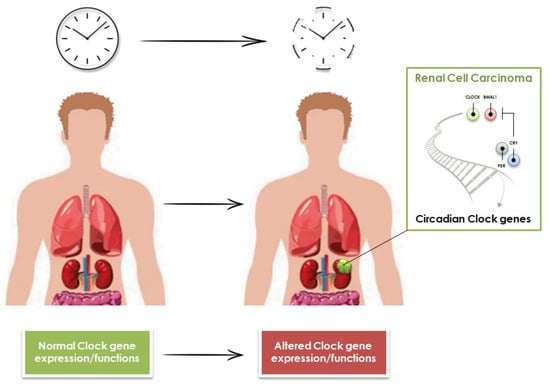
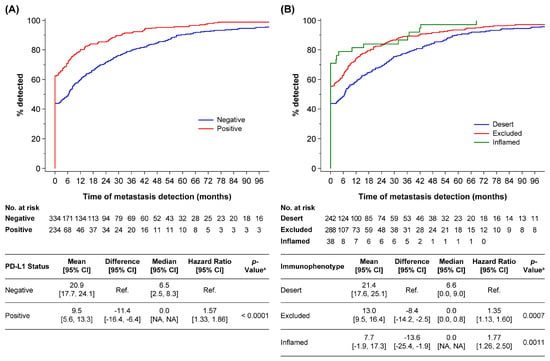
Clear cell renal cell carcinoma is a paradigmatic example of inter- and intra-tumor heterogeneity from morphological, immunohistochemical, and molecular viewpoints. It is also a model to study hypoxia-related carcinogenesis. The latest findings on the spatial and temporal evolutionary patterns detected in this tumor are opening up new promising possibilities for more successful treatments. In addition, the identification of metastatic phenotypes will allow the early detection of aggressive genotypes guiding specific patient management.
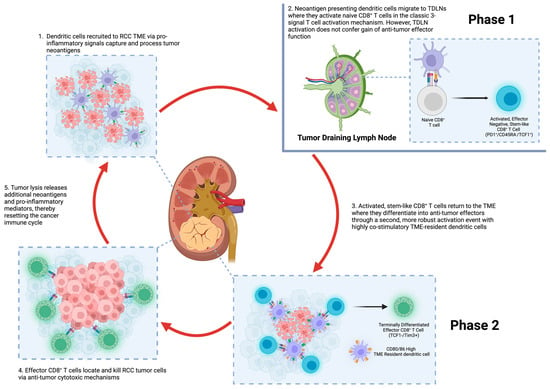
This tumor is also a good example to investigate the complexity of tumor/tumor and tumor/environment relationships from an ecological perspective. A deeper identification of the varied internal tumor self-organization through the specialization of cell clones and subclones as local invaders and metastasizers, on one hand, and the interactions of specific subsets of tumor cells with the local host microenvironment, on the other, will enrich significantly our knowledge of this neoplasm. Finally, alternative approaches such as game theory have provided, in recent years, promising mathematical models to unveil the diversity of possible behaviors of this polyedrical disease.
Clear cell renal cell carcinoma is also a paradigmatic test bench for antiangiogenic and immune checkpoint blockage therapies. The refinement of these therapeutic tools administered alone or in combination is a hot issue in oncology, and several international trials are underway.
All the aforementioned aspects, and still others, make advisable this Topical Collection, which intends to serve as a multidisciplinary platform where urologists, oncologists, pathologists, radiologists, and basic researchers interested in clear cell renal cell carcinoma may meet and collaborate.
Dr. José I. López
Dr. Claudia Manini
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- clear cell renal cell carcinoma
- pathology
- diagnosis
- hypoxia
- intratumor heterogeneity
- immune checkpoint blockage antiangiogenic therapy