Amino Compound-Synthesized Gold Nanoparticles for SARS-CoV-2 Antigen Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of AuNPs Reduced and Capped with Amino Compounds
2.2. Analysis of the Size Distribution and Zeta Potential of the AuNPs
2.3. Conjugation of AuNPs and RBD Protein
2.4. Mice Immunization
2.5. IgG Antibody Levels, Subclass, and Affinity
2.6. SARS-CoV-2-Neutralizing Effects of Anti-RBD Antibodies
2.7. Statistical Analysis
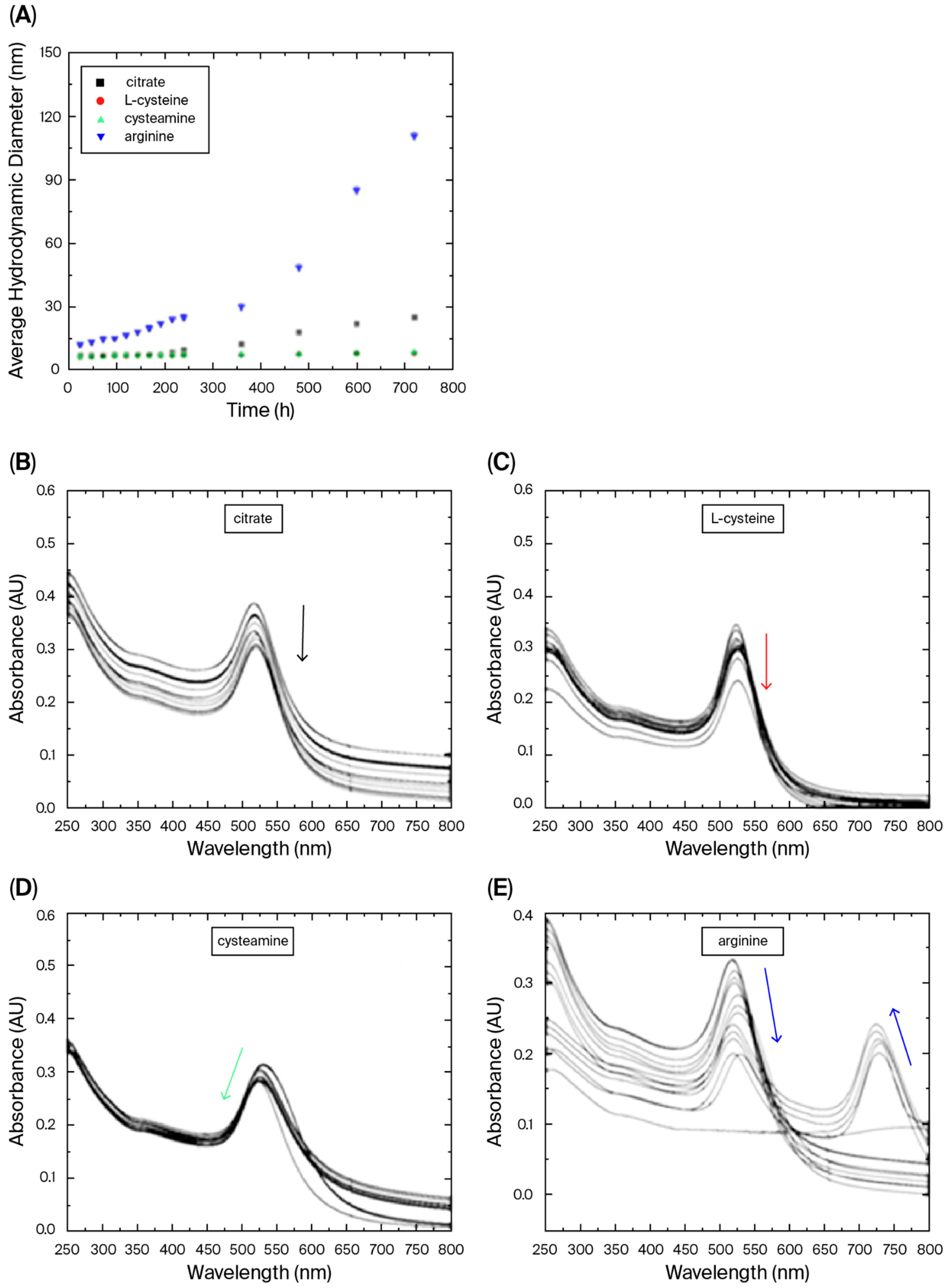
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, X.; Ito, A. Tailoring inorganic nanoadjuvants towards next generation vaccines. Chem. Soc. Rev. 2018, 47, 4954–4980. [Google Scholar] [CrossRef]
- Ferrando, R.M.; Lay, L.; Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle-Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Farfán-Castro, S.; García Soto, M.J.; Comas-GarcТакжеía, M.; Arévalo-Villalobos, J.I.; Palestino, G.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef] [PubMed]
- Farfán-Castro, S.; García-Soto, M.J.; Betancourt-Mendiola, L.; Cervantes, J.; Segura, R.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and evaluation of gold nanoparticles conjugated with five antigenic peptides derived from the spike protein of SARS-CoV-2 for vaccine development. Front. Nanotechnol. 2024, 6, 1335346. [Google Scholar] [CrossRef]
- Salazar, V.A.; Comenge, J.; Suárez-López, R.; Burger, J.A.; Sanders, R.W.; Bastús, N.G.; Jaime, C.; Joseph-Munne, J.; Puntes, V. Gold nanoparticle virus-like particles presenting SARS-CoV-2 spike protein: Synthesis, biophysical properties and immunogenicity in BALB/c mice. Vaccines 2024, 12, 829. [Google Scholar] [CrossRef]
- Miauton, A.; Audran, R.; Besson, J.; Maby-El Hajjami, H.; Karlen, M.; Warpelin-Decrausaz, L.; Sene, L.; Schaufelberger, S.; Faivre, V.; Faouzi, M.; et al. Safety and immunogenicity of a synthetic nanoparticle-based, T cell priming peptide vaccine against dengue in healthy adults in Switzerland: A double-blind, randomized, vehicle-controlled, phase 1 study. eBioMedicine 2024, 99, 104922. [Google Scholar] [CrossRef]
- Sangabathuni, S.; Vasudeva, M.R.; Chaudhary, P.M.; Surve, M.; Banerjee, A.; Kikkeri, R. Glyco-gold nanoparticle shapes enhance carbohydrate–protein interactions in mammalian cells. Nanoscale 2016, 8, 12729–12735. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.H.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Ma, H.; Chen, X. Ultrasound-assisted green synthesis of gold nanoparticles using citrus peel extract and their enhanced anti-inflammatory activity. Ultrason. Sonochem. 2022, 83, 105940. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpeti, C.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of size and shape on the anatomical distribution of endotoxin-free gold nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Figat, A.M.; Bartosewicz, B.; Liszewska, M.; Budner, B.; Norek, M.; Jankiewicz, B.J. α-Amino Acids as Reducing and Capping Agents in Gold Nanoparticles Synthesis Using the Turkevich Method. Langmuir 2023, 39, 8646–8657. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.K.; Booth, J.M.; Agrawal, S.; Coloe, P.; Kar, G. Gold nanoparticle formation during bromoaurate reduction by amino acids. Langmuir 2005, 21, 5949–5956. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Sakthipandi, K.; Sethuraman, B.; Venkatesan, K.; Alhashmi, B.; Purushothaman, G.; Ansari, I.A. Ultrasound-Based Sonochemical Synthesis of Nanomaterials. In Handbook of Vibroacoustics, Noise and Harshness; Garg, N., Gautam, C., Rab, S., Wan, M., Agarwal, R., Yadav, S., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Dalibera, N.C.; Rodrigues-Jesus, M.J.; Andreata-Santos, R.; Ramos Janini, L.M.; Oliveira, A.F.; Azzoni, A.R.; Ferreira, L.S.; Favaro, M.T.P. SARS-CoV-2 Nanovaccine Composed of Microfluidic-Produced Gold Nanoparticles Induces Neutralizing Immune Responses. ACS Appl. Nano Mater. 2023, 6, 22774–22783. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C. Precise Analysis of Nanoparticle Size Distribution in TEM Image. Methods Protoc. 2023, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; Demaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Araujo, D.B.; Machado, R.R.G.; Amgarten, D.E.; Malta, F.d.M.; de Araujo, G.G.; Monteiro, C.O.; Candido, E.D.; Soares, C.P.; de Menezes, F.G.; Pires, A.C.C.; et al. SARS-CoV-2 isolation from the first reported patients in Brazil and establishment of a coordinated task network. Mem. Inst. Oswaldo Cruz 2020, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Rebrov, E.V. Microreactors for Gold Nanoparticles Synthesis: From Faraday to Flow. Processes 2014, 2, 466–493. [Google Scholar] [CrossRef]
- Chen, H.J.; Wen, D. Ultrasonic-aided fabrication of gold nanofluids. Nanoscale Res. Lett. 2011, 6, 198. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, V.G.; Cardozo-Mata, F.; Pescador-Rojas, J.A. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultraso. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Carlucci, F.; Tabucchi, A. Capillary electrophoresis in the evaluation of aminothiols in body fluids. J. Chromatogr. B 2009, 877, 3347–3357. [Google Scholar] [CrossRef]
- Ghasemitarei, M.; Privat-Maldonado, A.; Yusupov, M.; Rahnama, S.; Bogaerts, A.; Ejtehadi, M.R. Effect of Cysteine Oxidation in SARS-CoV-2 Receptor-Binding Domain on Its Interaction with Two Cell Receptors: Insights from Atomistic Simulations. Comput. Biochem. 2021, 62, 129–141. [Google Scholar] [CrossRef]
- Krebs, F.; Scheller, C.; Grove-Heike, K.; Pohl, L.; Wätzig, H. Isoelectric point determination by imaged CIEF of commercially available SARS-CoV-2 proteins and the hACE2 receptor. Electrophoresis 2021, 42, 687–692. [Google Scholar] [CrossRef]
- Toma, H.E.; Zamarion, V.M.; Toma, S.H.; Araki, K. The coordination chemistry of gold nanoparticles. J. Braz. Chem. Soc. 2010, 21, 1158–1176. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Berlina, A.N.; Ivanov, V.S.; Zherdev, A.V.; Dzantiev, B.B. Adsorption of proteins on gold nanoparticles: One or more layers? Colloids Surf. B Biointerfaces 2019, 173, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Vangala, K.; Vo, T.; Zhang, D.; Fitzkee, N.C. A Three-Step Model for Protein–Gold Nanoparticle Adsorption. Phys. Chem. C 2014, 118, 8134–8142. [Google Scholar] [CrossRef]
- Mutwiri, G.; Gerdts, V.; Van Drunen Littel-Van Den Hurk, S.; Auray, G.; Eng, N.; Garlapati, S.; Babiuk, L.A.; Potter, A. Combination adjuvants: The next generation of adjuvants? Expert Rev. Vaccines 2014, 10, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Zhang, C.; Hu, J.; Xu, D. Recent advances in understanding the roles of T cells in pressure overload-induced cardiac hypertrophy and remodeling. J. Mol. Cell. Cardiol. 2019, 129, 293–302. [Google Scholar] [CrossRef]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef]
- Dykman, L.A.; Staroverov, S.A.; Fomin, A.S.; Khanadeev, V.A.; Khlebtsov, B.N.; Bogatyrev, V.A. Gold nanoparticles as an adjuvant: Influence of size, shape, and technique of combination with CpG on antibody production. Int. Immunopharmacol. 2018, 54, 163–168. [Google Scholar] [CrossRef]
- Favaro, M.T.P.; Alamo, P.; Roher, N.; Chillon, M.; Lascorz, J.; Márquez, M.; Corchero, J.L.; Mendoza, R.; Martínez-Torró, C.; Ferrer-Miralles, N.; et al. Zinc-Assisted Microscale Granules Made of the SARS-CoV-2 Spike Protein Trigger Neutralizing, Antivirus Antibody Responses. ACS Mater. Lett. 2024, 6, 954–962. [Google Scholar] [CrossRef]
- Delechiave, G.; Silva, M.A.; de Castro-Amarante, M.F.; Camarena, D.E.M.; Venceslau-Carvalho, A.A.; Ferreira, L.C.S.; Catalani, L.H. Layer-by-Layer Assembly of Polymeric Nanoparticles with Heparin-RBD Complexes as an Adjuvant for SARS-CoV-2 Protein-Based Vaccines. ACS Appl. Nano Mater. 2024, 7, 4068–4077. [Google Scholar] [CrossRef]
- Favaro, M.T.P.; Rodrigues-Jesus, M.J.; Venceslau-Carvalho, A.A.; Alves, R.P.S.; Pereira, L.R.; Pereira, S.S.; Andreata-Santos, R.; Ferreira, L.C.S. Nanovaccine based on self-assembling nonstructural protein 1 boosts antibody responses to Zika virus. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102334. [Google Scholar] [CrossRef]
- Javadi, M.M.; Hosseinzadeh, M.T.; Soleimani, N.; Rommasi, F. Evaluating the immunogenicity of gold nanoparticles conjugated RBD with Freund’s adjuvant as a potential vaccine against SARS-CoV-2. Microb. Pathog. 2022, 170, 105687. [Google Scholar] [CrossRef]
- Morais, T.; Soares, M.E.; Duarte, J.A.; Soares, L.; Maia, S.; Gomes, P.; Pereira, G.; Fraga, S.; Carmo, H.; Bastos, M.L. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur. J. Pharm. Biopharm. 2012, 80, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Niespodziana, K.; Karin Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.E.; Berge, T.B.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Towards scaling up the sonochemical synthesis of Pt-nanocatalysts. Ultrason. Sonochem. 2024, 103, 106794. [Google Scholar] [CrossRef]
- Abramov, O.V.; Gedanken, A.; Koltypin, Y.; Perkas, N.; Perelshtein, I.; Joyce, E.; Mason, T.J. Pilot scale sonochemical coating of nanoparticles onto textiles to produce biocidal fabrics. Surf. Coat. Technol. 2009, 204, 718–722. [Google Scholar] [CrossRef]
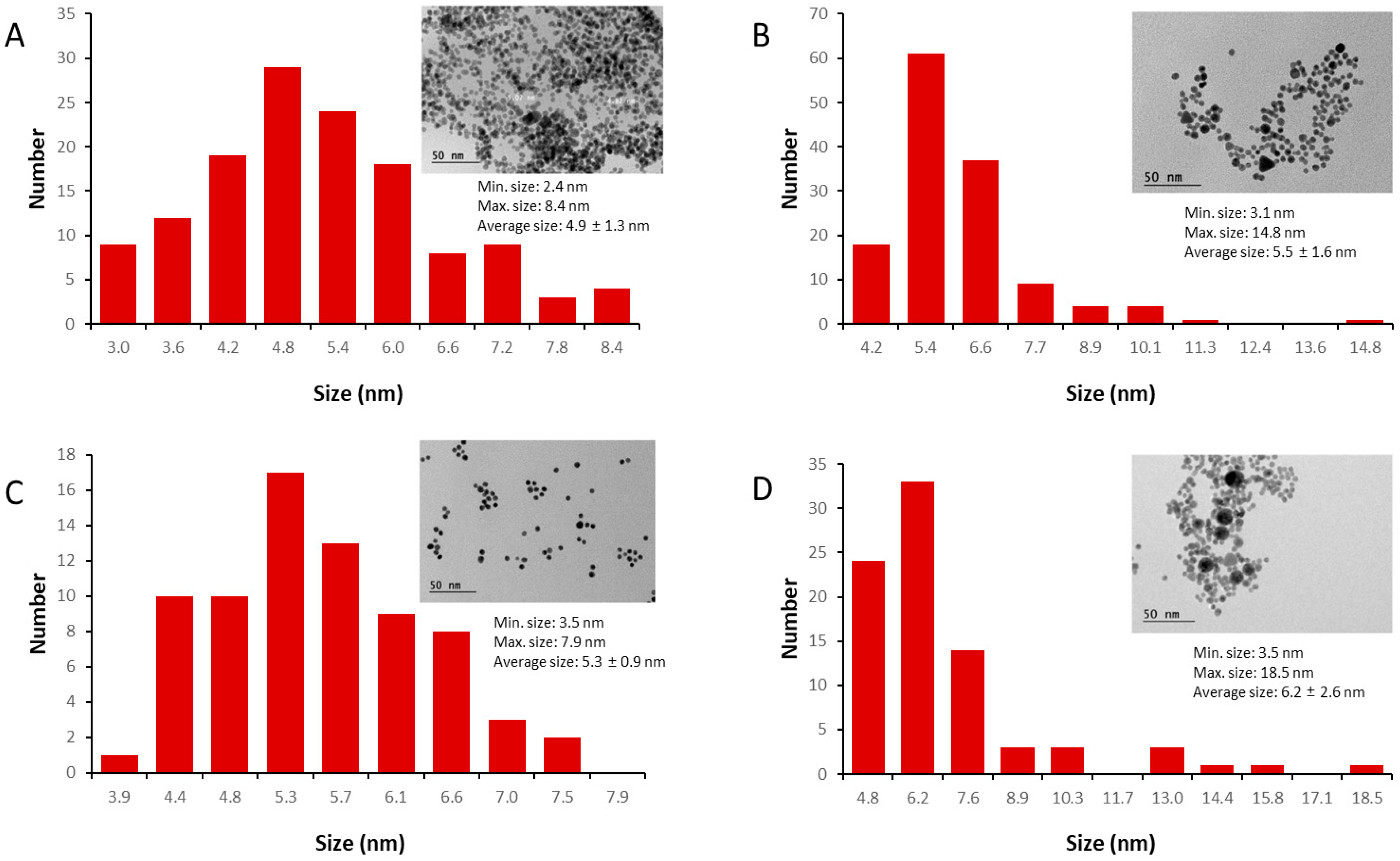

| Reducing Agent | Size (nm) TEM | Size (nm) DLS a | PDI | Zeta Potential (mV) | λmáx (nm) | Reaction Yield (%) |
|---|---|---|---|---|---|---|
| AuNP-Citrate | 4.9 ± 1.3 | 7.2 ± 0.5 | 0.198 ± 0.018 | −40.1 ± 1.8 | 525 | 99.8 |
| AuNP-Cysteamine | 5.3 ± 0.9 | 6.4 ± 1.4 | 0.138 ± 0.028 | +36.5 ± 1.9 | 525 | 101.3 |
| AuNP-Cysteine | 5.5 ± 1.6 | 6.3 ± 1.8 | 0.541 ± 0.128 | −25.6 ± 1.3 | 525 | 91.2 |
| AuNP-Arginine | 6.2 ± 2.6 | 12.4 ± 2.8 | 0.313 ± 0.067 | −40.5 ± 1.5 | 530 | 80.2 |
| Gold Nanoparticle | STD ** | |
|---|---|---|
| AuNP-Citrate | 23.7 | 0.76 |
| AuNP-Cysteine | 16.7 | 0.60 |
| AuNP-Cysteamine | 32.5 | 0.11 |
| AuNP-Arginine | 4.13 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rego, L.S.; Favaro, M.T.P.; Rodrigues-Jesus, M.J.; Andreata-Santos, R.; Janini, L.M.R.; Seckler, M.M.; Ferreira, L.C.d.S.; Azzoni, A.R. Amino Compound-Synthesized Gold Nanoparticles for SARS-CoV-2 Antigen Delivery. Pharmaceutics 2025, 17, 1211. https://doi.org/10.3390/pharmaceutics17091211
Rego LS, Favaro MTP, Rodrigues-Jesus MJ, Andreata-Santos R, Janini LMR, Seckler MM, Ferreira LCdS, Azzoni AR. Amino Compound-Synthesized Gold Nanoparticles for SARS-CoV-2 Antigen Delivery. Pharmaceutics. 2025; 17(9):1211. https://doi.org/10.3390/pharmaceutics17091211
Chicago/Turabian StyleRego, Layane Souza, Marianna Teixeira Pinho Favaro, Monica Josiane Rodrigues-Jesus, Robert Andreata-Santos, Luiz Mário Ramos Janini, Marcelo Martins Seckler, Luis Carlos de Souza Ferreira, and Adriano Rodrigues Azzoni. 2025. "Amino Compound-Synthesized Gold Nanoparticles for SARS-CoV-2 Antigen Delivery" Pharmaceutics 17, no. 9: 1211. https://doi.org/10.3390/pharmaceutics17091211
APA StyleRego, L. S., Favaro, M. T. P., Rodrigues-Jesus, M. J., Andreata-Santos, R., Janini, L. M. R., Seckler, M. M., Ferreira, L. C. d. S., & Azzoni, A. R. (2025). Amino Compound-Synthesized Gold Nanoparticles for SARS-CoV-2 Antigen Delivery. Pharmaceutics, 17(9), 1211. https://doi.org/10.3390/pharmaceutics17091211







