Surgical Trends and Complications in Partial and Radical Nephrectomy: Results from the GRAND Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. GeRmAn Nationwide Inpatient Data (GRAND)
2.2. Selection Criteria
2.3. Data Synthesis and Statistical Analysis
3. Results
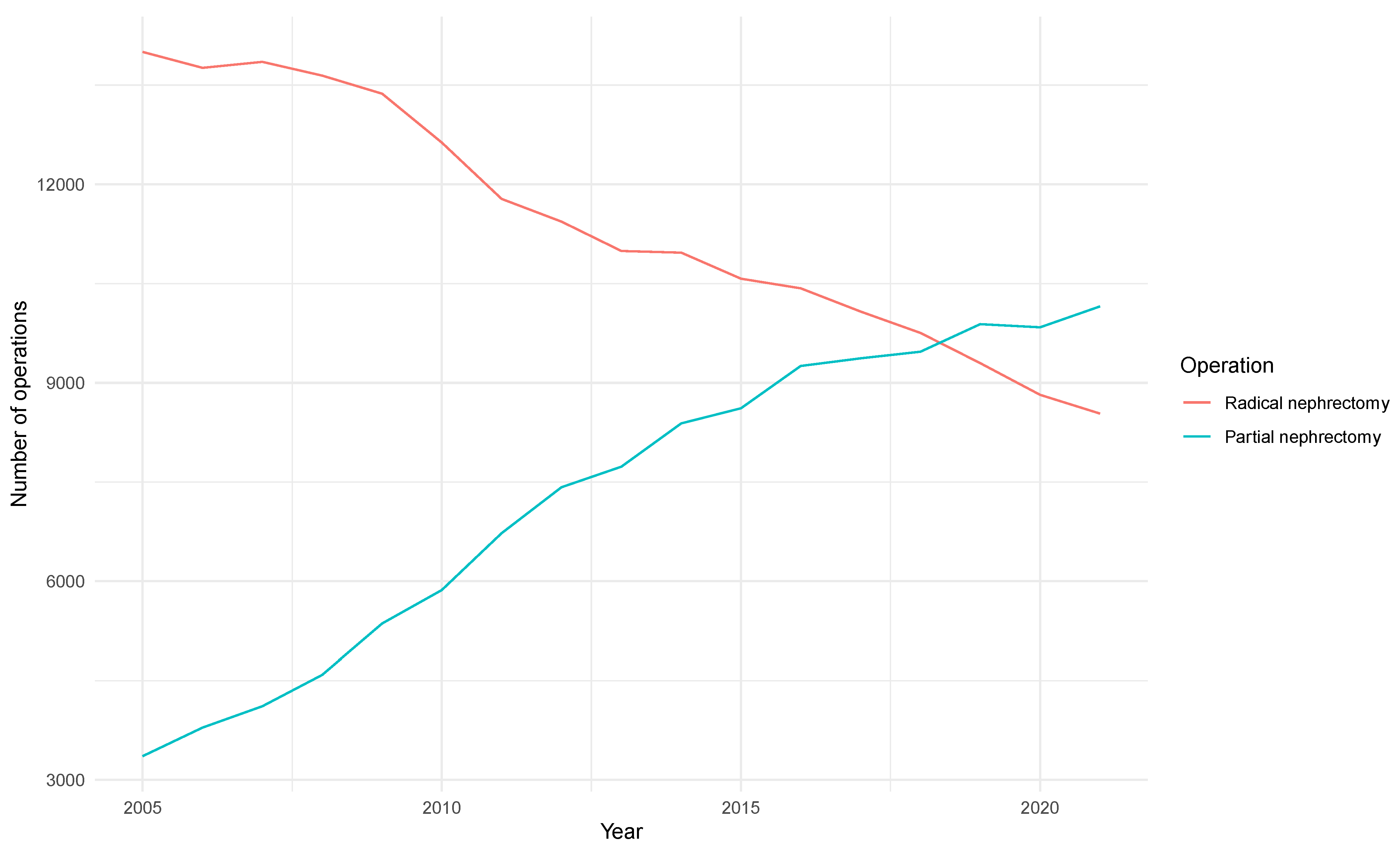
3.1. Baseline Characteristics
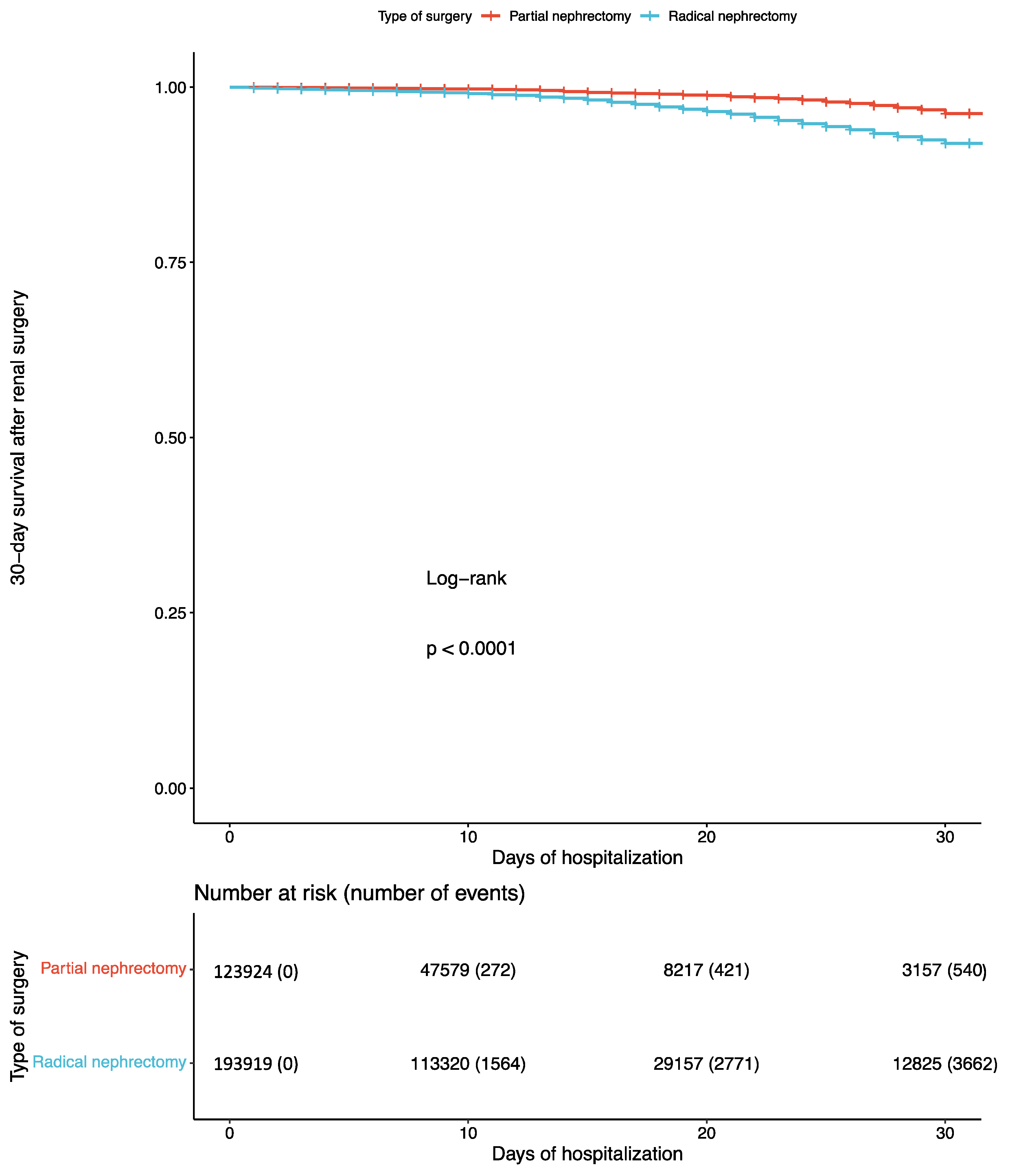
3.2. Effects of Renal Surgery on Perioperative Morbidity, Mortality, Hospital Stay, and Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Leung, D.K.-W.; Chan, E.O.-T.; Lok, V.; Leung, S.; Wong, I.; Lao, X.-Q.; Zheng, Z.-J.; Chiu, P.K.-F.; Ng, C.-F.; et al. A Global Trend Analysis of Kidney Cancer Incidence and Mortality and Their Associations with Smoking, Alcohol Consumption, and Metabolic Syndrome. Eur. Urol. Focus 2022, 8, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Klatte, T.; Amparore, D.; Bertolo, R.; Carbonara, U.; Erdem, S.; Ingels, A.; Kara, O.; Marandino, L.; Marchioni, M.; et al. Screening programs for renal cell carcinoma: A systematic review by the EAU young academic urologists renal cancer working group. World J. Urol. 2023, 41, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Fossett, L.K.; Ramchandani, P.; Tomaszewski, J.E.; Siegelman, E.S.; Banner, M.P.; Van Arsdalen, K.N.; Wein, A.J.; Malkowicz, S.B. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 2006, 68, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Ali, M.; Correa, R.J.M.; Muacevic, A.; Ponsky, L.; Ellis, R.J.; Lo, S.S.; Onishi, H.; Swaminath, A.; McLaughlin, M.; et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: An individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022, 23, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Marechal, J.-M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A Prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2007, 51, 1606–1615. [Google Scholar] [CrossRef]
- Kim, S.P.; Thompson, R.H.; Boorjian, S.A.; Weight, C.J.; Han, L.C.; Murad, M.H.; Shippee, N.D.; Erwin, P.J.; Costello, B.A.; Chow, G.K.; et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J. Urol. 2012, 188, 51–57. [Google Scholar] [CrossRef]
- Mir, M.C.; Derweesh, I.; Porpiglia, F.; Zargar, H.; Mottrie, A.; Autorino, R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. 2017, 71, 606–6177. [Google Scholar] [CrossRef]
- Crocerossa, F.; Carbonara, U.; Cantiello, F.; Marchioni, M.; Ditonno, P.; Mir, M.C.; Porpiglia, F.; Derweesh, I.; Hampton, L.J.; Damiano, R.; et al. Robot-assisted Radical Nephrectomy: A Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. 2021, 80, 428–439. [Google Scholar] [CrossRef]
- Sun, M.; Bianchi, M.; Trinh, Q.-D.; Abdollah, F.; Schmitges, J.; Jeldres, C.; Shariat, S.F.; Graefen, M.; Montorsi, F.; Perrotte, P.; et al. Hospital volume is a determinant of postoperative complications, blood transfusion and length of stay after radical or partial nephrectomy. J. Urol. 2012, 187, 405–410. [Google Scholar] [CrossRef]
- Hsu, R.C.J.; Salika, T.; Maw, J.; Lyratzopoulos, G.; Gnanapragasam, V.J.; Armitage, J.N. Influence of hospital volume on nephrectomy mortality and complications: A systematic review and meta-analysis stratified by surgical type. BMJ Open 2017, 7, e016833. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Pulido, J.E.; Chelluri, R.R.; Strother, M.C.; Taylor, B.L.; Raman, J.D.; Guzzo, T.J. Hospital volume and outcomes of robot-assisted partial nephrectomy. BJU Int. 2018, 121, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Bruins, H.M.; Veskimäe, E.; Hernández, V.; Neuzillet, Y.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Espinós, E.L.; Lorch, A.; et al. The Importance of Hospital and Surgeon Volume as Major Determinants of Morbidity and Mortality after Radical Cystectomy for Bladder Cancer: A Systematic Review and Recommendations by the European Association of Urology Muscle-invasive and Metastatic Bladder Cancer Guideline Panel. Eur. Urol. Oncol. 2020, 3, 131–144. [Google Scholar] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Walter, D.; Taksler, G.B.; Huang, W.C.; Wysock, J.S.; Sivarajan, G.; Loeb, S.; Taneja, S.S.; Makarov, D.V. National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology 2013, 82, 1283–1290. [Google Scholar] [CrossRef]
- Campbell, S.C.; Uzzo, R.G.; Karam, J.A.; Chang, S.S.; Clark, P.E.; Souter, L. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-up: AUA Guideline: Part II. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Fero, K.; Hamilton, Z.A.; Bindayi, A.; Murphy, J.D.; Derweesh, I.H. Utilization and quality outcomes of cT1a, cT1b and cT2a partial nephrectomy: Analysis of the national cancer database. BJU Int. 2018, 121, 565–574. [Google Scholar] [CrossRef]
- Plante, K.; Stewart, T.M.; Wang, D.; Bratslavsky, G.; Formica, M. Treatment trends, determinants, and survival of partial and radical nephrectomy for stage I renal cell carcinoma: Results from the National Cancer Data Base, 2004–2013. Int. Urol. Nephrol. 2017, 49, 1375–1381. [Google Scholar] [CrossRef]
- Hsu, R.C.J.; Barclay, M.; Loughran, M.A.; Lyratzopoulos, G.; Gnanapragasam, V.J.; Armitage, J.N. Time trends in service provision and survival outcomes for patients with renal cancer treated by nephrectomy in England 2000–2010. BJU Int. 2018, 122, 599–609. [Google Scholar] [CrossRef]
- Chen, K.; Lee, A.; Huang, H.H.; Tay, K.J.; Sim, A.; Lee, L.S.; Cheng, C.W.S.; Ng, L.G.; Ho, H.S.S.; Yuen, J.S.P. Evolving trends in the surgical management of renal masses over the past two decades: A contemporary picture from a large prospectively-maintained database. Int. J. Urol. 2019, 26, 465–474. [Google Scholar] [CrossRef]
- Yildirim, H.; Schuurman, M.S.; Widdershoven, C.V.; Lagerveld, B.W.; Brink, L.v.D.; Ruiter, A.E.C.; Beerlage, H.P.; van Moorselaar, R.J.A.; Graafland, N.M.; Bex, A.; et al. Variation in the management of cT1 renal cancer by surgical hospital volume: A nationwide study. BJUI Compass 2023, 4, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, M.; Koru-Sengul, T.; Moore, K.J.; Miao, F.; Sávio, L.F.; Nahar, B.; Prakash, N.S.; Venkatramani, V.; Jue, J.S.; Punnen, S.; et al. Trends in Utilization of Robotic and Open Partial Nephrectomy for Management of cT1 Renal Masses. Eur. Urol. Focus 2019, 5, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Talwar, R.; Taylor, B.L.; Shin, M.H.; Berger, I.B.; Sperling, C.D.; Chelluri, R.R.; Zambrano, I.A.; Raman, J.D.; Guzzo, T.J. National trends and disparities of minimally invasive surgery for localized renal cancer, 2010 to 2015. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 182.e17–182.e27. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Cadeddu, J.A.; Cestari, A.; Gill, I.S.; Jewett, M.A.; Joniau, S.; Kirkali, Z.; Marberger, M.; Patard, J.J.; Staehler, M.; et al. Contemporary management of small renal masses. Eur. Urol. 2011, 60, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; A Mistretta, F.; Knipper, S.; Mazzone, E.; Pecoraro, A.; Tian, Z.; Perrotte, P.; Antonelli, A.; Montorsi, F.; Shariat, S.F.; et al. Assessment of local tumor ablation and non-interventional management versus partial nephrectomy in T1a renal cell carcinoma. Minerva Urol. Nefrol. 2020, 72, 350–359. [Google Scholar] [CrossRef]
- Su, Z.T.; Patel, H.D.; Huang, M.M.; Alam, R.; Cheaib, J.G.; Pavlovich, C.P.; Allaf, M.E.; Pierorazio, P.M. Active Surveillance versus Immediate Intervention for Small Renal Masses: A Cost-Effectiveness and Clinical Decision Analysis. J. Urol. 2022, 208, 794–803. [Google Scholar] [CrossRef]
- Correa, R.J.; Louie, A.V.; Zaorsky, N.G.; Lehrer, E.J.; Ellis, R.; Ponsky, L.; Kaplan, I.; Mahadevan, A.; Chu, W.; Swaminath, A.; et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2019, 5, 958–969. [Google Scholar] [CrossRef]
- Flegar, L.; Thoduka, S.G.; Mahnken, A.H.; Figiel, J.; Heers, H.; Aksoy, C.; Eisenmenger, N.; Groeben, C.; Huber, J.; Zacharis, A. Focal Therapy for Renal Cancer: Comparative Trends in the USA and Germany from 2006 to 2020 and Analysis of the German Health Care Landscape. Urol. Int. 2023, 107, 396–405. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.-H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Teng, Z.; Han, Z. Partial nephrectomy versus radical nephrectomy for cT2 or greater renal tumors: A systematic review and meta-analysis. Minerva Urol. Nefrol. 2019, 71, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Johnson, M.H.; Patel, H.D.; Sozio, S.M.; Sharma, R.; Iyoha, E.; Bass, E.B.; Allaf, M.E. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J. Urol. 2016, 196, 989–999. [Google Scholar] [CrossRef]
| Characteristic | Overall, n = 317,843 | Partial Nephrectomy, n = 123,924 | Radical Nephrectomy, n = 193,919 | p-Value |
|---|---|---|---|---|
| Age (years) | 66 (56–74) | 65 (56–73) | 67 (56–75) | <0.001 |
| Males | 188,123 (59%) | 77,117 (62%) | 111,006 (57%) | <0.001 |
| Diabetes | 57,155 (18%) | 21,319 (17%) | 35,836 (18%) | <0.001 |
| Chronic heart failure | 20,140 (6.3%) | 5722 (4.6%) | 14,418 (7.4%) | <0.001 |
| Chronic obstructive pulmonary disease | 23,142 (7.3%) | 8694 (7%) | 14,448 (7.5%) | <0.001 |
| Chronic kidney disease | 65,605 (21%) | 16,380 (13%) | 49,225 (25%) | <0.001 |
| Cerebrovascular disease | 7607 (2.4%) | 2126 (1.7%) | 5481 (2.8%) | <0.001 |
| Dementia | 3689 (1.2%) | 693 (0.6%) | 2996 (1.5%) | <0.001 |
| Hypertension | 179,386 (56%) | 70,382 (57%) | 109,004 (56%) | 0.001 |
| Obesity | 32,008 (10%) | 12,384 (10%) | 19,624 (10%) | 0.25 |
| Operative technique | <0.001 | |||
| Open | 249,333 (78%) | 89,227 (72%) | 160,106 (83%) | |
| Laparoscopic | 44,994 (14%) | 15,528 (13%) | 29,466 (15%) | |
| Robotic | 23,516 (7.4%) | 19,169 (15%) | 4347 (2.2%) |
| Complications | Partial Nephrectomy | Radical Nephrectomy | |
|---|---|---|---|
| Transfusion | Events | 15,315 (12%) | 49,169 (25%) |
| OR (95% CI) | - | 2 (1.9, 2) | |
| p-Value | - | <0.001 | |
| Sepsis | Events | 1260 (1%) | 6018 (3.1%) |
| OR (95% CI) | - | 2.6 (2.4, 2.8) | |
| p-Value | - | <0.001 | |
| Acute respiratory failure | Events | 4448 (3.6%) | 10,438 (5.4%) |
| OR (95% CI) | - | 1.6 (1.5, 1.7) | |
| p-Value | - | <0.001 | |
| Acute kidney disease | Events | 4985 (4%) | 11,408 (5.9%) |
| OR (95% CI) | - | 1.6 (1.5, 1.7) | |
| p-Value | - | <0.001 | |
| Acute thromboembolism | Events | 630 (0.5%) | 1802 (0.9%) |
| OR (95% CI) | - | 1.9 (1.7, 2) | |
| p-Value | - | <0.001 | |
| Surgical wound infection | Events | 391 (0.3%) | 1369 (0.7%) |
| OR (95% CI) | - | 2 (1.8, 2.2) | |
| p-Value | - | <0.001 |
| Complications | Partial Nephrectomy | Radical Nephrectomy | |
|---|---|---|---|
| Ileus | Events | 1623 (1.3%) | 3903 (2%) |
| OR (95% CI) | - | 1.4 (1.3, 1.5) | |
| p-Value | - | <0.001 | |
| 30-Day mortality | Events | 540 (0.4%) | 3662 (1.9%) |
| OR (95% CI) | - | 3.5 (3.2, 3.9) | |
| p-Value | - | <0.001 | |
| ICU admission | Events | 20,116 (16%) | 41,355 (21%) |
| OR (95% CI) | - | 1.2 (1.2, 1.2) | |
| p-Value | - | <0.001 | |
| Length of hospital stay | Days | 9 (7–11) | 10 (8–15) |
| Beta (95% CI) | - | 1.9 (1.9, 2) | |
| p-Value | - | <0.001 | |
| Costs | EUR | 7087 (6484–8122) | 7400 (6484–10,492) |
| Beta (95% CI) | - | 1778 (1694, 1862) | |
| p-Value | - | <0.001 | |
| Pancreatitis | Events | 166 (0.1%) | 636 (0.3%) |
| OR (95% CI) | - | 2 (1.8, 2.5) | |
| p-Value | - | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrgidis, N.; Schulz, G.B.; Stief, C.; Blajan, I.; Ivanova, T.; Graser, A.; Staehler, M. Surgical Trends and Complications in Partial and Radical Nephrectomy: Results from the GRAND Study. Cancers 2024, 16, 97. https://doi.org/10.3390/cancers16010097
Pyrgidis N, Schulz GB, Stief C, Blajan I, Ivanova T, Graser A, Staehler M. Surgical Trends and Complications in Partial and Radical Nephrectomy: Results from the GRAND Study. Cancers. 2024; 16(1):97. https://doi.org/10.3390/cancers16010097
Chicago/Turabian StylePyrgidis, Nikolaos, Gerald Bastian Schulz, Christian Stief, Iulia Blajan, Troya Ivanova, Annabel Graser, and Michael Staehler. 2024. "Surgical Trends and Complications in Partial and Radical Nephrectomy: Results from the GRAND Study" Cancers 16, no. 1: 97. https://doi.org/10.3390/cancers16010097
APA StylePyrgidis, N., Schulz, G. B., Stief, C., Blajan, I., Ivanova, T., Graser, A., & Staehler, M. (2024). Surgical Trends and Complications in Partial and Radical Nephrectomy: Results from the GRAND Study. Cancers, 16(1), 97. https://doi.org/10.3390/cancers16010097







