Age-Related Outcomes of [177Lu]Lu-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Retrospective Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcomes Measured
2.3. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Prior Therapies
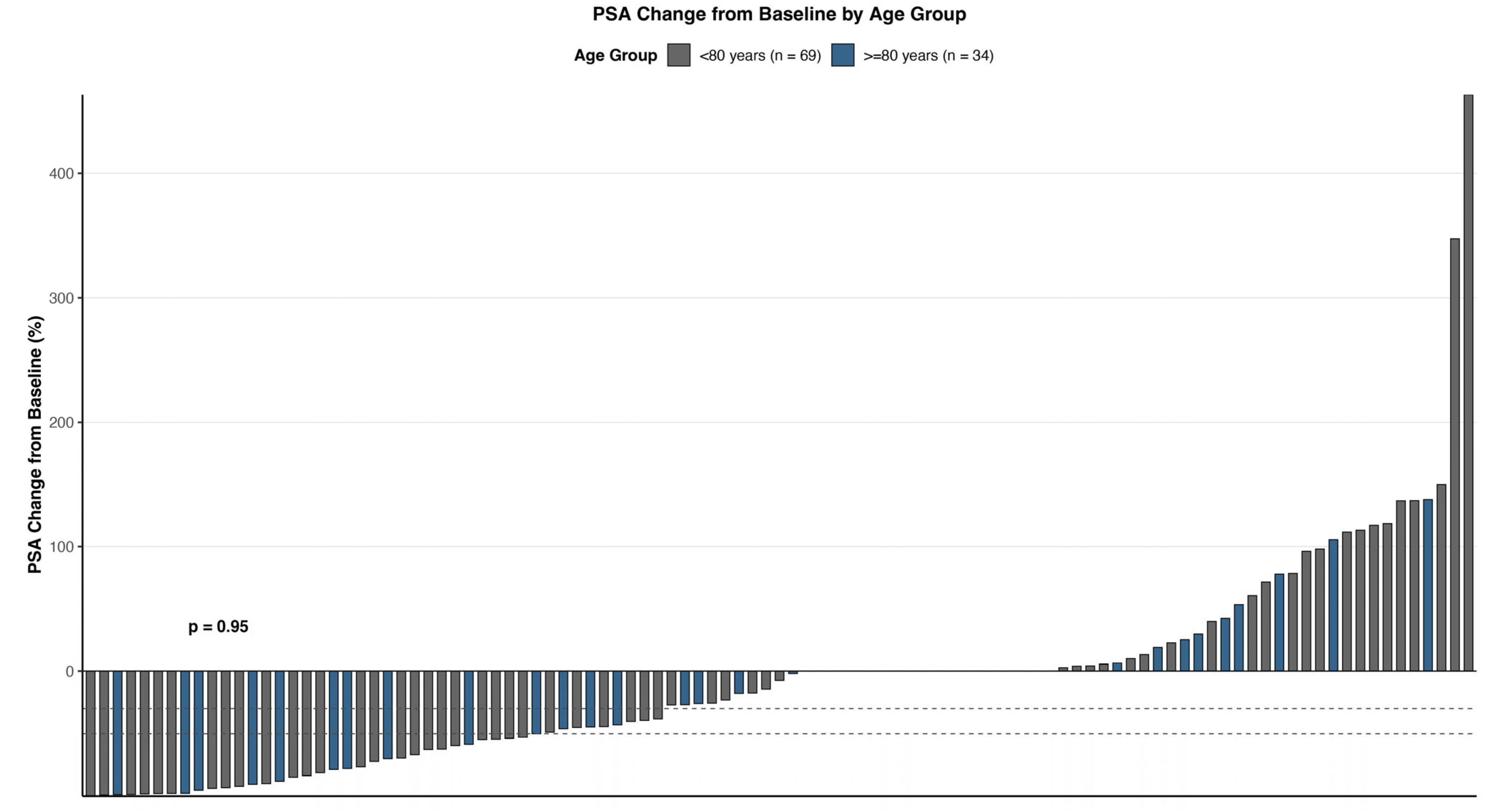
3.3. PSA Response
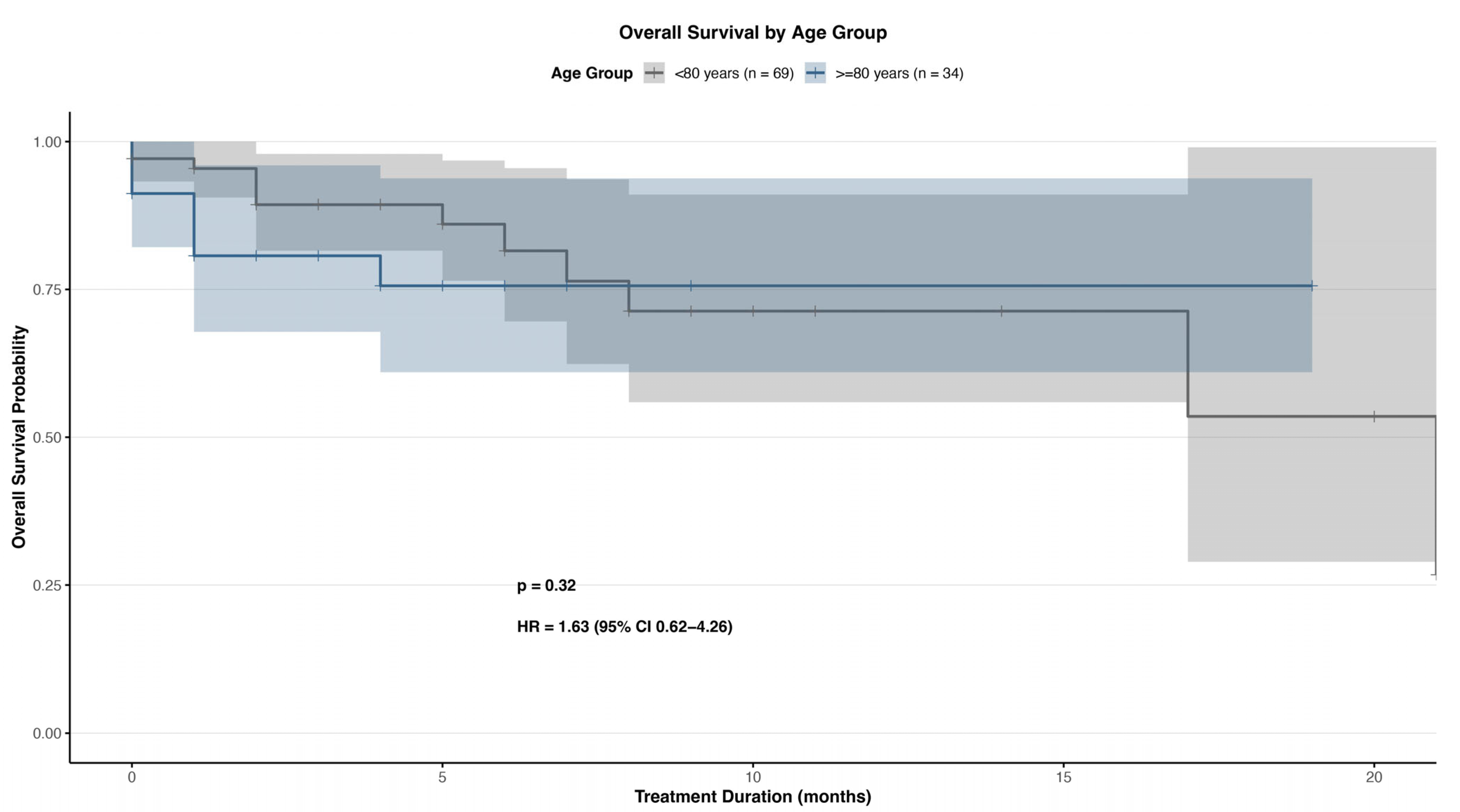
3.4. Survival Outcomes
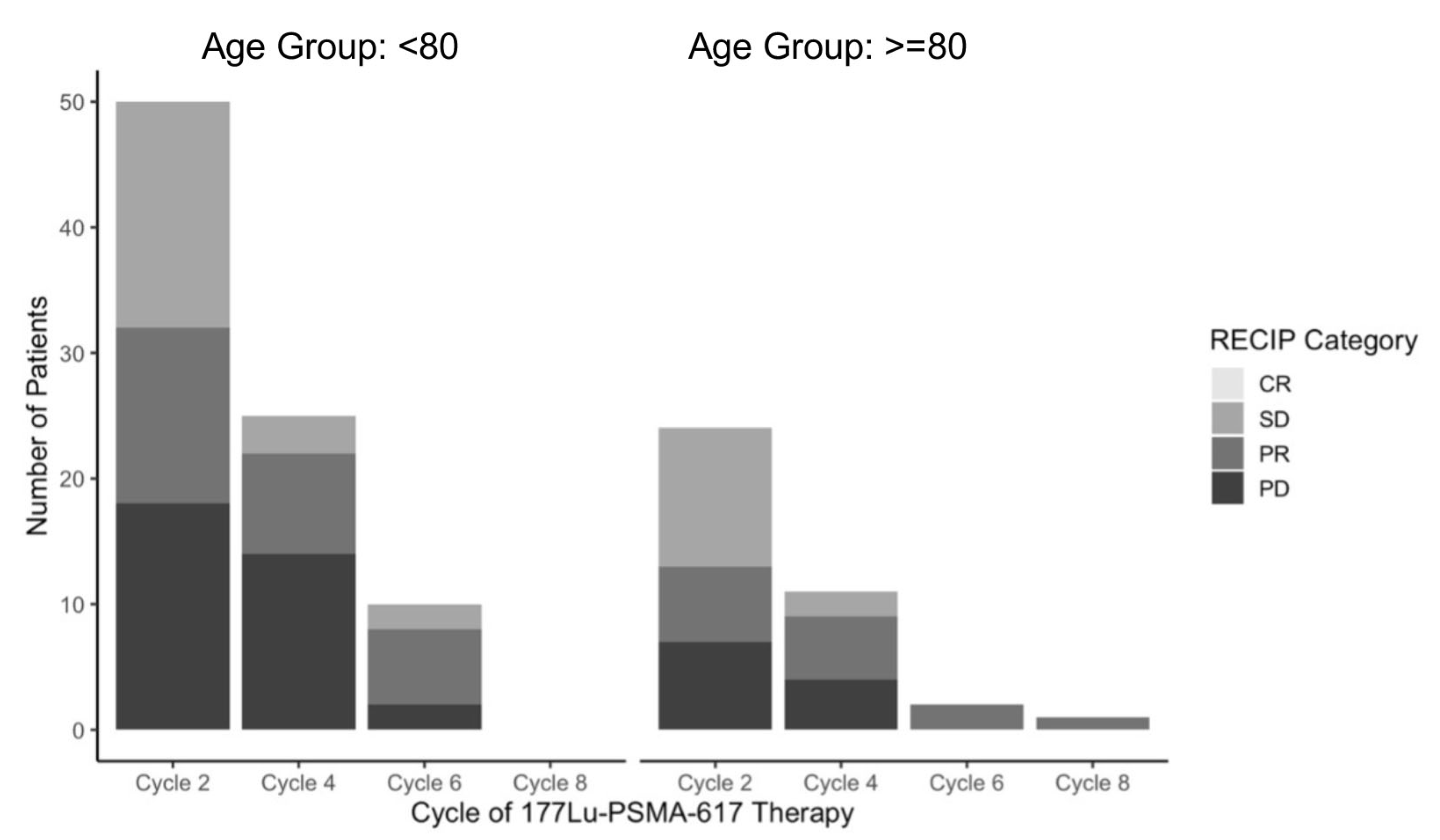
3.5. RECIP Imaging Analysis
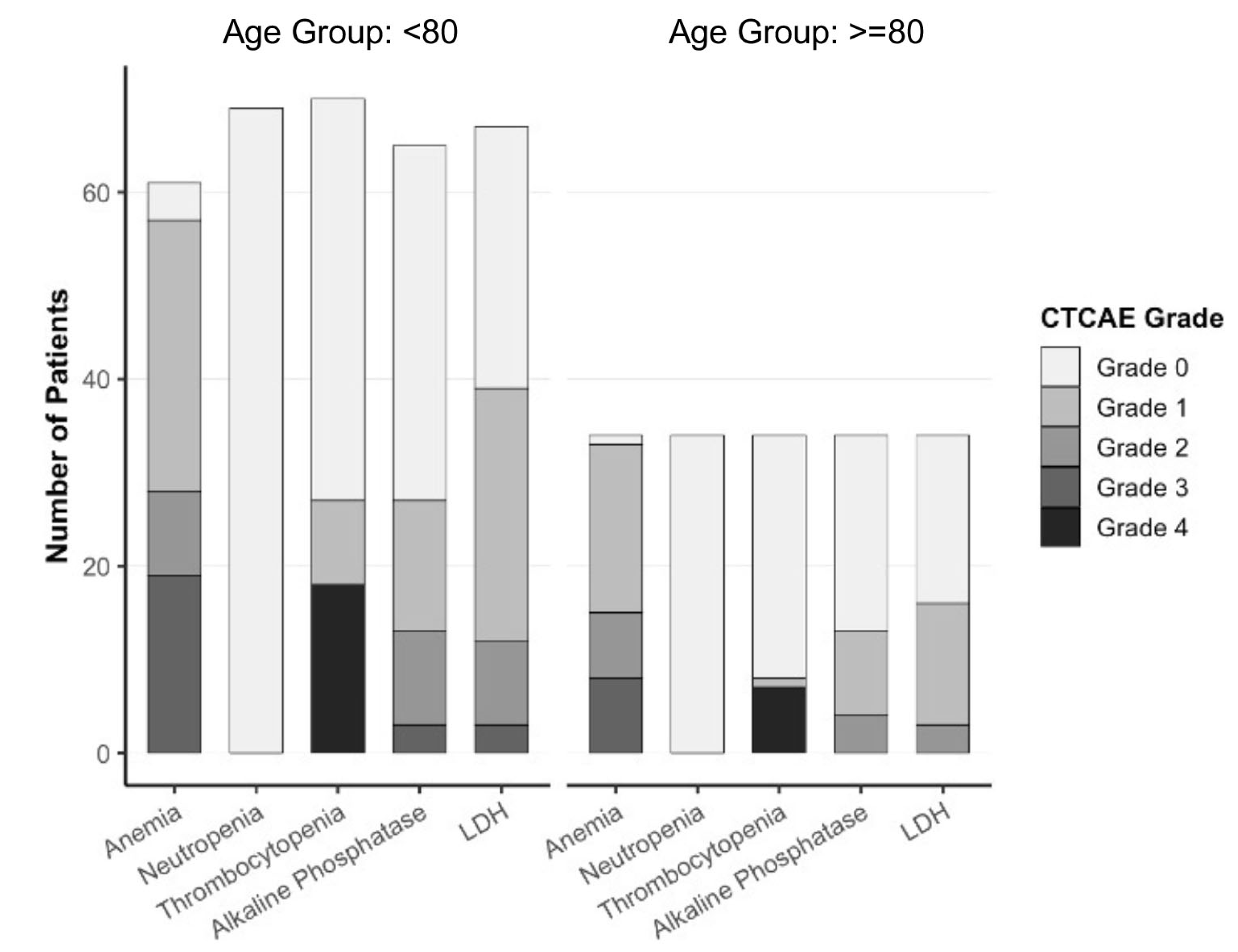
3.6. Toxicity Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [177Lu]Lu-PSMA-617 | Lutetium-177–Prostate-Specific Membrane Antigen 617 |
| [177Lu]Lu-PSMA-I&T | Lutetium-177–Prostate-Specific Membrane Antigen I&T |
| ABI | Abiraterone acetate |
| ADT | Androgen deprivation therapy |
| ALT | Alanine aminotransferase |
| AP | Alkaline phosphatase |
| ARPI | Androgen receptor pathway inhibitor |
| AS | Aspartate aminotransferase |
| CABA | Cabazitaxel |
| CN | Central nervous system |
| CR | Complete response (per RECIP 1.0 criteria) |
| CTX | Chemotherapy |
| CTCAE | Common Terminology Criteria for Adverse Events (version 5.0) |
| DOCE | Docetaxel |
| ECOG | Eastern Cooperative Oncology Group performance status |
| ENZ | Enzalutamide |
| FD | Fluorodeoxyglucose |
| GBq | Gigabecquerel |
| LD | Lactate dehydrogenase |
| LN | Lymph node |
| mCRPC | Metastatic castration-resistant prostate cancer |
| OS | Overall survival |
| PCa | Prostate cancer |
| PCWG3 | Prostate Cancer Clinical Trials Working Group 3 |
| PD | Progressive disease (per RECIP 1.0 criteria) |
| PET/CT | Positron emission tomography/Computed tomography |
| PR | Partial response (per RECIP 1.0 criteria) |
| PSA | Prostate-specific antigen |
| PSA30 | Reduction in PSA of ≥30% from baseline |
| PSA50 | Reduction in PSA of ≥50% from baseline |
| PSA90 | Reduction in PSA of ≥90% from baseline |
| PSMA | Prostate-specific membrane antigen |
| Ra223 | Radium-223 dichloride |
| rPFS | Radiographic Progression-Free Survival |
| RECIP 1.0 | Response Evaluation Criteria in PSMA Imaging in Prostate Cancer (version 1.0) |
| RLT | Radioligand therapy |
| RTX | Radiotherapy |
| SD | Stable disease (per RECIP 1.0 criteria) |
| ULN | Upper limit of normal |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Henriques, V.; Wenzel, M.; Demes, M.-C.; Köllermann, J. Das metastasierte kastrationsresistente Prostatakarzinom. Pathologe 2021, 42, 431–441. [Google Scholar] [CrossRef]
- He, M.X.; Cuoco, M.S.; Crowdis, J.; Bosma-Moody, A.; Zhang, Z.; Bi, K.; Kanodia, A.; Su, M.-J.; Ku, S.-Y.; Garcia, M.M.; et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat. Med. 2021, 27, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Palihati, M.; Das, J.P.; Yeh, R.; Capaccione, K. Emerging PET Imaging Agents and Targeted Radioligand Therapy: A Review of Clinical Applications and Trials. Tomography 2025, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Retz, M.; D’Alessandria, C.; Rauscher, I.; Scheidhauer, K.; Maurer, T.; Storz, E.; Janssen, F.; Schottelius, M.; Wester, H.-J.; et al. Systemic Radioligand Therapy with 177 Lu Labeled Prostate Specific Membrane Antigen Ligand for Imaging and Therapy in Patients with Metastatic Castration Resistant Prostate Cancer. J. Urol. 2016, 196, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, E.A.M.; van Vliet, N.; Dalm, S.U.; de Blois, E.; van Gent, D.C.; Haeck, J.; de Ridder, C.; Stuurman, D.; Konijnenberg, M.W.; van Weerden, W.M.; et al. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur. J. Nucl. Med. 2020, 48, 1339–1350. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Eppard, E.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gärtner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488. [Google Scholar] [CrossRef]
- Topal, E.; Kovan, B.; IrIbas, A.; Kuyumcu, S.; Basaran, M.; Demirtaş, A.M.; Sanli, O.; Sanli, Y. Impact of extended [177Lu] Lu-PSMA-617 therapy on absorbed kidney dose and CKD-EPI values: How long can therapy be safely continued? Eur. J. Nucl. Med. 2025, 52, 3135–3144. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Morris, M.J.; Castellano, D.; Herrmann, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Lehnert, W.; Koehler, D.; Shenas, F.; Kisters, A.; Apostolova, I.; Klutmann, S.; Adam, G.; Sauer, M. Overview of selected completed prospective studies on PSMA-targeted radioligand therapy with [177Lu]Lu-PSMA-617 in metastatic castration-resistant prostate cancer. Rofo Fortschritte Geb. Rontgenstrahlen Bild. Verfahr. 2025, 197, 1033–1042. [Google Scholar] [CrossRef]
- Ling, S.W.; Sablonière, Q.d.L.d.l.; Ananta, M.; de Blois, E.; Koolen, S.L.W.; Drexhage, R.C.; Hofland, J.; Robbrecht, D.G.J.; Veldt, A.A.M.v.d.; Verburg, F.A.; et al. First real-world clinical experience with [177Lu]Lu-PSMA-I&T in patients with metastatic castration-resistant prostate cancer beyond VISION and TheraP criteria. Eur. J. Nucl. Med. 2025, 52, 2034–2040. [Google Scholar] [CrossRef]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lützen, U.; Prasad, V.; Heinzel, A.; et al. [177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer]. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Gafita, A.; Djaileb, L.; Rauscher, I.; Fendler, W.P.; Hadaschik, B.; Rowe, S.P.; Herrmann, K.; Calais, J.; Rettig, M.; Eiber, M.; et al. Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) in Metastatic Castration-resistant Prostate Cancer. Radiology 2023, 308, e222148. [Google Scholar] [CrossRef]
- von Amsberg, G.; Busenbender, T.; Coym, A.; Kaune, M.; Strewinsky, N.; Ekrutt, J.; Tilki, D.; Dyshlovoy, S. Management of Metastatic Prostate Cancer. Oncol. Res. Treat. 2025, 1–22. [Google Scholar] [CrossRef]
- Bent, E.H.; Morris, M.J. Prostate Cancer Radioligand Therapy: PSMA and Beyond, Current Landscape and Future Directions. Curr. Oncol. Rep. 2025, 27, 1170–1184. [Google Scholar] [CrossRef]
- Jalloul, W.; Ghizdovat, V.; Saviuc, A.; Jalloul, D.; Grierosu, I.C.; Stefanescu, C. Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management. Pharmaceuticals 2025, 18, 1215. [Google Scholar] [CrossRef]
- Schwinger, M.; Kalogirou, C.; Scheper, V.; Däuwel, M.; Weber, S.; Seitz, A.K.; Kübler, H.; Buck, A.K.; Werner, R.A.; Hartrampf, P.E. Radioligand treatment with [177Lu]Lu-PSMA I&T in elderly Patients—Safety, efficacy, and prognostic factors for survival. Eur. J. Nucl. Med. 2025, 1–10. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2023, 25, 99–107. [Google Scholar] [CrossRef]
- Tauber, R.; Knorr, K.; Retz, M.; Rauscher, I.; Grigorascu, S.; Hansen, K.; D’aLessandria, C.; Wester, H.-J.; Gschwend, J.; Weber, W.; et al. Safety and Efficacy of [177Lu]-PSMA-I&T Radioligand Therapy in Octogenarians with Metastatic Castration-Resistant Prostate Cancer: Report on 80 Patients over the Age of 80 Years. J. Nucl. Med. 2023, 64, 1244–1251. [Google Scholar] [CrossRef]
- Bastian, M.B.; Wörl, B.; Blickle, A.M.; Burgard, C.; Speicher, T.; Wessendorf, J.; Bartholomä, M.; Schaefer-Schuler, A.; Maus, S.; Ezziddin, S.; et al. PSMA Radioligand Therapy in Advanced Age. Clin. Nucl. Med. 2025. [Google Scholar] [CrossRef]
- Sahin, E.; Elboga, U.; Cimen, U.; Okuyan, M.; Cayirli, Y.B. 177Lu-PSMA-617 Radioligand Treatment in Elderly Patients with Metastatic Castration-resistant Prostate Cancer: Therapeutic Efficacy and Safety Assessment. Curr. Radiopharm. 2024, 17, 356–363. [Google Scholar] [CrossRef]
| All Patients (n = 103) | <80 Years (n = 69) | ≥80 Years (n = 34) | |
|---|---|---|---|
| Median (Range)/n (%) | Median (Range)/n (%) | Median (Range)/n (%) | |
| Age | 75 (54–88) | 71 (54–79) | 82 (80–88) |
| ECOG Performance Status Score of 0 or 1–Nr. (%) | 93 (90.29%) | 63 (91.30%) | 30 (88.24%) |
| Sites of Metastasis—n (%) | |||
| Primary Metastasis | 96 (93.20%) | 65 (94.20%) | 31 (91.18%) |
| Pelvic LN | 31 (30.10%) | 21 (30.43%) | 10 (29.41%) |
| Extrapelvic LN | 53 (51.46%) | 36 (52.17%) | 17 (50.00%) |
| LN Only | 2 (1.94%) | 1 (1.45%) | 1 (2.94%) |
| Bone | 97 (94.17%) | 64 (92.75%) | 33 (97.06%) |
| Bone + LN | 62 (60.19%) | 38 (55.07%) | 24 (70.59%) |
| Bone Only | 32 (31.07%) | 25 (36.23%) | 7 (20.59%) |
| Visceral | 12 (11.65%) | 6 (8.70%) | 6 (17.65%) |
| Lung | 11 (10.68%) | 6 (8.70%) | 5 (14.71%) |
| Liver | 3 (2.91%) | 0 (0.00%) | 3 (8.82%) |
| Adrenal | 4 (3.88%) | 3 (4.35%) | 1 (2.94%) |
| CNS | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Visceral Organs Only | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| PSA Level ng/mL (Range) | 266.67 (0.02–2782.43) | 201.09 (0.25–2484.05) | 137.27 (0.02–2782.43) |
| Alkaline Phosphatase IU/Liter (Range) | 178.36 (43.00–1422.00) | 200.77 (44.00–1422.00) | 132.88 (43.00–561.00) |
| LDH IU/Liter (Range) | 314.17 (14.00–1974.00) | 334.70 (14.00–1974.00) | 272.53 (161.00–537.00) |
| Gleason Score—n (%) | |||
| 7 | 31 (30.10%) | 23 (33.33%) | 10 (29.41%) |
| 8–10 | 48 (46.60%) | 35 (50.72%) | 13 (38.24%) |
| Unknown | 21 (20.39%) | 10 (14.49%) | 11 (32.35%) |
| Prior Therapies—n (%) | 398 | 276 | 122 |
| ADT | 100 (97.09%) | 67 (97.10%) | 33 (97.06%) |
| ABI | 58 (56.31%) | 36 (52.17%) | 22 (64.71%) |
| ENZ | 40 (38.83%) | 22 (31.88%) | 18 (52.94%) |
| ABI + ENZ | 25 (24.27%) | 12 (17.39%) | 13 (38.24%) |
| DOCE | 73 (70.87%) | 54 (78.26%) | 19 (55.88%) |
| CABA | 21 (20.39%) | 14 (20.29%) | 7 (20.59%) |
| DOCE + CABA | 21 (20.39%) | 14 (20.29%) | 7 (20.59%) |
| CTX | 74 (71.84%) | 55 (79.91%) | 19 (55.88%) |
| RTX | 30 (29.13%) | 24 (34.78%) | 6 (17.65%) |
| Ra223 | 3 (2.91%) | 1 (1.45%) | 2 (5.88%) |
| Prior Therapies > 3 | 72 (69.90%) | 56 (84.06%) | 23 (67.65%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweigert, N.; Strewinsky, N.; Köhler, D.; Lehnert, W.; Ekrutt, J.; Karimzadeh, A.; Klutmann, S.; von Amsberg, G.; Sauer, M. Age-Related Outcomes of [177Lu]Lu-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Retrospective Analysis. Cancers 2025, 17, 3515. https://doi.org/10.3390/cancers17213515
Schweigert N, Strewinsky N, Köhler D, Lehnert W, Ekrutt J, Karimzadeh A, Klutmann S, von Amsberg G, Sauer M. Age-Related Outcomes of [177Lu]Lu-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Retrospective Analysis. Cancers. 2025; 17(21):3515. https://doi.org/10.3390/cancers17213515
Chicago/Turabian StyleSchweigert, Nikolaus, Nadja Strewinsky, Daniel Köhler, Wencke Lehnert, Jonas Ekrutt, Amir Karimzadeh, Susanne Klutmann, Gunhild von Amsberg, and Markus Sauer. 2025. "Age-Related Outcomes of [177Lu]Lu-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Retrospective Analysis" Cancers 17, no. 21: 3515. https://doi.org/10.3390/cancers17213515
APA StyleSchweigert, N., Strewinsky, N., Köhler, D., Lehnert, W., Ekrutt, J., Karimzadeh, A., Klutmann, S., von Amsberg, G., & Sauer, M. (2025). Age-Related Outcomes of [177Lu]Lu-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Retrospective Analysis. Cancers, 17(21), 3515. https://doi.org/10.3390/cancers17213515





