Non-Invasive Physical Plasma as an Oncological Therapy Option: Modulation of Cancer Cell Growth, Motility, and Metabolism Without Induction of Cancer Resistance Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Non-Invasive Physical Plasma Device
2.3. NIPP Treatment
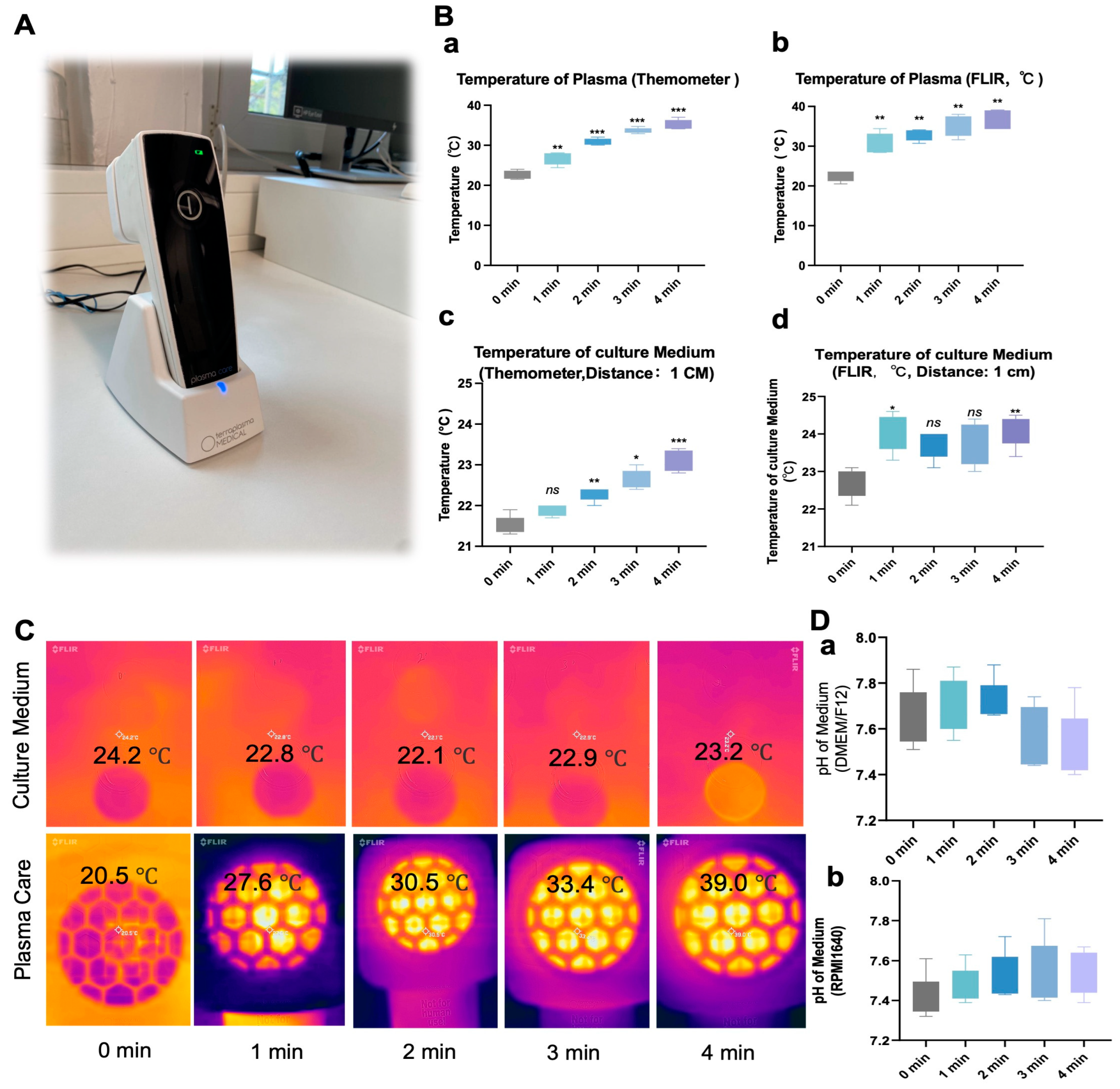
2.4. pH and Temperature Measurement in Culture Media
2.5. Viable Cell Count Detection
2.6. Wound-Healing Assay
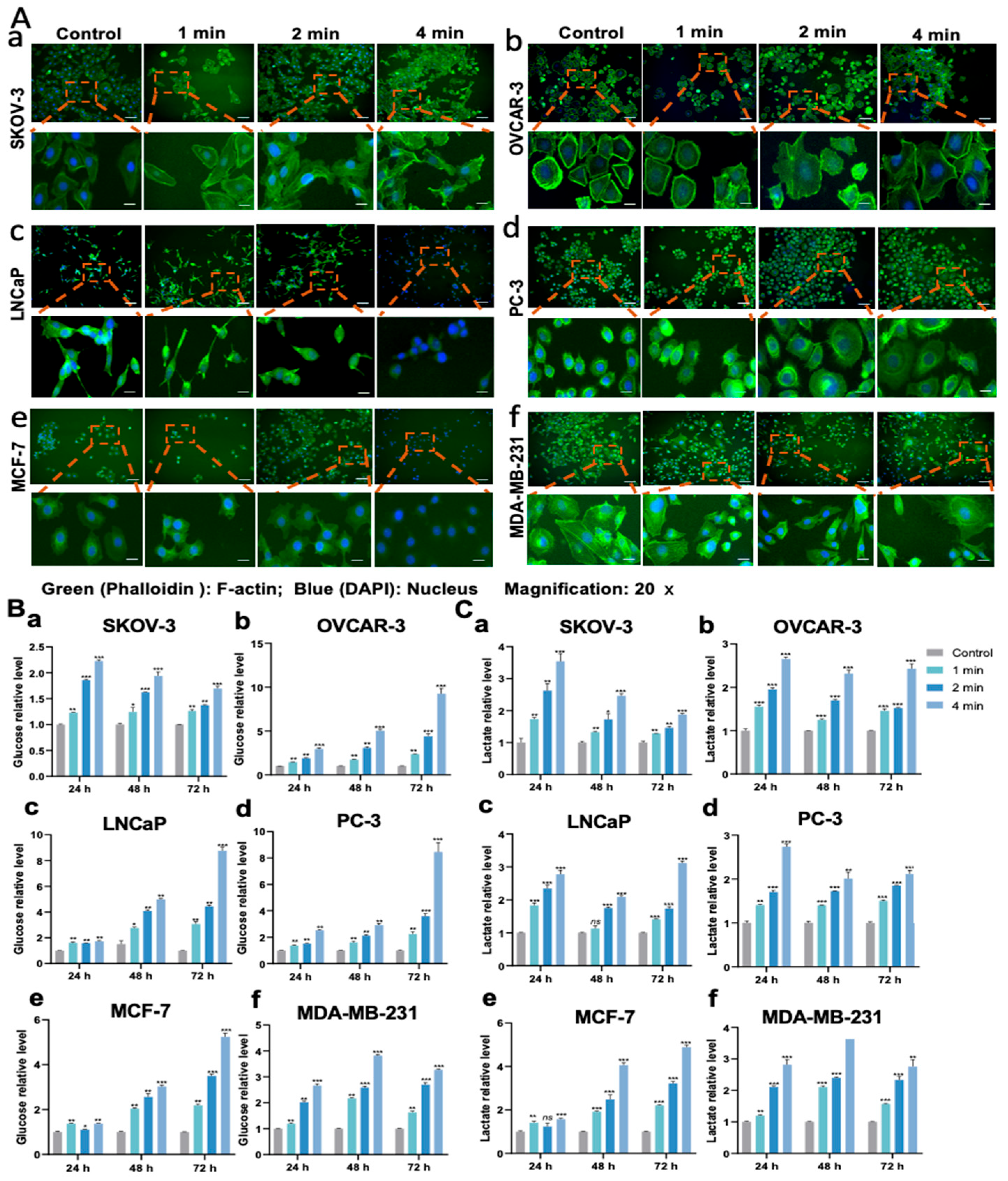
2.7. Fluorescence Microscopy
2.8. Western Blot Analysis
2.9. Evaluation of Mitochondria Membrane Potential
2.10. Evaluation of Glucose Level
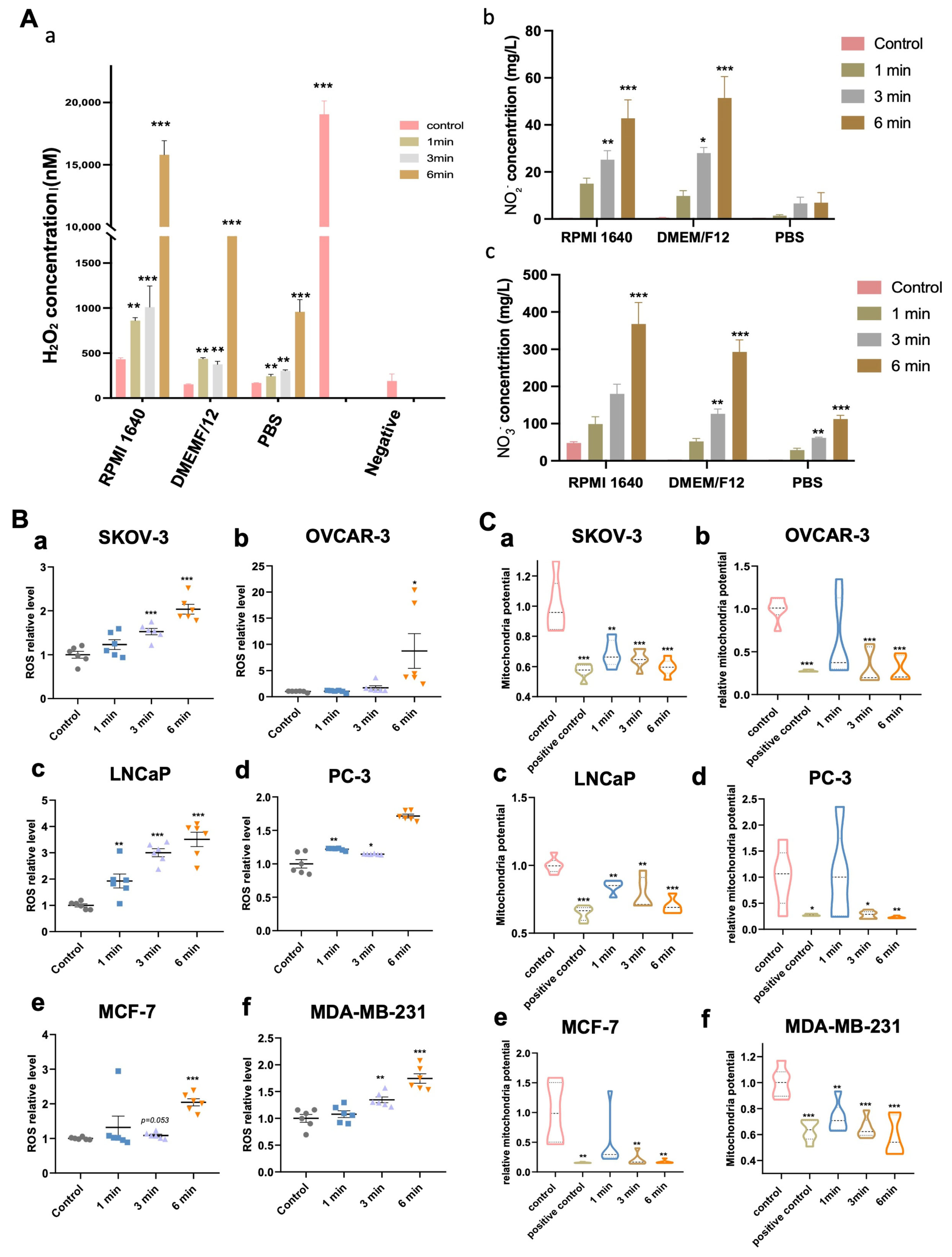
2.11. Evaluation of H2O2, NO2− and NO3− Level in Medium
2.12. Evaluation of ROS Level Intracellular
2.13. Evaluation of Lactate Level
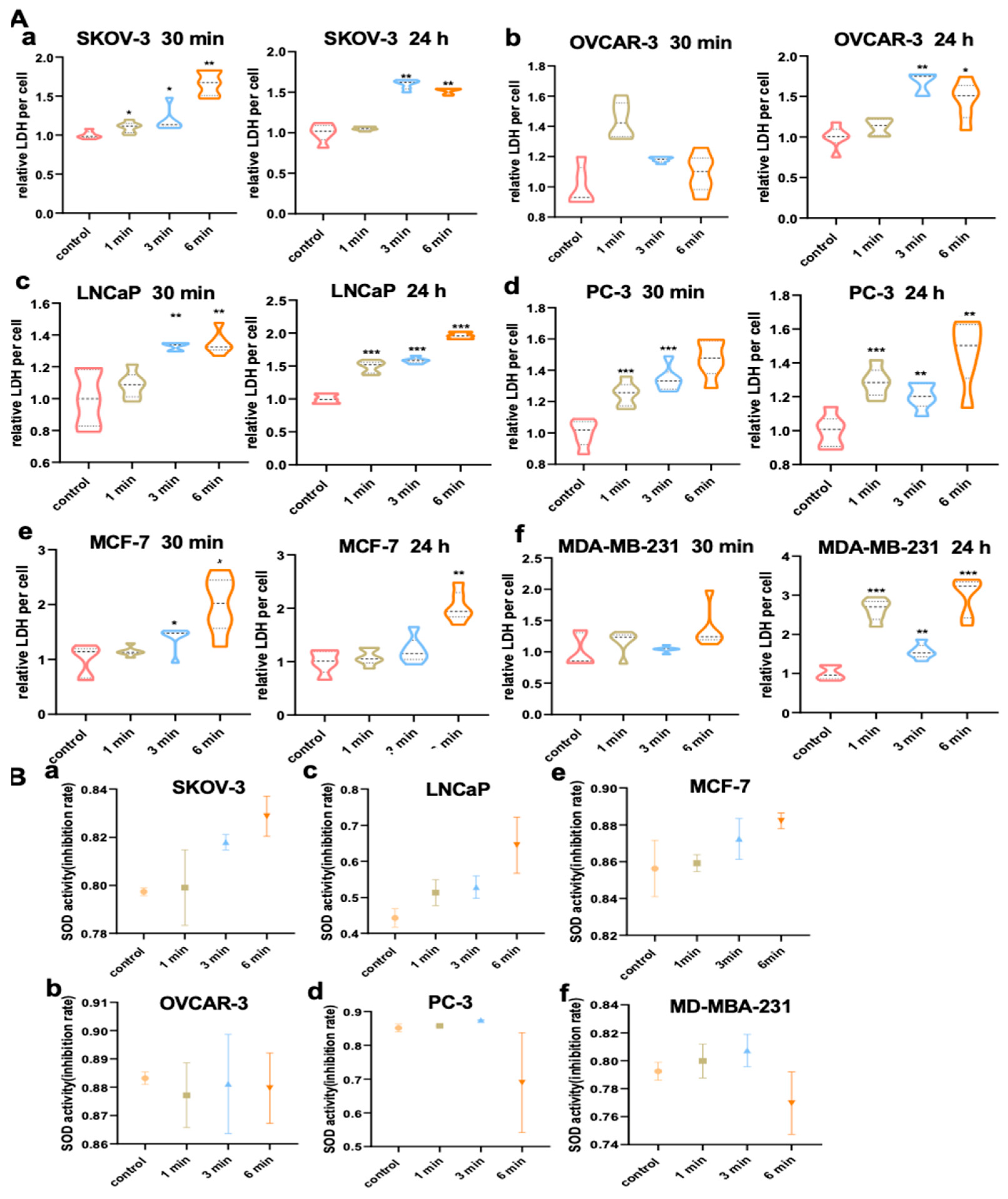
2.14. Evaluation of Intracellular Lactate Dehydrogenase (LDH) Level
2.15. Evaluation of Intracellular Superoxide Dismutase Activity
2.16. Statistical Analysis
3. Results
3.1. NIPP Did Not Significantly Alter the Culture Medium Temperature and pH
3.2. NIPP-Induced Accumulation of Hydrogen Peroxide (H2O2), Nitrite (NO2−) and Nitrate (NO3−) in the Medium
3.3. NIPP-Induced Accumulation of Intracellular ROS of Tumor Cells
3.4. NIPP-Induced Mitochondria Membrane Potential Decrease
3.5. NIPP Attenuates Tumor Cell Growth
3.6. NIPP Affects Tumor Cells’ Migration Ability and Cell Morphology
3.7. NIPP-Induced Mass Glucose in the Medium
3.8. NIPP Upregulated Lactate Levels in the Media
3.9. NIPP Elevated LDH Activity of Tumor Cells
3.10. NIPP Did Not Alter SOD Activity of Tumor Cells
3.11. NIPP Did Not Induce HSP27 Expression in Tumor Cells
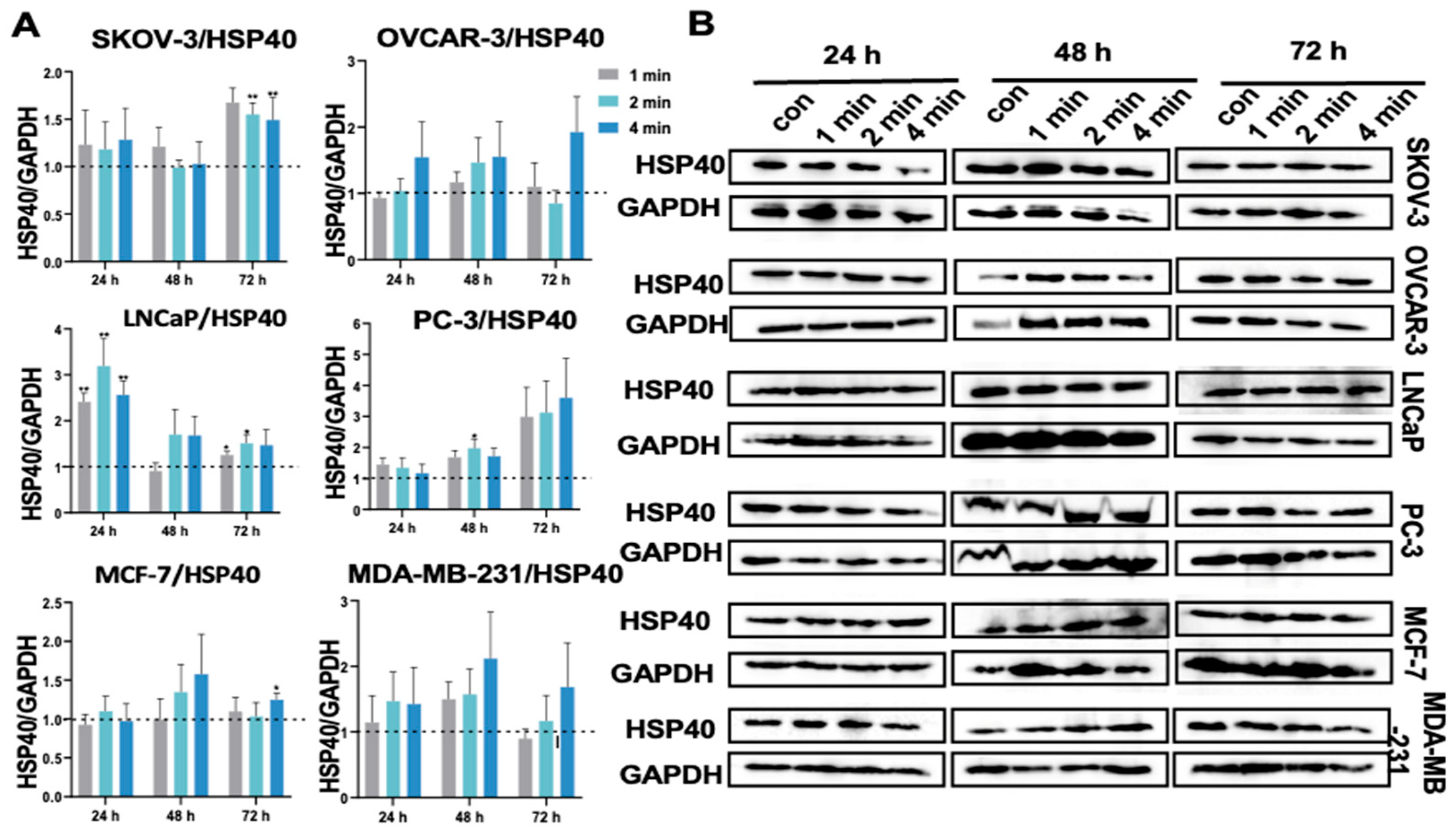
3.12. NIPP Did Not Significantly Change HSP40 Expression in Tumor Cells
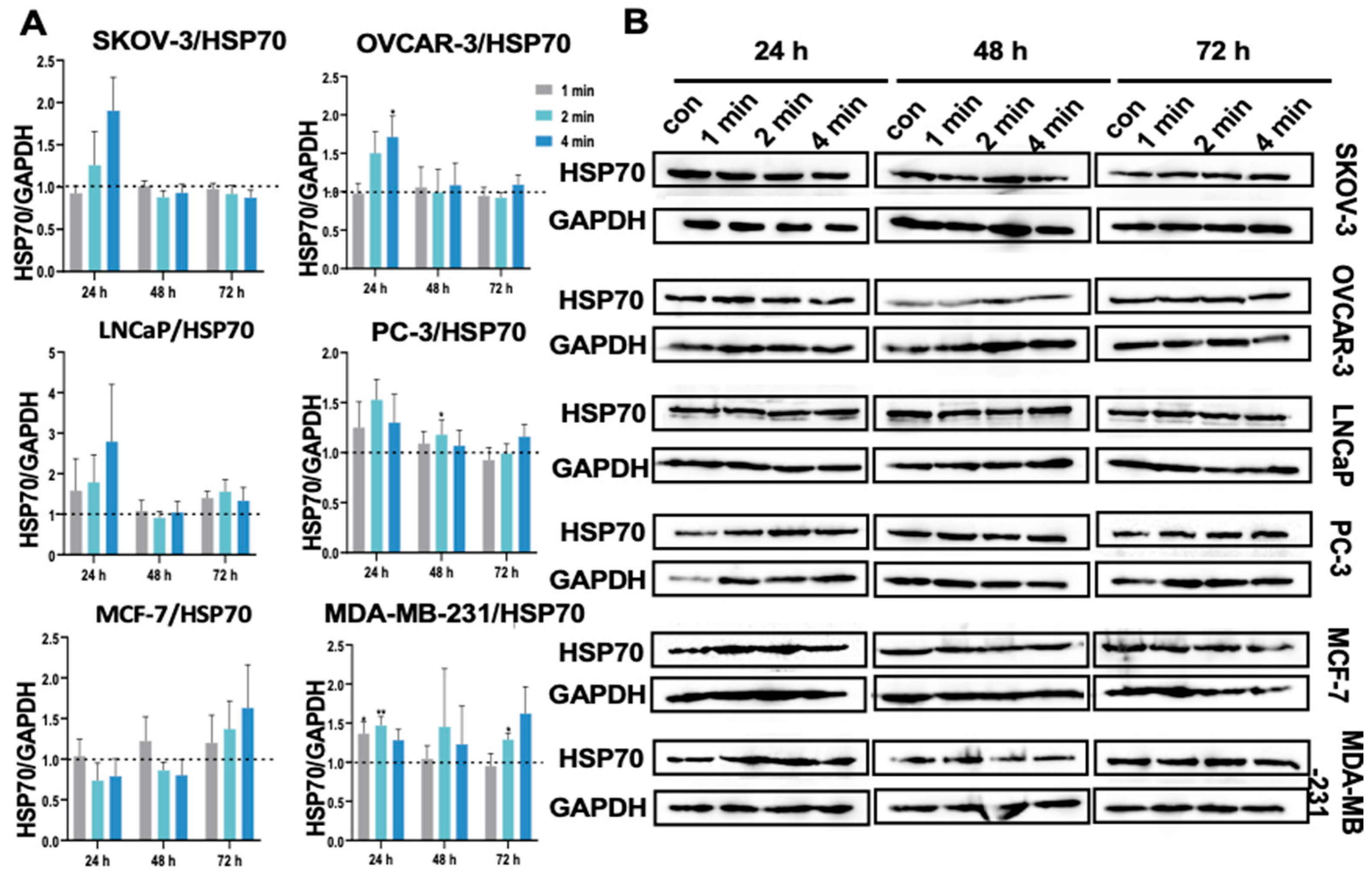
3.13. NIPP Did Not Alter HSP70 Expression in Tumor Cells
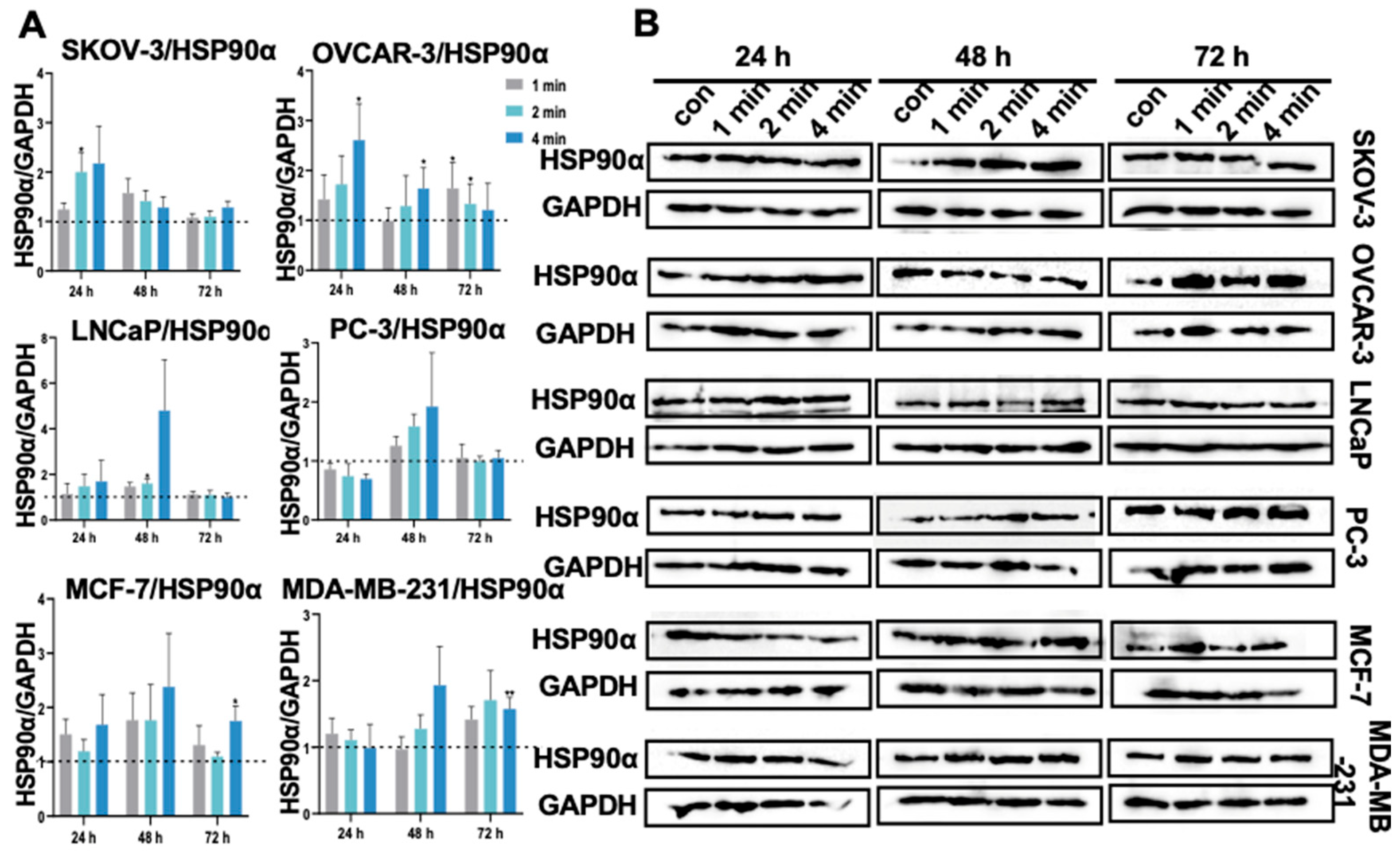
3.14. NIPP Did Not Induced HSP90α Expression in Tumor Cells
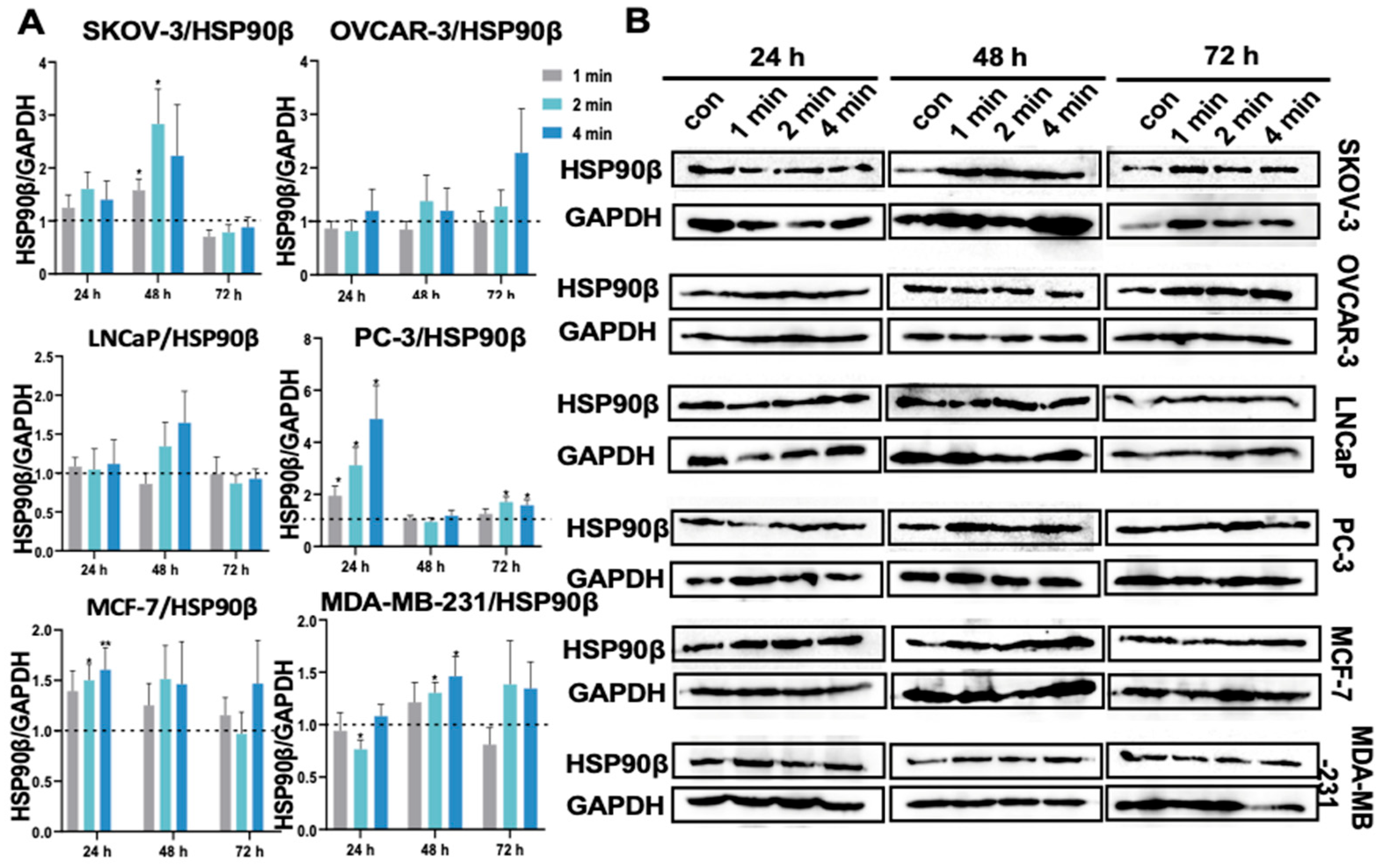
3.15. NIPP Did Not Boost HSP90β Expression in Tumor Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBD | Dielectric Barrier Discharge |
| DOX | Doxorubicin |
| GSH | Glutathione |
| GSSG | Oxidized Glutathione |
| GLUT | Glucose Transporter |
| HSP | Heat Shock Protein |
| LDH | Lactate Dehydrogenase |
| MCT | Monocarboxylate Transporters |
| MMP | Mitochondrial Membrane Potential |
| NIPP | Non-invasive Physical Plasma |
| PBS | Phosphate-Buffered Saline |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| TCA | Tricarboxylic Acid Cycle |
References
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Stope, M.B. Physical plasma: A new treatment option in gynecological oncology. Arch. Gynecol. Obstet. 2018, 298, 853–855. [Google Scholar] [CrossRef]
- Burm, K. Plasma: The fourth state of matter. Plasma Chem. Plasma Process. 2012, 32, 401–407. [Google Scholar] [CrossRef]
- Kletschkus, K.; Haralambiev, L.; Mustea, A.; Bekeschus, S.; Stope, M.B. Review of innovative physical therapy methods: Introduction to the principles of cold physical plasma. In Vivo 2020, 34, 3103–3107. [Google Scholar] [CrossRef]
- Maza, S.R.; Frishman, W.H. Therapeutic options to minimize free radical damage and thrombogenicity in ischemic/reperfused myocardium. Am. Heart J. 1987, 114, 1206–1215. [Google Scholar] [CrossRef]
- Morfill, G.E.; Kong, M.G.; Zimmermann, J.L. Focus on Plasma Medicine. New J. Phys. 2009, 11, 115011. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kramer, A.; Schmidt, A. Gas plasma-augmented wound healing in animal models and veterinary medicine. Molecules 2021, 26, 5682. [Google Scholar] [CrossRef]
- Hahn, O.; Waheed, T.O.; Sridharan, K.; Huemerlehner, T.; Staehlke, S.; Thürling, M.; Boeckmann, L.; Meister, M.; Masur, K.; Peters, K. Cold Atmospheric Pressure Plasma-Activated Medium Modulates Cellular Functions of Human Mesenchymal Stem/Stromal Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 4944. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Chang, C.H.; Yano, K.i.; Sato, T. Nanosecond pulsed current under plasma-producing conditions induces morphological alterations and stress fiber formation in human fibrosarcoma HT-1080 cells. Arch. Biochem. Biophys. 2020, 681, 108252. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold atmospheric plasma apoptotic and oxidative effects on MCF7 and HCC1806 human breast cancer cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Du, M.; Wang, Y.; Ju, S.; Ma, R.; Jiao, Z. Subcellular mechanism of microbial inactivation during water disinfection by cold atmospheric-pressure plasma. Water Res. 2021, 188, 116513. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. The Warburg effect: Why and how do cancer cells activate glycolysis in the presence of oxygen? Anti-Cancer Agents Med. Chem. 2008, 8, 305–312. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and mechanism of atmospheric-pressure dielectric barrier discharge cold plasmaon lactate dehydrogenase (LDH) enzyme. Sci. Rep. 2015, 5, 10031. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S. Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.P.; Tornín, J.; Canal, C. Cold atmospheric plasma enhances doxorubicin selectivity in metastasic bone cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef]
- Nitsch, A.; Sieb, K.F.; Qarqash, S.; Schoon, J.; Ekkernkamp, A.; Wassilew, G.I.; Niethard, M.; Haralambiev, L. Selective effects of cold atmospheric plasma on bone sarcoma cells and human osteoblasts. Biomedicines 2023, 11, 601. [Google Scholar] [CrossRef]
- Brünnert, D.; Langer, C.; Zimmermann, L.; Bargou, R.C.; Burchardt, M.; Chatterjee, M.; Stope, M.B. The heat shock protein 70 inhibitor VER155008 suppresses the expression of HSP27, HOP and HSP90β and the androgen receptor, induces apoptosis, and attenuates prostate cancer cell growth. J. Cell. Biochem. 2020, 121, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K. Plasma-Induced Death of HepG2 Cancer Cells: Intracellular Effects of Reactive Species. Plasma Process. Polym. 2012, 9, 59–66. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, Z.; Mao, X.; Meng, D.; Lei, Q.; Li, Y.; Deng, P.; Chen, M.; Tu, M.; Lu, X.; et al. Atmospheric pressure room temperature plasma jets facilitate oxidative and nitrative stress and lead to endoplasmic reticulum stress dependent apoptosis in HepG2 cells. PLoS ONE 2013, 27, e73665. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef]
- Ni, C.H.; Yu, C.S.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Chen, P.Y.; Wu, S.H.; Ip, S.W.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol- induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol. 2014, 29, 740–749. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2021, 184, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Alhumaydhi, F.A.; Gowder, S.J.; Rahmani, A.H. The biochemical and clinical perspectives of lactate dehydrogenase: An enzyme of active metabolism. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2020, 20, 855–868. [Google Scholar] [CrossRef]
- Bresciani, G.; da Cruz, I.B.M.; González-Gallego, J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar]
- Masuda, D.; Nakanishi, I.; Ohkubo, K.; Ito, H.; Matsumoto, K.I.; Ichikawa, H.; Chatatikun, M.; Klangbud, W.K.; Kotepui, M.; Imai, M.; et al. Mitochondria play essential roles in intracellular protection against oxidative stress—Which molecules among the ROS generated in the mitochondria can escape the mitochondria and contribute to signal activation in Cytosol? Biomolecules 2024, 14, 128. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Milzani, A.; Di Simplicio, P.; Colombo, R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 2001, 31, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, G.; Bellaye, P.; Micheau, O.; Bonniaud, P. Small heat shock proteins and the cytoskeleton: An essential interplay for cell integrity? Int. J. Biochem. Cell Biol. 2012, 44, 1680–1686. [Google Scholar] [CrossRef]
- Sun, Q.; Kim, O.s.; He, Y.; Lim, W.; Ma, G.; Kim, B.; Kim, Y.; Kim, O. Role of E2F1/SPHK1 and HSP27 during irradiation in a PMA-induced inflammatory model. Photobiomodul. Photomed. Laser Surg. 2020, 38, 512–520. [Google Scholar]
- Kim, S.A.; Chang, S.; Yoon, J.H.; Ahn, S.G. TAT-Hsp40 inhibits oxidative stress-mediated cytotoxicity via the inhibition of Hsp70 ubiquitination. FEBS Lett. 2008, 582, 734–740. [Google Scholar] [CrossRef]
- Barrett, M.J.; Alones, V.; Wang, K.X.; Phan, L.; Swerdlow, R.H. Mitochondria-derived oxidative stress induces a heat shock protein response. J. Neurosci. Res. 2004, 78, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Anjali Aggarwal, A.A.; Ashutosh, A.; Gulab Chandra, G.C.; Singh, A. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with α-tocopherol acetate. Trop. Anim. Health Prod. 2013, 45, 239–245. [Google Scholar] [CrossRef]
- Hirata, I.; Naito, Y.; Handa, O.; Hayashi, N.; Mizushima, K.; Adachi, S.; Omatsu, T.; Okayama, T.; Kishimoto, E.; Ichikawa, H.; et al. Heat-shock protein 70-overexpressing gastric epithelial cells are resistant to indomethacin-induced apoptosis. Digestion 2009, 79, 243–250. [Google Scholar] [CrossRef]
- Thanner, J.; Bekos, C.; Veraar, C.; Janik, S.; Laggner, M.; Boehm, P.M.; Schiefer, A.I.; Müllauer, L.; Klepetko, W.; Ankersmit, H.J.; et al. Heat shock protein 90α in thymic epithelial tumors and non-thymomatous myasthenia gravis. Oncoimmunology 2020, 9, 1756130. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Abdallah, M.E.; Aslam, A.; Bader, A.; Vassallo, A.; Tommasi, N.D.; Malki, W.H.; Gouda, A.M.; Mukhtar, M.H.; El-Readi, M.Z.; et al. Synergistic anti leukemia effect of a novel Hsp90 and a pan cyclin dependent kinase inhibitors. Molecules 2020, 25, 2220. [Google Scholar] [CrossRef]
- Alsaeed, S.A.; Toss, M.; Alsaleem, M.; Aleskandarany, M.; Joseph, C.; Kurozumi, S.; Ball, G.; Mongan, N.; Green, A.; Rakha, E. Prognostic significance of heat shock protein 90AA1 (HSP90α) in invasive breast cancer. J. Clin. Pathol. 2022, 75, 263–269. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, L.L.; Li, C.P.; Hsu, Y.T.; Jiang, S.S.; Fan, C.S.; Chua, K.V.; Huang, S.X.; Shyr, Y.M.; Chen, L.T.; et al. Myeloid-derived macrophages and secreted HSP90α induce pancreatic ductal adenocarcinoma development. Oncoimmunology 2018, 7, e1424612. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ji, J.H.; Park, K.T.; Lee, J.H.; Kang, K.W.; Park, J.H.; Hwang, S.W.; Lee, E.H.; Cho, Y.J.; Jeong, Y.Y.; et al. High-level expression of Hsp90β is associated with poor survival in resectable non-small-cell lung cancer patients. Histopathology 2015, 67, 509–519. [Google Scholar] [CrossRef]
- Meng, J.; Liu, Y.; Han, J.; Tan, Q.; Chen, S.; Qiao, K.; Zhou, H.; Sun, T.; Yang, C. Hsp90β promoted endothelial cell-dependent tumor angiogenesis in hepatocellular carcinoma. Mol. Cancer 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hance, M.W.; Nolan, K.D.; Isaacs, J.S. The Double-Edged Sword: Conserved Functions of Extracellular Hsp90 in Wound Healing and Cancer. Cancers 2014, 6, 1065–1097. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Pak, J.H.; Choi, W.H.; Park, J.Y.; Nam, J.H.; Kim, J.H. The relationship between cisplatin resistance and histone deacetylase isoform overexpression in epithelial ovarian cancer cell lines. J. Gynecol. Oncol. 2012, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Stope, M.B.; Schubert, T.; Staar, D.; Rönnau, C.; Streitbörger, A.; Kroeger, N.; Kubisch, C.; Zimmermann, U.; Walther, R.; Burchardt, M. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J. Urol. 2012, 30, 327–331. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.X.; He, T.; Dong, W.W.; Yuan, Y.; Zhao, X.; Chen, X.Y.; Zhang, N.; Zou, Z.F.; Zhang, Y.; et al. A Novel Method for Estimating the Dosage of Cold Atmospheric Plasmas in Plasma Medical Applications. Appl. Sci. 2021, 11, 11135. [Google Scholar] [CrossRef]
- Volkov, A.G. Cold atmospheric pressure He-plasma jet and plasma ball interactions with the Venus flytrap: Electrophysiology and side effects. Bioelectrochemistry 2021, 140, 107833. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Lau, H.W.; Hermans, C.; Lin, A.; Lardon, F.; et al. Auranofin and cold atmospheric plasma synergize to trigger distinct cell death mechanisms and immunogenic responses in glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Estarabadi, H.; Atyabi, S.A.; Tavakkoli, S.; Noormohammadi, Z.; Gholami, M.R.; Ghiaseddin, A.; Irani, S. Cold atmospheric plasma induced genotoxicity and cytotoxicity in esophageal cancer cells. Mol. Biol. Rep. 2021, 48, 1323–1333. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Jacoby, J.M.; Strakeljahn, S.; Bekeschus, S.; Mustea, A.; Ekkernkamp, A.; Stope, M.B. Cold atmospheric plasma treatment of chondrosarcoma cells affects proliferation and cell membrane permeability. Int. J. Mol. Sci. 2020, 21, 2291. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An innovative therapeutic option for the treatment of skeletal sarcomas: Elimination of osteo-and Ewing’s sarcoma cells using physical gas plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Kang, S.; Cho, J.; Chang, J.; Shin, Y.; Kim, K.; Park, J.; Yang, S.; Lee, J.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Ando, T.; Suzuki-Karasaki, M.; Ito, T.; Onoe-Takahashi, A.; Ochiai, T.; Soma, M.; Suzuki-Karasaki, Y. Plasma-stimulated medium kills TRAIL-resistant human malignant cells by promoting caspase-independent cell death via membrane potential and calcium dynamics modulation. Int. J. Oncol. 2018, 52, 697–708. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Marches, A.; Clement, E.; Albérola, G.; Rols, M.P.; Cousty, S.; Simon, M.; Merbahi, N. Cold atmospheric plasma jet treatment improves human keratinocyte migration and wound closure capacity without causing cellular oxidative stress. Int. J. Mol. Sci. 2022, 23, 10650. [Google Scholar] [CrossRef]
- Tahmasebi, G.; Eslami, E.; Naserzadeh, P.; Seydi, E.; Pourahmad, J. Role of mitochondria and lysosomes in the selective cytotoxicity of cold atmospheric plasma on retinoblastoma cells. Iran. J. Pharm. Res. IJPR 2020, 19, 203. [Google Scholar] [PubMed]
- Pereira-Nunes, A.; Simoes-Sousa, S.; Pinheiro, C.; Miranda-Goncalves, V.; Granja, S.; Baltazar, F. Targeting lactate production and efflux in prostate cancer. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165894. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Proc. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef]
- Soni, V.; Adhikari, M.; Lin, L.; Sherman, J.H.; Keidar, M. Theranostic potential of adaptive cold atmospheric plasma with temozolomide to checkmate glioblastoma: An in vitro study. Cancers 2022, 14, 3116. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2021, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Gümbel, D.; Hanschmann, E.M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold atmospheric plasma treatment induces anti-proliferative effects in prostate cancer cells by redox and apoptotic signaling pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Park, D.; Choi, E.H. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS ONE 2014, 9, e103349. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2016, 8, 15977. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Chen, X.; Zheng, Z.; Chen, Z.; Tian, R.; Zhao, Y.G. SOD1 is delivered to lysosomes via autophagy to maintain lysosomal function and integrity. J. Cell Biol. 2025, 224, e202501007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abazid, A.; Badendieck, S.; Mustea, A.; Stope, M.B. Impact of non-invasive physical plasma on heat shock protein functionality in eukaryotic cells. Biomedicines 2023, 11, 1471. [Google Scholar] [CrossRef]
- Bolouki, N.; Hsu, Y.N.; Hsiao, Y.C.; Jheng, P.R.; Hsieh, J.H.; Chen, H.L.; Mansel, B.W.; Yeh, Y.Y.; Chen, Y.H.; Lu, C.X.; et al. Cold atmospheric plasma physically reinforced substances of platelets-laden photothermal-responsive methylcellulose complex restores burn wounds. Int. J. Biol. Macromol. 2021, 192, 506–515. [Google Scholar] [CrossRef]
- Ebrahimi-Shaghaghi, F.; Noormohammadi, Z.; Atyabi, S.M.; Razzaghi-Abyaneh, M. Inhibitory effects of cold atmospheric plasma on the growth, virulence factors and HSP90 gene expression in Candida albicans. Arch. Biochem. Biophys. 2021, 700, 108772. [Google Scholar] [CrossRef]
- Abdo, A.I.; Kopecki, Z. Comparing redox and intracellular signalling responses to cold plasma in wound healing and cancer. Curr. Issues Mol. Biol. 2024, 46, 4885–4923. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. The inducible role of autophagy in cell death: Emerging evidence and future perspectives. Cell Commun. Signal. 2025, 23, 151. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Eggers, B.; Abazid, A.; Erb, H.H.H.; Stope, M.B. Non-Invasive Physical Plasma as an Oncological Therapy Option: Modulation of Cancer Cell Growth, Motility, and Metabolism Without Induction of Cancer Resistance Factors. Cancers 2025, 17, 3517. https://doi.org/10.3390/cancers17213517
Wang Y, Eggers B, Abazid A, Erb HHH, Stope MB. Non-Invasive Physical Plasma as an Oncological Therapy Option: Modulation of Cancer Cell Growth, Motility, and Metabolism Without Induction of Cancer Resistance Factors. Cancers. 2025; 17(21):3517. https://doi.org/10.3390/cancers17213517
Chicago/Turabian StyleWang, Yanqing, Benedikt Eggers, Alexander Abazid, Holger H. H. Erb, and Matthias B. Stope. 2025. "Non-Invasive Physical Plasma as an Oncological Therapy Option: Modulation of Cancer Cell Growth, Motility, and Metabolism Without Induction of Cancer Resistance Factors" Cancers 17, no. 21: 3517. https://doi.org/10.3390/cancers17213517
APA StyleWang, Y., Eggers, B., Abazid, A., Erb, H. H. H., & Stope, M. B. (2025). Non-Invasive Physical Plasma as an Oncological Therapy Option: Modulation of Cancer Cell Growth, Motility, and Metabolism Without Induction of Cancer Resistance Factors. Cancers, 17(21), 3517. https://doi.org/10.3390/cancers17213517






