Analysis and Excavation of Unique Metabolic Components of Wheat Cultivated in Saline–Alkaline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Materials
2.2. Sample Preparation and Extraction
2.3. Analysis Conditions
- (1)
- Chromatographic column: Agilent SB-C18 (1.8 µm, 2.1 mm × 100 mm).
- (2)
- Mobile phase: pure water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B).
- (3)
- A gradient program was employed for sample measurement. The elution gradient proceeded as follows: initial condition—95% A, 5% B; a linear increase in B to 95% within 9 min; a decrease in A to 5%, maintained for 1 min; and an adjustment to 95% A and 5% B in 1.1 min, and then equilibrium for 14 min.
- (4)
- During elution, the flow velocity was 0.35 mL/min; the column oven was set to 40 °C; the injection volume was 2 μL. The effluent was connected to an ESI-Q TRAP-MS/MS system.
2.4. Multivariate Statistical Analysis
3. Results
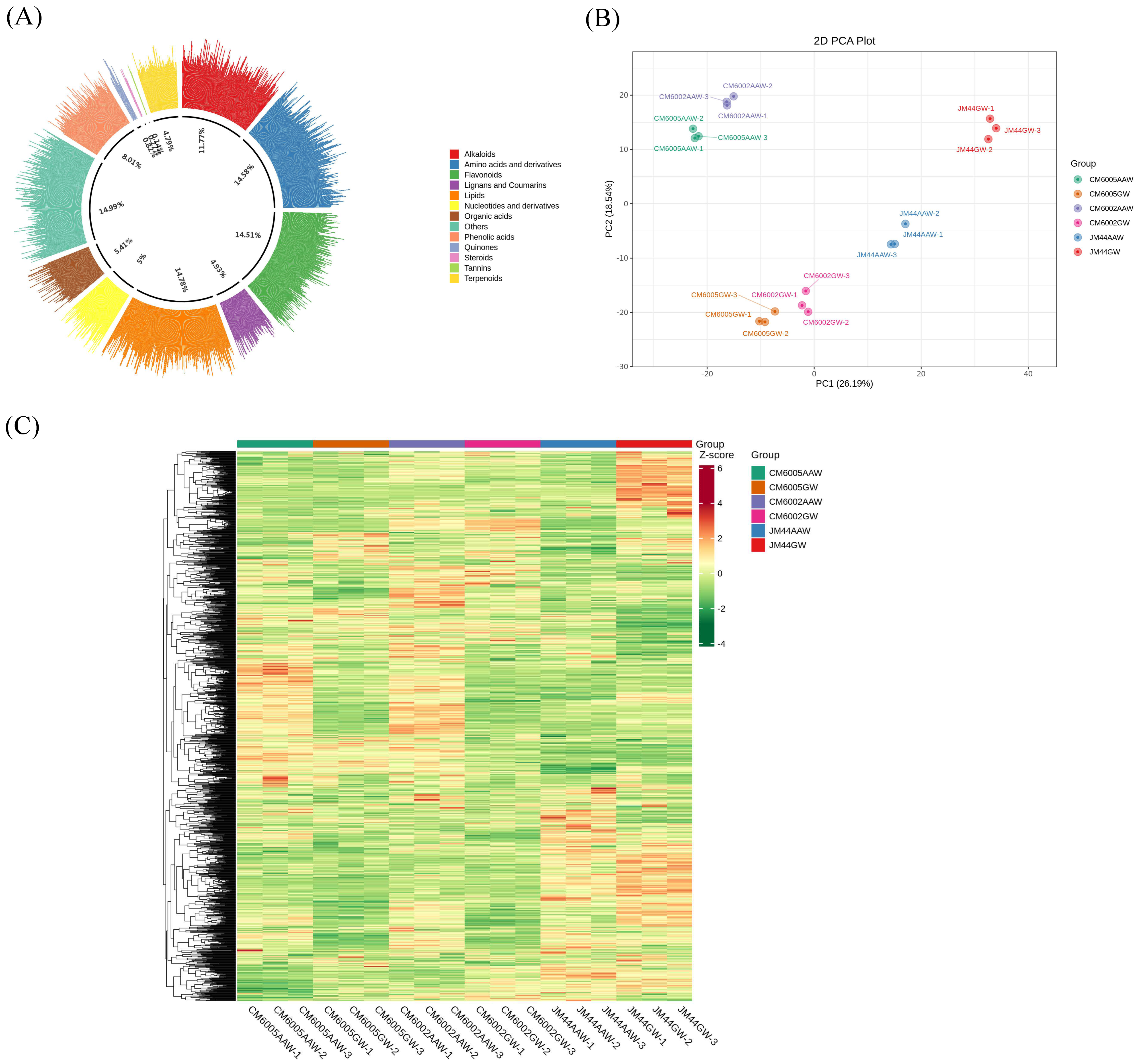
3.1. Metabolic Profiling
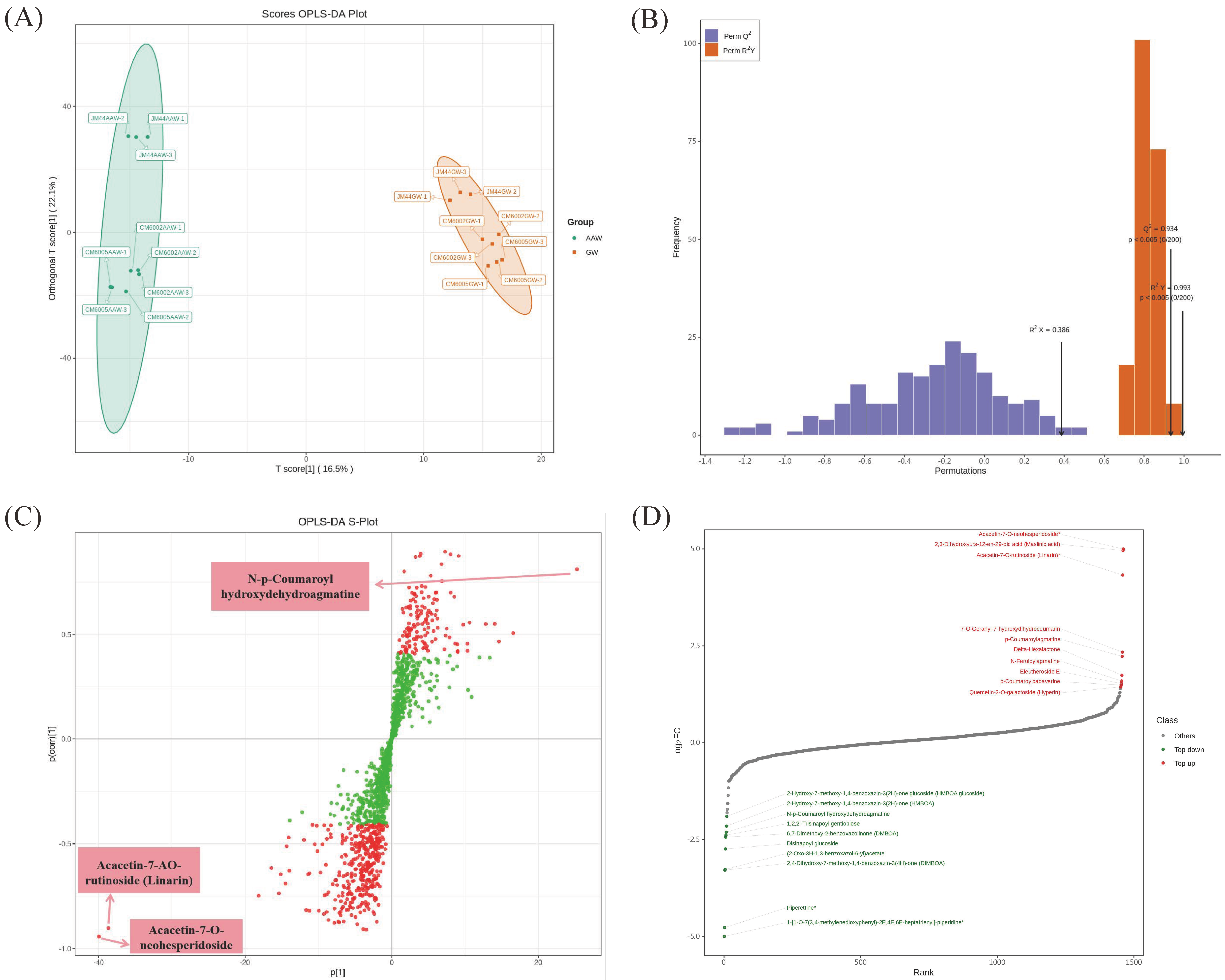
3.2. Multivariate Statistical Analysis
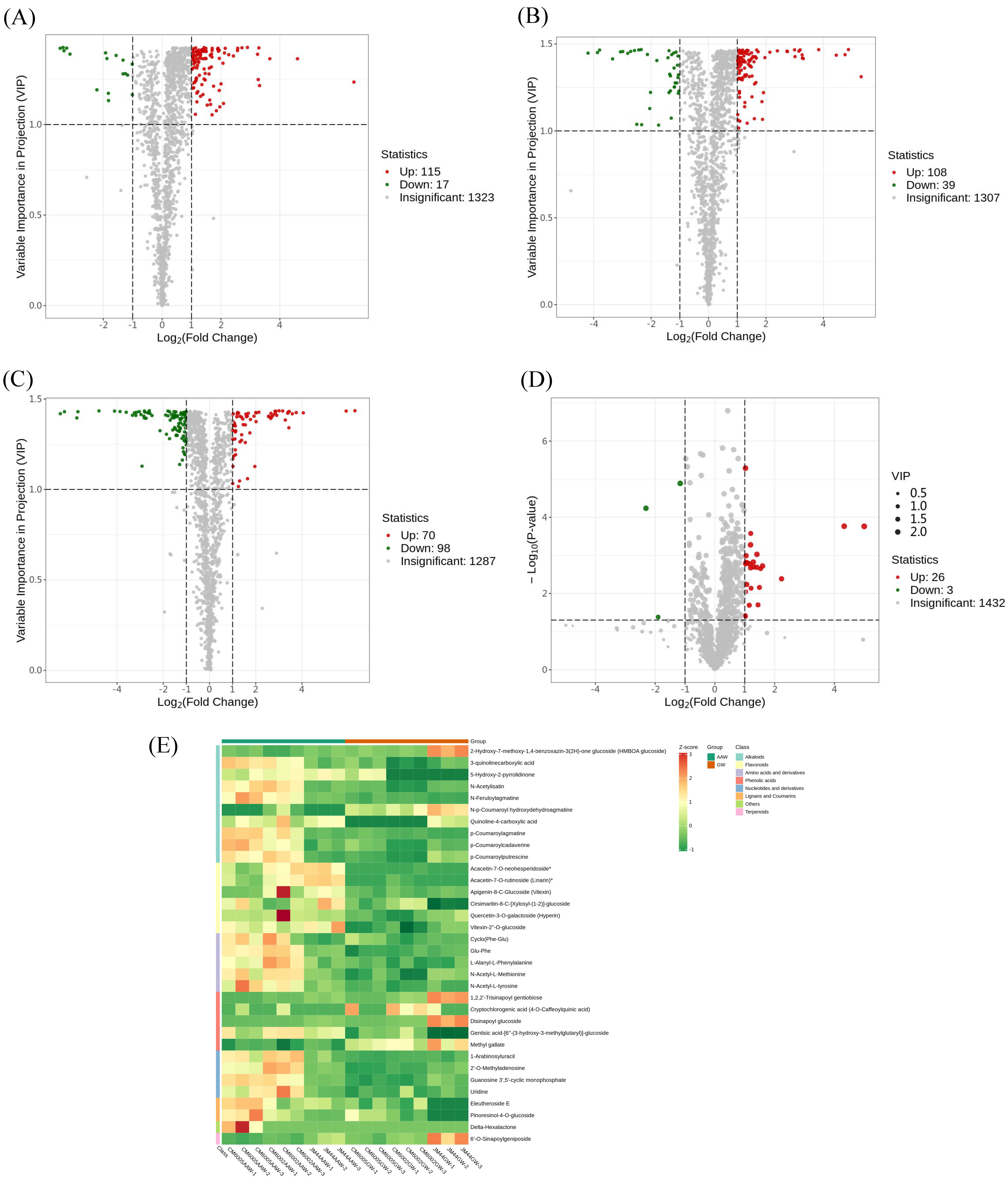
3.3. Identification of Differential Metabolites
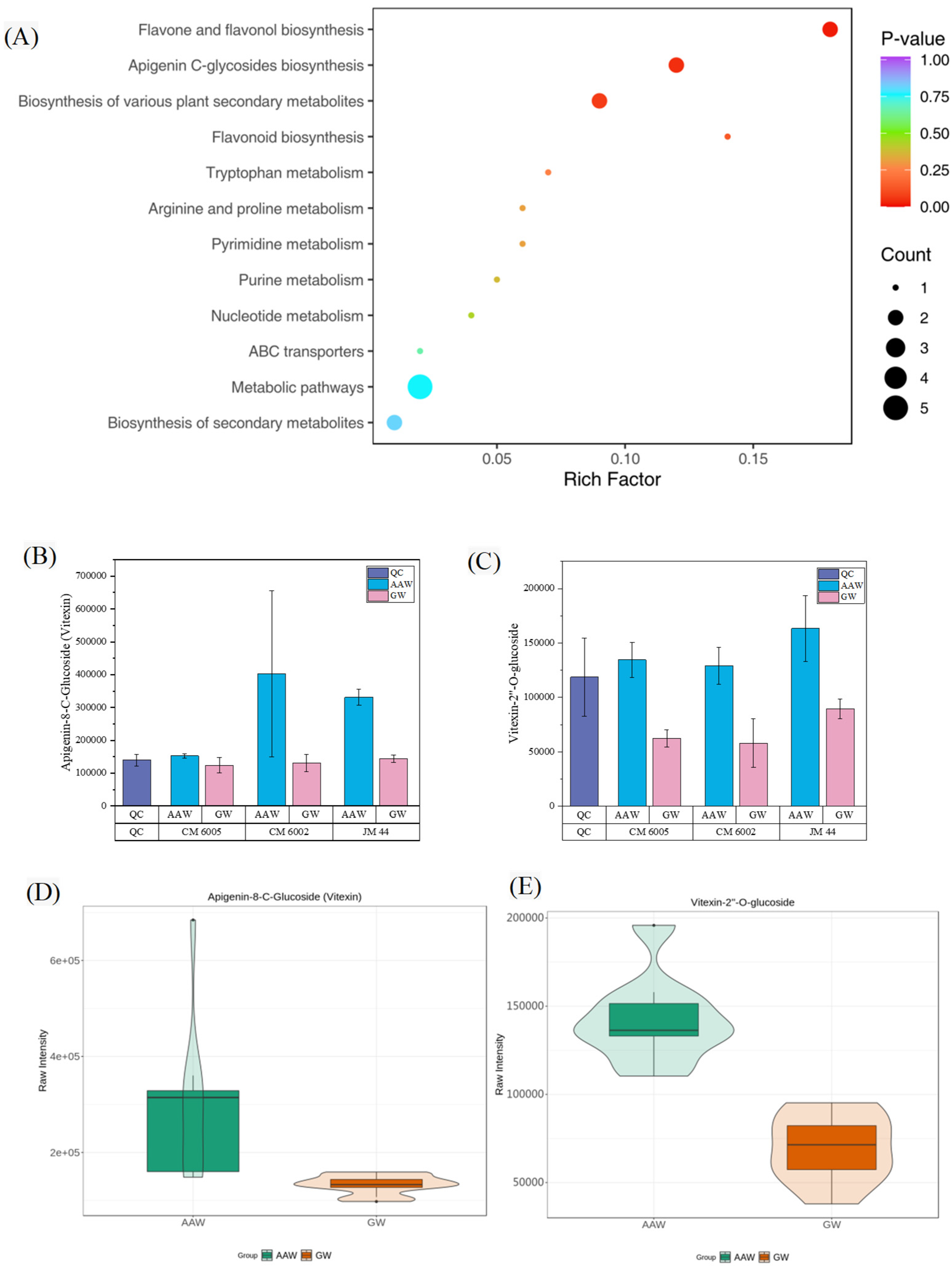
3.4. KEGG Annotation and Enrichment Analysis of Differential Metabolites
4. Discussion
4.1. Differential Metabolites of AAW and GW
4.2. Effects of Drought and Saline–Alkaline Stress on Wheat Metabolites
4.3. Specific Metabolic Changes Under Drought and Saline–Alkaline Stress Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAW | Wheat cultivated in saline–alkaline soil at Zhong Jie Industrial Park |
| GW | Generally grown wheat at Xian Huanyuan Village |
| PCA | Principal component analysis |
| HCA | Hierarchical cluster analysis |
| OPLS-DA | Orthogonal partial least squares–discriminant analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, H.W.; Wang, J.T.; Macdonald, C.A.; Singh, P.; Cong, V.T.; Klein, M.; Delgado-Baquerizo, M.; Singh, B.K. Drought-induced plant microbiome and metabolic enrichments improve drought resistance. Cell Host Microbe 2025, 33, 882–900. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Wang, L.; Liu, L.; Liu, L.; Zhang, Z.; Yan, N. Metabolomic analysis of flavonoid diversity and biosynthetic pathways in whole grains. Food Res. Int. 2025, 211, 116359. [Google Scholar] [CrossRef]
- Tenenboim, H.; Brotman, Y. Omic Relief for the Biotically Stressed: Metabolomics of Plant Biotic Interactions. Trends Plant Sci. 2016, 21, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Paucean, A.; Muresan, V.; Maria-Man, S.; Chis, M.S.; Muresan, A.E.; Serban, L.R.; Pop, A.; Muste, S. Metabolomics as a Tool to Elucidate the Sensory, Nutritional and Safety Quality of Wheat Bread-A Review. Int. J. Mol. Sci. 2021, 22, 8945. [Google Scholar] [CrossRef]
- Tinte, M.M.; Chele, K.H.; van Der Hooft, J.J.J.; Tugizimana, F. Metabolomics-Guided Elucidation of Plant Abiotic Stress Responses in the 4IR Era: An Overview. Metabolites 2021, 11, 445. [Google Scholar] [CrossRef]
- Beleggia, R.; Platani, C.; Nigro, F.; De Vita, P.; Cattivelli, L.; Papa, R. Effect of genotype, environment and genotype-by-environment interaction on metabolite profiling in durum wheat (Triticum durum Desf.) grain. J. Cereal Sci. 2013, 57, 183–192. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Gregorova, Z.; Kovacik, J.; Klejdus, B.; Maglovski, M.; Kuna, R.; Hauptvogel, P.; Matusikova, I. Drought-Induced Responses of Physiology, Metabolites, and PR Proteins in Triticum aestivum. J. Agric. Food Chem. 2015, 63, 8125–8133. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics Response for Drought Stress Tolerance in Chinese Wheat Genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef]
- Lv, L.; Chen, X.; Li, H.; Huang, J.; Liu, Y.; Zhao, A. Different adaptive patterns of wheat with different drought tolerance under drought stresses and rehydration revealed by integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2022, 13, 1008624. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.N.; Oba, S.; Ercisli, S.; Marc, R.A.; Golokhvast, K.S. Salinity Stress Ameliorates Pigments, Minerals, Polyphenolic Profiles, and Antiradical Capacity in Lalshak. Antioxidants 2023, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Q.; Li, P.H.; Ma, S.S.; Rupassara, S.I.; Bohnert, H.J. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 2005, 44, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.; Behnke, K.; Schnitzler, J.-P.; Kanawati, B.; Schmitt-Kopplin, P.; Polle, A. Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol. 2010, 10, 150. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Dai, S.; Ding, H.; Wang, Q.; Li, X.; Ding, G.; Wang, P.; Guan, Y.; Liu, W. Integrative analyses of transcriptomics and metabolomics upon seed germination of foxtail millet in response to salinity. Sci. Rep. 2020, 10, 13660. [Google Scholar] [CrossRef]
- Minh, L.T.; Khang, D.T.; Ha, P.T.T.; Tuyen, P.T.; Xuan, T.D. Effects of Salinity Stress on Growth and Phenolics of Rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Wang, F.; Ren, G.; Li, F.; Qi, S.; Xu, Y.; Wang, B.; Yang, Y.; Ye, Y.; Zhou, Q.; Chen, X. A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol. Plant. 2018, 40, 97. [Google Scholar] [CrossRef]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef]
- Li, J.-X.; Li, R.-Z.; Sun, A.; Zhou, H.; Neher, E.; Yang, J.-S.; Huang, J.-M.; Zhang, Y.-Z.; Jiang, Z.-B.; Liang, T.-L.; et al. Metabolomics and integrated network pharmacology analysis reveal Tricin as the active anti-cancer component of Weijing decoction by suppression of PRKCA and sphingolipid signaling. Pharmacol. Res. 2021, 171, 105574. [Google Scholar] [CrossRef] [PubMed]
- Tounekti, T.; Vadel, A.M.; Ennajeh, M.; Khemira, H.; Munne-Bosch, S. Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). J. Plant Nutr. Soil Sci. 2011, 174, 504–514. [Google Scholar] [CrossRef]
- Yin, B.; Jia, J.; Sun, X.; Hu, X.; Ao, M.; Liu, W.; Tian, Z.; Liu, H.; Li, D.; Tian, W.; et al. Dynamic metabolite QTL analyses provide novel biochemical insights into kernel development and nutritional quality improvement in common wheat. Plant Commun. 2024, 5, 100792. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparative Analysis of Phenolic Compound Characterization and Their Biosynthesis Genes between Two Diverse Bread Wheat (Triticum aestivum) Varieties Differing for Chapatti (Unleavened Flat Bread) Quality. Front. Plant Sci. 2016, 7, 1870. [Google Scholar] [CrossRef]
- Li, Z.; Kekeli, M.A.; Jiang, Y.; Rui, Y. Progress and Prospect of Saline-Alkaline Soil Management Technology: A Review. Appl. Sci. 2025, 15, 4567. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Shafi, M.; Bakht, J.; Hassan, M.J.; Raziuddin, M.; Zhang, G. Effect of Cadmium and Salinity Stresses on Growth and Antioxidant Enzyme Activities of Wheat (Triticum aestivum L.). Bull. Environ. Contam. Toxicol. 2009, 82, 772–776. [Google Scholar] [CrossRef]
- Steinbrenner, A.D.; Gomez, S.; Osorio, S.; Fernie, A.R.; Orians, C.M. Herbivore-Induced Changes in Tomato (Solanum lycopersicum) Primary Metabolism: A Whole Plant Perspective. J. Chem. Ecol. 2011, 37, 1294–1303. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Environ. 2016, 39, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zheng, W.; Zhang, Y.; Gao, B.; Yu, L. Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach. Foods 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Islam, M.; Begum, M.C.; Kabir, A.H.; Alam, M.F. Molecular and biochemical mechanisms associated with differential responses to drought tolerance in wheat (Triticum aestivum L.). J. Plant Interact. 2015, 10, 195–201. [Google Scholar] [CrossRef]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef]
- Saeed, M.; Quraishi, U.M.; Mustafa, G.; Farooqi, A.; Greger, M.; Malik, R.N. Metabolomics profiling reveals the detoxification and tolerance behavior of two bread wheat (Triticum aestivum L.) varieties under arsenate stress. Food Chem. 2024, 443, 138612. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biololy 2020, 9, 367. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Qi, Z.; Yang, C.; Ding, D.; Xiao, B.; Wang, S.; Yang, C. Regulation of Root Exudation in Wheat Plants in Response to Alkali Stress. Plants 2024, 13, 1227. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Fu, J.; Zhou, J.; Wu, X. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 2015, 388, 99–117. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Feng, S.; Yao, Y.-T.; Wang, B.-B.; Li, Y.-M.; Li, L.; Bao, A.-K. Flavonoids are involved in salt tolerance through ROS scavenging in the halophyte Atriplex canescens. Plant Cell Rep. 2024, 43, 5. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Yang, S.-H.; Liao, P.-H.; Pan, Y.-F.; Chen, S.-L.; Chou, S.-S.; Chou, M.-Y. The Novel p53-Dependent Metastatic and Apoptotic Pathway Induced by Vitexin in Human Oral Cancer OC2 Cells. Phytother. Res. 2013, 27, 1154–1161. [Google Scholar] [CrossRef]
- Can, O.D.; Ozkay, U.D.; Ucel, U.I. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
| Planting Site | Altitudes [m] | Precipitation [mm] | Soil Horizon [cm] | pH (Mean ± s.d.) | Total Salt Content (Mean ± s.d.) [g/kg] |
|---|---|---|---|---|---|
| Xian Huanyuan village | 8 | 735.1 | 0–60 | 7.77 ± 0.20 | 1.37 ± 0.21 |
| Zhong Jie industrial park | 2.5 | 689.4 | 0–60 | 8.26 ± 0.32 | 2.70 ± 1.86 |
| Kernel | Compounds | CAS | Class I | Class II | Type |
|---|---|---|---|---|---|
| 1 | 2-Hydroxy-7-methoxy-1,4-benzoxazin-3(2H)-one glucoside (HMBOA glucoside) | 17622-26-3 | Alkaloids | Alkaloids | down |
| 2 | 3-quinolinecarboxylic acid | - | Alkaloids | Quinoline alkaloids | up |
| 3 | 5-Hydroxy-2-pyrrolidinone | 62312-55-4 | Alkaloids | Pyrrole alkaloids | up |
| 4 | N-Acetylisatin | 574-17-4 | Alkaloids | Plumerane | up |
| 5 | N-Feruloylagmatine | - | Alkaloids | Phenolamine | up |
| 6 | N-p-Coumaroyl hydroxydehydroagmatine | - | Alkaloids | Phenolamine | down |
| 7 | Quinoline-4-carboxylic acid | 486-74-8 | Alkaloids | Quinoline alkaloids | up |
| 8 | p-Coumaroylagmatine | 7295-86-5 | Alkaloids | Phenolamine | up |
| 9 | p-Coumaroylcadaverine | - | Alkaloids | Phenolamine | up |
| 10 | p-Coumaroylputrescine | 34136-53-3 | Alkaloids | Phenolamine | up |
| 11 | Cyclo(Phe-Glu) | - | Amino acids and derivatives | Amino acids and derivatives | up |
| 12 | Glu-Phe | 20556-22-3 | Amino acids and derivatives | Amino acids and derivatives | up |
| 13 | L-Alanyl-L-Phenylalanine | 3061-90-3 | Amino acids and derivatives | Amino acids and derivatives | up |
| 14 | N-Acetyl-L-Methionine | 65-82-7 | Amino acids and derivatives | Amino acids and derivatives | up |
| 15 | N-Acetyl-L-tyrosine | 537-55-3 | Amino acids and derivatives | Amino acids and derivatives | up |
| 16 | Acacetin-7-O-neohesperidoside | 20633-93-6 | Flavonoids | Flavones | up |
| 17 | Acacetin-7-O-rutinoside (Linarin) | 480-36-4 | Flavonoids | Flavones | up |
| 18 | Apigenin-8-C-Glucoside (Vitexin) | 3681-93-4 | Flavonoids | Flavones | up |
| 19 | Cirsimaritin-8-C-[Xylosyl-(1-2)]-glucoside | - | Flavonoids | Flavones | up |
| 20 | Quercetin-3-O-galactoside (Hyperin) | 482-36-0 | Flavonoids | Flavonols | up |
| 21 | Vitexin-2″-O-glucoside | 61360-94-9 | Flavonoids | Flavones | up |
| 22 | Eleutheroside E | 39432-56-9 | Lignans and Coumarins | Lignans | up |
| 23 | Pinoresinol-4-O-glucoside | 41607-20-9 | Lignans and Coumarins | Lignans | up |
| 24 | 1-Arabinosyluracil | 3083-77-0 | Nucleotides and derivatives | Nucleotides and derivatives | up |
| 25 | 2′-O-Methyladenosine | 2140-79-6 | Nucleotides and derivatives | Nucleotides and derivatives | up |
| 26 | Guanosine 3′,5′-cyclic monophosphate | 7665-99-8 | Nucleotides and derivatives | Nucleotides and derivatives | up |
| 27 | Uridine | 58-96-8 | Nucleotides and derivatives | Nucleotides and derivatives | up |
| 28 | Delta-Hexalactone | 823-22-3 | Others | Lactones | up |
| 29 | 1,2,2′-Trisinapoyl gentiobiose | - | Phenolic acids | Phenolic acids | down |
| 30 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | 905-99-7 | Phenolic acids | Phenolic acids | down |
| 31 | Disinapoyl glucoside | - | Phenolic acids | Phenolic acids | down |
| 32 | Gentisic acid-[6″-(3-hydroxy-3-methylglutaryl)]-glucoside | - | Phenolic acids | Phenolic acids | up |
| 33 | Methyl gallate | 99-24-1 | Phenolic acids | Phenolic acids | down |
| 34 | 6′-O-Sinapoylgeniposide | 1012306-66-9 | Terpenoids | Monoterpenoids | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; Liu, Y.; Li, M.; Chang, L.; Guo, B. Analysis and Excavation of Unique Metabolic Components of Wheat Cultivated in Saline–Alkaline Soil. Foods 2025, 14, 3888. https://doi.org/10.3390/foods14223888
Song Q, Liu Y, Li M, Chang L, Guo B. Analysis and Excavation of Unique Metabolic Components of Wheat Cultivated in Saline–Alkaline Soil. Foods. 2025; 14(22):3888. https://doi.org/10.3390/foods14223888
Chicago/Turabian StyleSong, Qiaozhi, Yu Liu, Ming Li, Lei Chang, and Boli Guo. 2025. "Analysis and Excavation of Unique Metabolic Components of Wheat Cultivated in Saline–Alkaline Soil" Foods 14, no. 22: 3888. https://doi.org/10.3390/foods14223888
APA StyleSong, Q., Liu, Y., Li, M., Chang, L., & Guo, B. (2025). Analysis and Excavation of Unique Metabolic Components of Wheat Cultivated in Saline–Alkaline Soil. Foods, 14(22), 3888. https://doi.org/10.3390/foods14223888






