Efficacy of Immune Checkpoint Inhibitors vs. Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: Indirect Comparison of Disease-Free Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
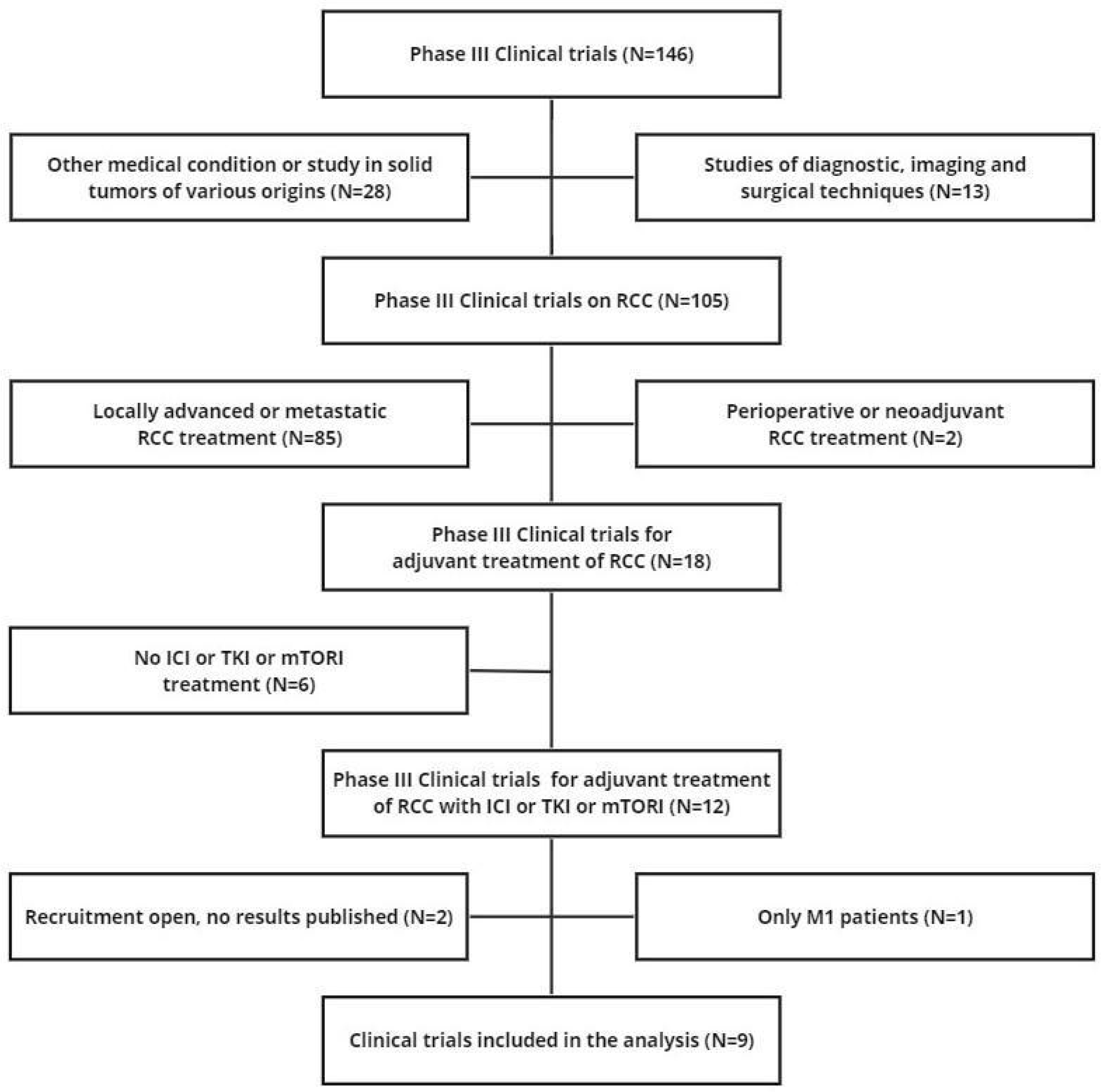
2.2. Literature Search
2.3. Reconstruction of Individual Patient Data
2.4. Statistical Analysis
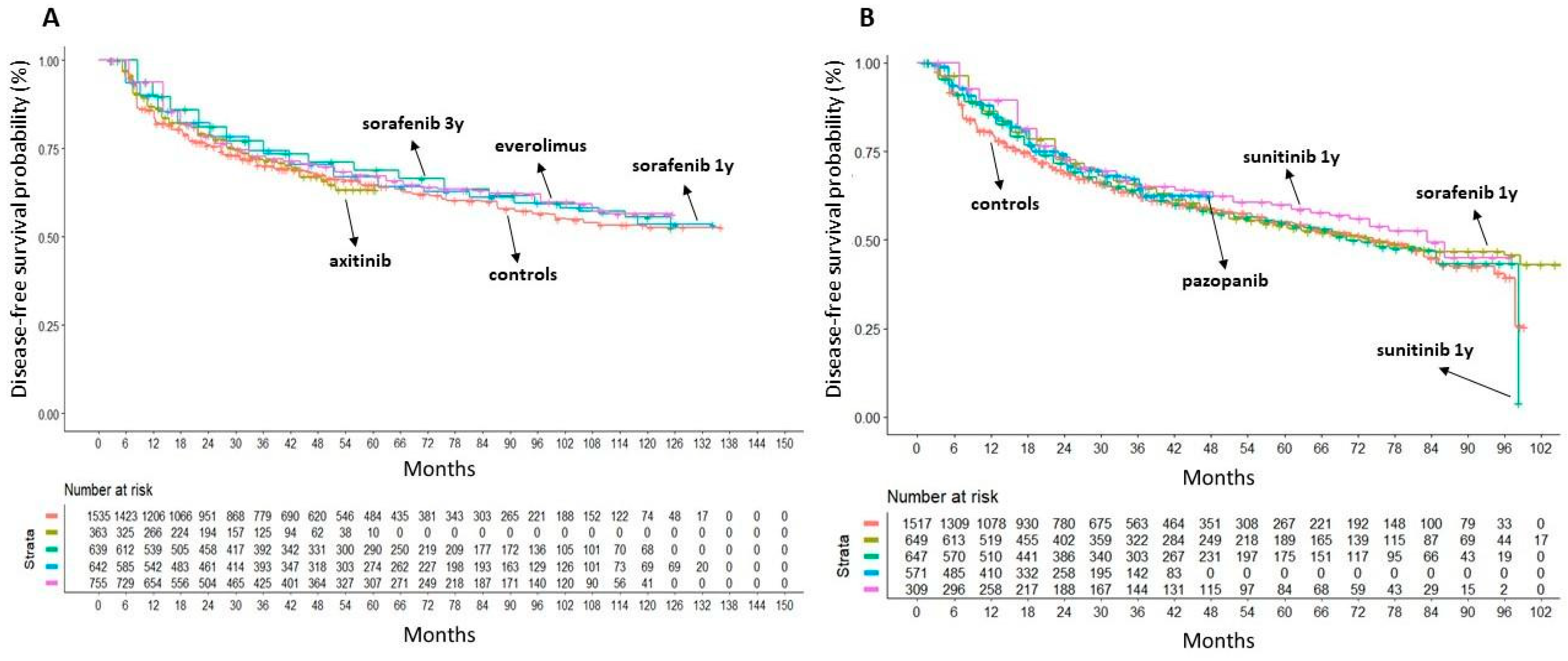
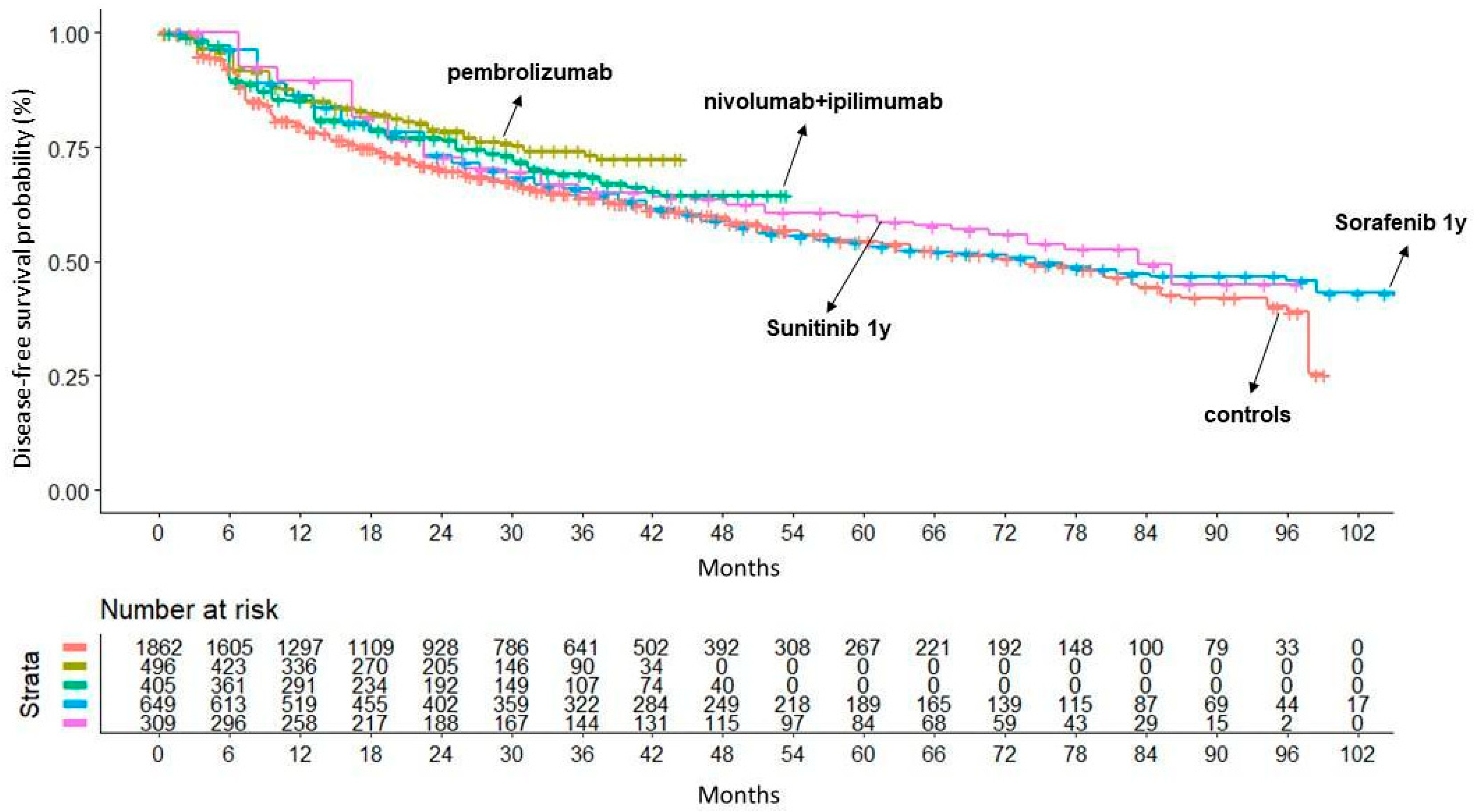
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; Escudier, B.; Motzer, R.J.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Ravaud, A.; et al. Everolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur. J. Cancer 2012, 48, 333–339. [Google Scholar] [CrossRef]
- Xu, J.X.; Maher, V.E.; Zhang, L.; Tang, S.; Sridhara, R.; Ibrahim, A.; Kim, G.; Pazdur, R. FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis. Oncologist 2017, 22, 311–317. [Google Scholar] [CrossRef]
- Lalani, A.A.; Heng, D.Y.C.; Basappa, N.S.; Wood, L.; Iqbal, N.; McLeod, D.; Soulières, D.; Kollmannsberger, C. Evolving landscape of first-line combination therapy in advanced renal cancer: A systematic review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221108685. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Mengato, D.; Chiumente, M.; Messori, A.; Damuzzo, V. Progression-Free and Overall Survival of First-Line Treatments for Advanced Renal Cell Carcinoma: Indirect Comparison of Six Combination Regimens. Cancers 2023, 15, 2029. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; de Velasco, G.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Martinez Chanza, N.; Tripathi, A.; Harshman, L.C. Adjuvant Therapy Options in Renal Cell Carcinoma: Where Do We Stand? Curr. Treat. Options Oncol. 2019, 20, 44. [Google Scholar] [CrossRef]
- Motzer, R.J.; Ravaud, A.; Patard, J.J.; Pandha, H.S.; George, D.J.; Patel, A.; Chang, Y.H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib for High-risk Renal Cell Carcinoma After Nephrectomy: Subgroup Analyses and Updated Overall Survival Results. Eur. Urol. 2018, 73, 62–68. [Google Scholar] [CrossRef]
- Harshman, L.C.; Xie, W.; Moreira, R.B.; Bossé, D.; Ruiz Ares, G.J.; Sweeney, C.J.; Choueiri, T.K. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: A trial-level meta-analysis. Cancer 2018, 124, 925–933. [Google Scholar] [CrossRef]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.H.; Lee, J.L.; et al. Investigators. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; Kwon, T.G.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.I.; Byun, S.S.; Lee, J.L.; Master, V.; Jin, J.; et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef]
- Eisen, T.; Frangou, E.; Oza, B.; Ritchie, A.W.S.; Smith, B.; Kaplan, R.; Davis, I.D.; Stockler, M.R.; Albiges, L.; Escudier, B.; et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results From the SORCE Randomized Phase III Intergroup Trial. J. Clin. Oncol. 2020, 38, 4064–4075. [Google Scholar] [CrossRef]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; Chang, Y.H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N. Engl. J. Med. 2016, 375, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.W.; Tangen, C.M.; Heath, E.I.; Stein, M.N.; Meng, M.V.; Alva, A.S.; Pal, S.K.; Puzanov, I.; Clark, J.I.; Choueiri, T.K.; et al. Adjuvant everolimus after surgery for renal cell carcinoma (EVEREST): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2023, 402, 1043–1051. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Messori, A. Synthetizing Published Evidence on Survival by Reconstruction of Patient-Level Data and Generation of a Multi-Trial Kaplan-Meier Curve. Cureus 2021, 13, e19422. [Google Scholar] [CrossRef]
- Messori, A.; Damuzzo, V.; Rivano, M.; Cancanelli, L.; Di Spazio, L.; Ossato, A.; Chiumente, M.; Mengato, D. Application of the IPDfromKM-Shiny Method to Compare the Efficacy of Novel Treatments Aimed at the Same Disease Condition: A Report of 14 Analyses. Cancers 2023, 15, 1633. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Messori, A. Reconstruction of individual-patient data from the analysis of Kaplan-Meier curves: The use of this method has extended from oncology to cardiology. Open Sci. Framew. 2023; preprint. Available online: https://osf.io/qejus (accessed on 15 November 2023).
- de Sá Marchi, M.F.; Calomeni, P.; Gauza, M.M.; Kanhouche, G.; Ravani, L.V.; Rodrigues, C.V.F.; Tarasoutchi, F.; de Brito, F.S., Jr.; Rodés-Cabau, J.; Van Mieghem, N.M.; et al. Impact of periprocedural myocardial injury after transcatheter aortic valve implantation on long-term mortality: A meta-analysis of Kaplan-Meier derived individual patient data. Front. Cardiovasc. Med. 2023, 10, 1228305. [Google Scholar] [CrossRef]
- Saluja, R.; Cheng, S.; Delos Santos, K.A.; Chan, K.K.W. Estimating hazard ratios from published Kaplan-Meier survival curves: A methods validation study. Res. Synth. Methods 2019, 10, 465–475. [Google Scholar] [CrossRef]
- Everest, L.; Blommaert, S.; Tu, D.; Pater, J.L.; Hay, A.; Cheung, M.C.; Chan, K.K.W. Validating restricted mean survival time estimates from reconstructed kaplan-meier data against original trial individual patient data from trials conducted by the canadian cancer trials group. Value Health 2022, 25, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Haas, N.B.; Bagheri, M.; Lane, B.R.; Coleman, J.; Hammers, H.; Bratslavsky, G.; Chauhan, C.; Kim, L.; Krishnasamy, V.P.; et al. Eligibility and Radiologic Assessment for Adjuvant Clinical Trials in Kidney Cancer. JAMA Oncol. 2020, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, T.; Welsh, S.J.; Zaki, K.; Mulders, P.; Eisen, T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin. Oncol. 2013, 40, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Giberti, C.; Oneto, F.; Martorana, G.; Rovida, S.; Carmignani, G. Radical nephrectomy for renal cell carcinoma: Long-term results and prognostic factors on a series of 328 cases. Eur. Urol. 1997, 31, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cella, D.; Manola, J.; DiPaola, R.S.; Wagner, L.I.; Haas, N.S.B. Fatigue among patients with renal cell carcinoma receiving adjuvant sunitinib or sorafenib: Patient-reported outcomes of ECOG-ACRIN E2805 trial. Support. Care Cancer 2018, 26, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Staehler, M.; Motzer, R.J.; George, D.J.; Pandha, H.S.; Donskov, F.; Escudier, B.; Pantuck, A.J.; Patel, A.; DeAnnuntis, L.; Bhattacharyya, H.; et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: Safety, therapy management, and patient-reported outcomes in the S-TRAC trial. Ann. Oncol. 2018, 29, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Symeonides, S.; Hajek, J.; Ferguson, T.; Chang, Y.H.; Lee, J.L.; Haas, N.; et al. Patient-Reported Outcomes in KEYNOTE-564: Adjuvant Pembrolizumab Versus Placebo for Renal Cell Carcinoma. Oncologist 2023, 17, oyad231. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, A.; Solimando, A.G.; Kerscher, A.; Bittrich, M.; Kalogirou, C.; Kübler, H.; Rosenwald, A. Subgroup-Independent Mapping of Renal Cell Carcinoma-Machine Learning Reveals Prognostic Mitochondrial Gene Signature Beyond Histopathologic Boundaries. Front. Oncol. 2021, 11, 621278. [Google Scholar] [CrossRef] [PubMed]



| # | Trial | Reference | Treatments under Comparison | DFS | |
|---|---|---|---|---|---|
| Treatment Group (Events/Patients) | Controls (Events/Patients) | ||||
| ARCC01 | KEYNOTE-564 (two-arm) | Powles et al. [11] | Pembrolizumab (200 mg) Q3W (max 17 cycles); Placebo Q3W. | 114/496 | 169/498 |
| ARCC02 | IMmotion010 (two-arm) | Pal et al. [12] | Atezolizumab (1200 mg) Q3W (max 16 cycles); Placebo Q3W. | 164/390 | 168/388 |
| ARCC03 | CheckMate914 (two-arm) | Motzer et al. [13] | Nivolumab (240 mg) Q2W (max 12 cycles) + Ipilimumab (1 mg/kg) Q6W (max 4 cycles); Placebo Q2W + Q6W. | 110/405 | 118/411 |
| TKI01 | ATLAS (two-arm) | Gross-Goupil et al. [14] | Axitinib (5 mg) BID (max 3 years); Placebo BID (max 3 years). | 96/363 | 107/361 |
| TKI02 | SORCE (three-arm) | Eisen et al. [15] | Sorafenib (400 mg) BID for 3 years | 245/639 | 167/430 |
| Sorafenib (400 mg) BID for 1 year plus placebo for 2 years; Placebo BID for 3 years. | 242/642 | ||||
| TKI03 | ASSURE (three-arm) | Haas et al. [16] | Sunitinib (50 mg) (4 weeks on/2 off) for 54 weeks; | 284/647 | 287/647 |
| Sorafenib (400 mg) BID for 54 weeks; Placebo for 54 weeks. | 284/649 | ||||
| TKI04 | PROTECT (two-arm) | Motzer et al. [17] | Pazopanib (600 mg) for 1 year; Placebo for 1 year. | 194/571 | 202/564 |
| TKI05 | S-TRAC (two-arm) | Ravaud et al. [18] | Sunitinib (50 mg) (4 weeks on/2 off) for 52 weeks; Placebo for 52 weeks. | 113/309 | 144/306 |
| mTORI01 | EVEREST (two-arm) | Ryan et al. [19] | Everolimus (10 mg) for 54 weeks; Placebo for 54 weeks. | 262/755 | 294/744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossato, A.; Gasperoni, L.; Del Bono, L.; Messori, A.; Damuzzo, V. Efficacy of Immune Checkpoint Inhibitors vs. Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: Indirect Comparison of Disease-Free Survival. Cancers 2024, 16, 557. https://doi.org/10.3390/cancers16030557
Ossato A, Gasperoni L, Del Bono L, Messori A, Damuzzo V. Efficacy of Immune Checkpoint Inhibitors vs. Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: Indirect Comparison of Disease-Free Survival. Cancers. 2024; 16(3):557. https://doi.org/10.3390/cancers16030557
Chicago/Turabian StyleOssato, Andrea, Lorenzo Gasperoni, Luna Del Bono, Andrea Messori, and Vera Damuzzo. 2024. "Efficacy of Immune Checkpoint Inhibitors vs. Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: Indirect Comparison of Disease-Free Survival" Cancers 16, no. 3: 557. https://doi.org/10.3390/cancers16030557
APA StyleOssato, A., Gasperoni, L., Del Bono, L., Messori, A., & Damuzzo, V. (2024). Efficacy of Immune Checkpoint Inhibitors vs. Tyrosine Kinase Inhibitors/Everolimus in Adjuvant Renal Cell Carcinoma: Indirect Comparison of Disease-Free Survival. Cancers, 16(3), 557. https://doi.org/10.3390/cancers16030557







