1. Introduction
The oral mucosa is increasingly recognized as a promising site for both local and systemic drug delivery. Its rich vascularization, relative permeability, and ease of access make it a compelling alternative to conventional routes such as oral ingestion or intravenous administration, especially for drugs that are susceptible to degradation in the gastrointestinal tract or extensive hepatic first-pass metabolism [
1,
2,
3,
4,
5]. Furthermore, oral mucosal delivery offers advantages such as avoidance of gastrointestinal irritation, ease of administration, and better patient compliance, particularly in populations with swallowing difficulties or chronic conditions [
6,
7,
8].
The oral cavity encompasses multiple distinct regions, each with different anatomical and physiological characteristics that influence drug absorption. These include the sublingual, buccal, gingival, and palatal mucosae. Among these, the buccal and sublingual regions are most commonly utilized for drug delivery due to their favorable permeability and accessibility [
2,
9,
10,
11,
12]. However, despite these advantages, the route is not without challenges. Salivary washout, enzymatic degradation, and limited surface area for adhesion all contribute to reduced drug retention and bioavailability [
2,
13,
14].
Glycosaminoglycans (GAGs) are linear polysaccharides composed of repeating disaccharides (an amino sugar with a uronic acid or galactose) and are classified into sulfated families—chondroitin/dermatan sulfate, heparin/heparan sulfate, keratan sulfate—and the non-sulfated hyaluronan. Their high anionic charge and polydispersity impart strong hydration, viscoelasticity, and wide protein-binding capacity [
15,
16,
17]. These physicochemical features are directly relevant to oromucosal delivery: hydrated interfacial layers support lubrication and mucoadhesion, while sequence- and position-specific sulfation motifs (“sulfation code”) govern selective interactions with cytokines and growth factors, enabling bioactive matrices [
14,
18,
19]. Hyaluronan further engages the CD44 receptor, widely expressed and often upregulated in inflamed or malignant oral tissues, providing a basis for tissue retention and receptor-mediated cell interactions exploitable in targeted formulations [
20,
21,
22]. The oral environment also contains native hyaluronan and hyaluronidase activity, highlighting the need to tune molecular weight, crosslinking, and formulation context to balance residence time with controlled biodegradation at the mucosal surface [
16,
17,
23]. Together, these structure–property relationships justify a focused appraisal of GAG-based films, hydrogels, and nanoengineered carriers as modular materials for oromucosal therapy, while clarifying where enzymatic turnover and receptor biology can be leveraged—or must be mitigated—in translational design [
21,
22,
24]. GAGs offer clear advantages for oromucosal delivery—biocompatibility, intrinsic mucoadhesion, tunable charge/hydration, and, in the case of hyaluronan, receptor-mediated interactions that can improve localization and retention [
16,
17]. These benefits are counterweighted by practical limitations, including batch-to-batch variability of natural sources, enzymatic degradation in saliva, challenges in maintaining mechanical integrity and palatability during wear, and incomplete standardization of mucoadhesion/permeation assays [
6,
25,
26]. Translation is further constrained by stability and sterilization requirements [
19,
27,
28], ambiguity around drug–device–combination classification [
29,
30,
31,
32], and a scarcity of head-to-head clinical data versus current standard care [
33,
34,
35,
36]. Taken together, GAG-based systems are most compelling when prolonged mucosal residence and gentle, bioactive matrices are required, but their routine adoption will depend on harmonized testing, robust life-cycle stability data, and patient-centric usability evidence. In response to these limitations, research has increasingly focused on developing advanced oral mucosal drug delivery systems (DDS) designed to prolong mucosal contact, enhance permeation, and enable controlled release. Mucoadhesive polymers, nanocarriers, and stimuli-responsive hydrogels represent key innovations in this area [
37,
38,
39,
40,
41,
42].
Of particular interest is the incorporation of glycosaminoglycans (GAGs)—a class of naturally occurring, biocompatible polysaccharides such as chitosan, hyaluronic acid, and alginate—into DDS formulations. These biomaterials exhibit high mucoadhesiveness, hydration capacity, and modifiability, making them ideal for tailoring drug release profiles and improving therapeutic outcomes [
1,
2,
6,
43,
44]. These polymers have moved from ancillary excipients to principal design elements that directly govern residence time, barrier negotiation, and on-site bioactivity in the oral cavity. Beyond generic mucoadhesion, GAGs provide tunable hydration layers, electrostatic and receptor-mediated interactions (e.g., HA–CD44), and stimuli-responsive matrices that collectively enable durable localization and controllable release in a highly dynamic, saliva-bathed environment [
17,
20,
21,
22]. Over just the past few years, their roles have expanded across clinically relevant formats—mucoadhesive films/wafers, smart hydrogels, nanoparticulate coatings, and even textile-inspired scaffolds [
45,
46,
47,
48]—bringing the field closer to translation while exposing specific standardization and regulatory gaps this review is positioned to clarify [
25,
26,
29,
30,
31,
32]. At the same time, comparative stability data [
19,
27,
28], pediatric-friendly wafers [
49,
50,
51,
52], and early clinical experiences with HA-based products [
45,
46,
47,
48] underscore real-world feasibility, strengthening the case for a targeted appraisal of GAG chemistry–function relationships and platform selection for distinct oral indications. Finally, recurring bottlenecks—non-harmonized mucoadhesion/permeation assays [
25,
27], imperfect in vitro–in vivo correlation [
26], and ambiguity around drug–device classification [
29,
30,
31,
32]—demand a consolidated, GAG-centric perspective to guide experimental design and translation.
In this review, the anatomical and physiological constraints that shape oromucosal delivery and clinical use cases are first framed. Current dosage forms and enabling technologies are then surveyed [
6,
53,
54], to delineate where GAGs add distinctive value. Next, the major GAG families and their structure–property–performance relationships relevant to oral applications (chitosan, HA, dextran, alginate) are dissected. Building on this foundation, GAG-based platforms—mucoadhesive films/wafers, hydrogels/nanogels, nanoparticulates/microparticulates, and emerging textile scaffolds—are compared, with emphasis on formulation, stability, and therapeutic use-cases. For this review, ‘mucosal DDS’ refers primarily to retentive mucoadhesive systems engineered to remain in situ (non-disintegrating or predictably eroding) and provide unidirectional or site-focused release. Dispersive intraoral formats (e.g., orodispersible films, lozenges, sprays) are discussed only where surface coating or rapid symptom relief is the therapeutic goal. Evaluation methodologies (release, permeability, and mucoadhesion) are then examined, and areas where standardization is most urgently needed are highlighted [
14,
25,
26]. Finally, patient-centric usability [
49,
50,
51,
52], manufacturing and regulatory considerations [
29,
30,
31,
32], and health-economic factors are discussed, and prioritized research gaps together with a translational outlook are presented.
2. Anatomical and Physiological Features of the Oral Mucosa Relevant to Drug Delivery
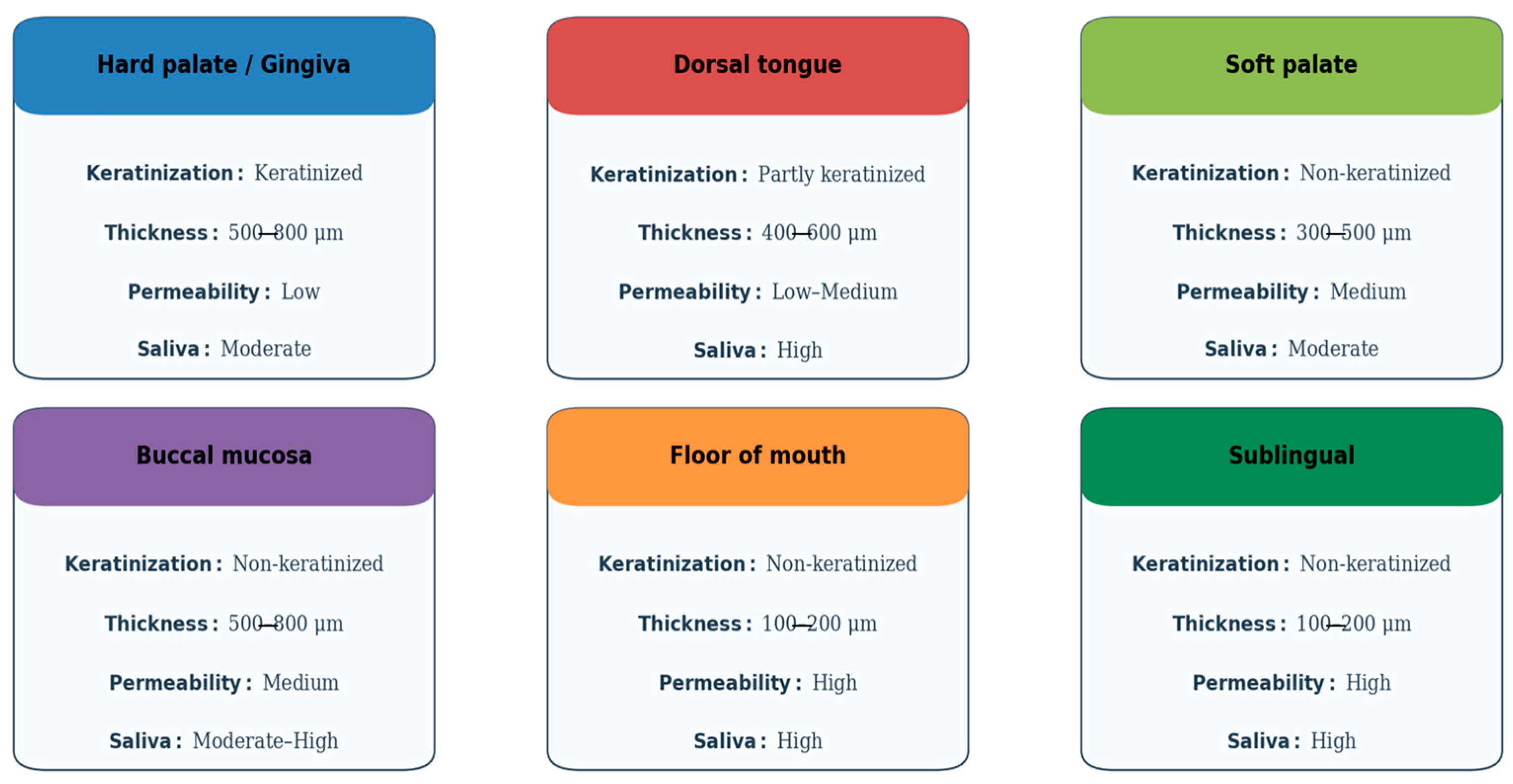
The oral cavity comprises several distinct subregions that differ in structure, function, and permeability, all of which influence the success of mucosal drug delivery. The oral mucosa is typically categorized into three types: masticatory (e.g., gingiva and hard palate), lining (e.g., buccal and sublingual areas), and specialized (e.g., dorsal tongue). Each type possesses unique histological and physiological characteristics that determine its suitability for drug absorption [
14,
55].
The buccal and sublingual mucosae are the most studied routes for transmucosal drug delivery. The buccal mucosa is approximately 500–800 μm thick and features a non-keratinized epithelium overlying a vascularized lamina propria, which facilitates drug permeation while providing a relatively stable environment for dosage form adherence. In contrast, the sublingual mucosa is thinner and more permeable but more prone to salivary washout, which can reduce the residence time of formulations [
14,
56,
57].
The permeability of the mucosa is heavily influenced by the degree of keratinization, lipid content, and the organization of intercellular junctions. Non-keratinized regions allow for easier diffusion of hydrophilic molecules, whereas keratinized epithelium, with its higher lipid content and tight intercellular junctions, serves as a formidable barrier [
56,
58]. Moreover, the buccal mucosa is less enzymatically active than gastrointestinal tissues, reducing the degradation risk for sensitive molecules like peptides or proteins [
59,
60].
Saliva also plays a critical role in drug dissolution, diffusion, and metabolism. Produced at a rate of approximately 0.5–1.5 L per day, saliva contains enzymes such as amylase and esterase that can influence drug stability. Its continuous flow and varying pH (ranging from 6.2 to 7.6) can lead to dilution and removal of the drug, emphasizing the need for mucoadhesive delivery systems that can withstand such clearance [
61,
62,
63,
64].
Another emerging consideration is the interaction between drug formulations and the oral microbiome. Changes in microbial composition—whether from disease, antibiotics, or the drug carrier itself—may influence both drug efficacy and mucosal health [
65,
66]. Additionally, immune cells present in the mucosa, such as Langerhans cells and dendritic cells, can recognize and respond to drug components, especially in vaccine delivery or immunotherapy contexts [
67,
68,
69].
From a histological perspective, the oral mucosa consists of a stratified squamous epithelium and a connective tissue lamina propria. While the epithelium acts as the primary barrier to drug permeation, the lamina propria is richly supplied with blood and lymphatic vessels, offering an efficient pathway for systemic absorption. The turnover rate of oral epithelial cells is also relatively rapid—about 5–10 days—which can influence both healing and absorption dynamics, particularly for chronically applied formulations [
55].
Vascular drainage also plays an important role. Drugs absorbed via the sublingual and buccal mucosa primarily enter the systemic circulation through the deep lingual and facial veins, bypassing hepatic first-pass metabolism. This pharmacokinetic advantage allows for lower dosing of certain drugs and reduces the risk of hepatic toxicity. However, regional variability in vascular density and blood flow may influence interindividual differences in absorption efficiency [
70,
71].
Mucosal hydration is another crucial factor. Adequate hydration maintains epithelial integrity and facilitates mucoadhesion and drug diffusion [
2,
13]. Conditions such as xerostomia (dry mouth), common in older adults and patients undergoing chemotherapy or radiotherapy, can compromise drug delivery by altering mucosal permeability and the performance of adhesive formulations [
72].
Bioadhesion refers to interfacial bonding between a material and a biological surface (cells, soft tissues, or mineralized substrates), whereas mucoadhesion denotes the mucus-specific case in which polymers adhere to the mucin layer and/or the epithelial glycocalyx of mucosal tissues. In oromucosal drug delivery, mucoadhesion is the principal design objective because it prolongs residence against salivary clearance and supports directional drug flux toward the epithelium. Mechanistically, mucoadhesion arises from a combination of wetting/adsorption, hydrogen-bonding and electrostatic interactions, and interpenetration of polymer and mucus chains; throughout this review we therefore use “mucoadhesion” unless broader bioadhesive principles are being discussed [
73,
74].
Importantly, the dynamics of oral muscle activity—including speech, chewing, and swallowing—can affect dosage form retention. These mechanical movements can displace mucoadhesive films or patches, limiting their effectiveness unless properly designed to anchor in place [
6,
75]. Thus, the success of mucosal delivery systems depends not only on the physicochemical compatibility between drug and tissue but also on the interplay between physiological activity and formulation robustness.
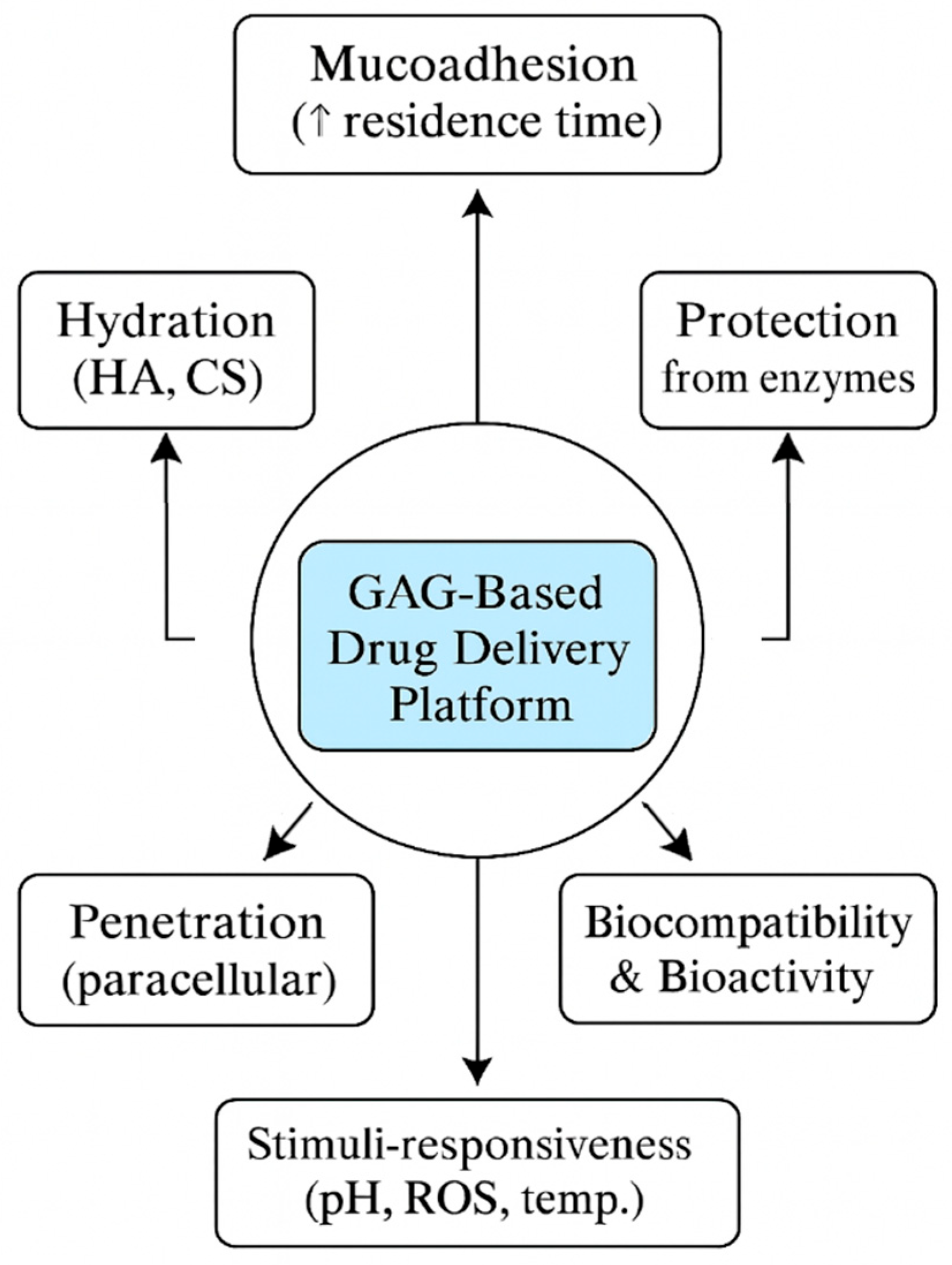
As discussed above, the oral mucosa presents a complex but highly promising environment for drug delivery. A thorough understanding of its anatomical compartments, vascular and immune infrastructure, enzymatic and microbiome context, and mechanical properties is essential for designing effective therapeutic systems that maximize both local and systemic bioavailability (
Figure 1). These complex anatomical and physiological factors further highlight the relevance of mucoadhesive glycosaminoglycan (GAG)-based systems, whose mechanisms of action are illustrated in
Figure 2.
3. Barriers and Limitations in Oral Mucosal Drug Delivery
While the oral mucosa offers multiple advantages as a drug delivery site, including accessibility, non-invasiveness, and avoidance of first-pass metabolism, it also presents significant physiological and biochemical barriers that can limit the efficacy of administered therapeutics [
60,
76]. Understanding these limitations is crucial for designing systems that can overcome them and deliver drugs effectively.
Salivary washout represents one of the most significant challenges. The average adult produces 0.5 to 1.5 L of saliva daily, and this continuous flow can rapidly dilute or remove a drug formulation, particularly in the sublingual area. Consequently, maintaining the residence time of drug carriers long enough to allow adequate absorption becomes a major formulation goal [
76,
77]. Mucoadhesive systems have been developed to resist this mechanical clearance, but their effectiveness can be compromised by movement of the tongue, swallowing, and variations in salivary viscosity [
78].
Enzymatic degradation is another critical barrier. Although the enzymatic activity in the oral cavity is lower than in the gastrointestinal tract, the presence of proteolytic enzymes like aminopeptidases, esterases, and lysozymes can still degrade susceptible drugs, especially peptides, nucleotides, and prodrugs. Enzyme inhibitors, protective coatings, or chemical modifications—such as polyethylene glycol (PEG)ylation—are often employed to enhance the stability of these molecules [
79,
80].
Disintegration of oromucosal formulations can leave insoluble or partially hydrated residues in the oral cavity—an effect reported especially for hydrocolloid-rich matrices that may not disperse completely [
81]. Residual fragments and perceived stickiness are recognized determinants of end-user acceptability, contributing to discomfort, unpleasant mouthfeel, and, in some users, transient interference with speech or normal oral function [
82]. Accordingly, excipient selection and dissolution kinetics should be engineered to promote complete clearance (e.g., rapidly dispersing maltodextrin-based ODFs) and verified with human acceptability panels that assess mouthfeel and ease of use alongside disintegration performance [
83,
84].
Backing-layer composition is a critical design variable in buccal films: impermeable layers (e.g., ethyl cellulose/Eudragit) are routinely employed to enforce unidirectional release, minimize drug loss into saliva, and direct flux toward the mucosa [
85,
86]. Where non-dissolvable backings are selected, the device should be removed after the intended dosing interval; alternatively, dissolvable/erodible backings may be used so that the assembly clears once drug release is complete [
87]. In the absence of an appropriate backing, release occurs from both faces of the film—diluting the mucosal dose and exposing non-target tissues—whereas bilayer designs demonstrably reduce donor-side loss and improve local availability [
88,
89].
Permeability limitations also exist. The epithelial structure of the oral mucosa, particularly in keratinized regions such as the hard palate and gingiva, presents a strong barrier to the diffusion of hydrophilic drugs and macromolecules. The tight junctions between epithelial cells restrict paracellular transport, making transcellular permeation the primary mechanism for most compounds. This restricts the passive diffusion of large and/or polar molecules [
2,
14,
90,
91].
To enhance permeability, chemical penetration enhancers such as surfactants, bile salts, fatty acids, and cyclodextrins have been investigated. However, many of these substances are associated with cytotoxicity, irritation, or disruption of mucosal integrity, raising concerns about long-term safety and user acceptability [
55,
92]. Finding a balance between sufficient permeation and biocompatibility remains a key challenge in this domain.
Salivary flow varies widely (unstimulated ~0.3–0.4 mL/min; stimulated ~1.5–2.0 mL/min), and hyposalivation/xerostomia (e.g., oncology, polypharmacy) depresses flow and alters pH—both materially affect mucoadhesion, dissolution, and taste masking. Stratify in vitro tests by flow rate and pH to mimic xerostomia vs. normal [
93].
Mucosal thickness/keratinization and receptor expression (e.g., CD44 in inflamed or malignant tissues) also vary across patients and lesions—report site-specific outcomes (buccal vs. sublingual) and, for HA-targeted systems, include CD44-status as a covariate [
94,
95].
Taste masking represents another formulation difficulty. Drugs that have a bitter or metallic taste may induce aversion or nausea, particularly in pediatric or geriatric populations. While taste-masking techniques such as flavoring agents, polymer encapsulation, or ion-exchange resins exist, they must not interfere with drug release kinetics or mucoadhesion [
96,
97].
Interindividual variability adds further complexity. Differences in saliva composition, mucosal thickness, enzymatic activity, and even the oral microbiome may affect drug absorption and therapeutic response between patients [
98,
99]. Conditions such as xerostomia or periodontal disease can further alter the local environment, reduce formulation effectiveness or increase side effects [
99,
100].
Moreover, pH fluctuations in the oral cavity, caused by diet, disease, or circadian rhythms, can affect drug solubility, ionization, and stability. For instance, weakly basic drugs may become ionized and less permeable in the relatively acidic sublingual region, necessitating the development of pH-modulating excipients or prodrugs [
101,
102].
The limited surface area of the oral mucosa, approximately 200 cm
2, further constrains the volume of drug that can be administered. This is particularly problematic for drugs requiring high doses or sustained plasma levels, which may necessitate repeated administration or the development of highly concentrated formulations [
7,
103].
Finally, formulation retention and user experience are vital. Large or poorly designed patches may be uncomfortable or interfere with speech and swallowing. Dosage forms must be discreet, comfortable, and capable of adhering to the mucosa without causing irritation, especially for long-term or chronic therapies [
104,
105].
To resolve these descriptions of barriers, there is an alignment for each challenge with a GAG-centric solution path. For salivary washout and shear, wet-adhesive catechol-modified HA or chitosan and thiolated chitosan enable durable adhesion via catechol bonding and mucin disulfides, respectively; bilayer films add unidirectional flux control (design readouts: detachment force, survival under simulated flow) [
106,
107]. For enzymatic degradation, moderately crosslinked HA/alginate networks and GAG-coated nanoparticles provide sacrificial protection while preserving release [
14]. For permeability limits, chitosan-based (including thiolated) matrices afford reversible tight-junction opening at tolerated doses, while HA–CD44 interactions support receptor-mediated uptake/retention in diseased tissues (readouts: TEER recovery, paracellular marker flux, CD44-stratified uptake) [
108,
109]. Finally, pH/ionic variability can be mitigated using ion-tunable alginate/HA blends and catechol-alginate surfaces that maintain adhesion in saliva [
110].
Where chemical enhancers are considered, risk–benefit should be disclosed explicitly. For example, 10% RAMEB is cytotoxic on reconstructed buccal epithelium, whereas 2–5% appears tolerated over repeated exposures; bile salts show efficacy with concentration-dependent epithelial stress; and 0.5–2% SLS exposures are linked to mucosal desquamation in humans (design mitigation: low-dose, pulsed exposure with full TEER/LDH recovery reporting) [
111]. Given real-world variability in salivary flow and mucosal status, in vitro performance should be profiled under xerostomia-mimicking and normosalivary conditions and, for HA-targeted systems, analyzed by CD44 expression [
93,
95].
Overcoming these limitations demands an interdisciplinary approach, combining insights from pharmaceutical sciences, biomaterials engineering, oral medicine, and patient-centered design. Advances in mucoadhesive biomaterials, nanocarrier technologies, and responsive systems (e.g., thermosensitive or pH-triggered gels) are beginning to address these challenges, offering promising avenues for the next generation of oral mucosal drug delivery platforms.
7. Analytical Methods in the Evaluation of Oral DDS
7.1. Structural Characterization of GAGs
The structural complexity of GAGs makes them particularly difficult to analyze. Mass spectrometry (MS) has become a key tool in this field due to its exceptional sensitivity, ability to detect subtle structural variations, and capacity to handle complex biological mixtures. Therefore, many studies show that researchers are gaining insight into GAG structures and connecting them to their biological roles, especially regarding their interactions with proteins, as past studies have highlighted the importance of GAG structure-function relationships [
201].
GAGs exhibit significant molecular heterogeneity, particularly in their uronic acid, hexosamine components, and sulfate group positions. Understanding their biological roles requires detailed detection and structural identification. Extraction typically involves enzymatic depolymerization using exogenous proteinases or sodium hydroxide. Physicochemical analyses are performed after enzymatic treatment, using techniques like ion-pair chromatography and MS [
202].
The structural characterization of GAGs is complex, requiring enzymatic depolymerization with specific bacterial enzymes followed by disaccharide analysis using Gel Permeation Chromatography (GPC) [
203], High-Performance Liquid Chromatography (HPLC) [
204], or Ultra-Performance Liquid Chromatography (UPLC) [
205], Nuclear Magnetic Resonance (NMR) spectroscopy [
206], Capillary Electrophoresis (CE) [
207], and Fluorophore-Assisted Carbohydrate Electrophoresis (FACE) [
208].
Liquid chromatography-mass spectrometry (LC-MS) and MS have become prevalent due to their ability to analyze GAGs without interference from biological impurities. Reverse-phase ion-pair Reverse-Phase Ion-Pair High-Performance Liquid Chromatography (RPIP-HPLC) employs volatile ion-pairing reagents that allow analytes to bind to hydrophobic stationary phases and remain compatible with electrospray ionization (ESI)-MS [
209]. This approach suits a range of GAGs, from unsulfated to highly sulfated. Reverse-Phase Ion-Pair Ultra-Performance Liquid Chromatography–Mass Spectrometry (RPIP-UPLC-MS) further improves resolution, sensitivity, and efficiency using high-pressure columns with small particle sizes. However, factors such as ion-pair reagent concentration, counter-ion type, and pH can influence separation outcomes [
210]. Routine GAG structural analysis often requires multiple enzymatic treatments, disaccharide isolations, chromatographic steps, and various MS detection methods, making it labor-intensive.
MS techniques like ESI and matrix-assisted laser desorption/ionization (MALDI) are widely used for structural analysis of GAG oligosaccharides. MALDI time-of-flight (TOF) MS and Electrospray Ionization Mass Spectrometry (ESI-MS) effectively analyze large polar macromolecules [
211]. NMR spectroscopy also offers detailed structural insights, including saccharide composition and sulfation patterns, but requires relatively large amounts of purified GAGs, limiting its routine use.
Accurate analysis of GAG structures continues to pose major analytical challenges [
212]. Unlike proteins and nucleic acids, which can be amplified or overexpressed, GAGs are synthesized through a non-template enzymatic process. This begins with a uniform copolymer that is later heavily modified by enzymes such as deacetylases, sulfotransferases, and epimerases, resulting in heterogeneous chains with varying levels of acetylation and sulfation. These modifications produce highly complex and diverse biological GAG mixtures, typically available only in limited quantities. Because of their high molecular weight and low abundance, analytical methods like NMR or X-ray diffraction are often impractical. This is why developing advanced MS techniques for GAG analysis has attracted significant research attention. Two key features of GAGs—their negative charge and the delicate nature of their sulfate groups—strongly influence the choice of MS methods.
Over the past decade, improvements in online separation techniques, ion activation methods, and software for automated MS/MS data interpretation have greatly advanced GAG structural analysis [
213]. Given their involvement in essential biological functions such as cell signaling, wound repair, and blood coagulation, GAGs are crucial targets for detailed structural studies.
Most linear GAG chains are built from repeating disaccharide units made up of a hexosamine and a hexuronic acid, with keratan sulfate (KS) being the main exception, as it contains hexosamine and galactose instead. Even without their attached protein cores, GAGs are highly intricate and varied molecules due to the wide range of possible combinations in chain lengths, sugar sequences, chemical compositions, sulfo group placements, and how these domains are arranged along the chain. The specific types of GAG chains—such as heparin/heparan sulfate (Hp/HS), chondroitin/dermatan sulfate (CS/DS), and keratan sulfate (KS)—are defined by their characteristic repeating disaccharide units, and these structural features play a key role in shaping how proteoglycans (PGs) are organized [
214]. New MS methodologies, including innovations in sample preparation and tandem MS approaches, have led to impressive gains in the accuracy and speed of GAG characterization.
7.2. Drug Release Studies and Permeation Assays
The development of oral drug delivery systems using biopolymers such as GAGs—including hyaluronic acid, chitosan, dextran, and alginate—has gained significant traction due to their biocompatibility, biodegradability, and mucoadhesive properties. However, evaluating these materials requires a multi-layered analytical approach that rigorously examines their performance, stability, and safety.
Synthetic membranes are often preferred over biological tissues because they are more readily available, cost-effective, and structurally simpler, allowing for large-scale studies and mechanistic investigations. Additionally, they provide more reproducible permeation data by eliminating in vivo variables such as skin age, sex, ethnicity, and anatomical location. Despite these advantages, artificial membrane studies still show notable variability [
215].
These assays help determine the drug release profile—whether immediate, sustained, or controlled—under simulated gastrointestinal conditions. Permeation assays across intestinal models (such as Caco-2 cell monolayers or ex vivo tissues) provide insight into the ability of the polymer–drug system to cross epithelial barriers, a crucial step for oral bioavailability.
To reflect GAG chemistry, dissolution/release tests were adapted as follows: (i) mucin-containing saliva simulants to capture rheological synergism that predicts mucoadhesion and residence; we report ΔG′/viscosity shift alongside release (bioadhesive liquids show positive synergy with mucin) [
154]; (ii) Calcium dynamics for alginate systems: internal gelation using CaCO
3/GDL and phosphate/ionic-strength challenges to map crosslink stability and release (alginate release is sensitive to Ca
2+ availability and competing anions) [
216,
217,
218]; (iii) Enzyme-triggered conditions (e.g., hyaluronidase for HA networks) to evaluate on-demand degradation-mediated release [
219].
Permeability refers to how easily a molecule can pass through a biological membrane. It is typically measured as a velocity, expressed in units such as centimeters per second (cm/s), representing the distance the molecule travels across the membrane per unit time. This measurement applies regardless of whether the molecule moves via active transport or passive diffusion mechanisms [
220].
For transport and safety, TR146 oral epithelium is a relevant buccal model (with TEER and paracellular markers), while Caco-2 can be used as a comparative tight-junction reference [
130]. Chitosan and thiolated chitosan can transiently open tight junctions and increase permeation under controlled conditions, so TEER recovery should be documented [
221]. For hyaluronan carriers, CD44-aware uptake or retention assays are justified because CD44 is present and can be upregulated in oral tissues [
222,
223]. Cell-free artificial barriers (for example, PermeaPad) are best used as screening tools and cross-checked against TR146 or ex vivo buccal tissue [
224]. Mucin interaction and ionic crosslinking govern adhesion and release for many anionic GAG matrices (for example, alginate), and thiolated chitosan adds reversible tight-junction modulation; ignoring these features can misestimate real-world performance [
152].
Critically, many studies focus on release kinetics (zero-order, first-order, Higuchi, or Korsmeyer–Peppas models) but often lack in-depth correlation with in vivo pharmacokinetics, highlighting a gap between bench testing and clinical translation.
7.3. Bioadhesion Testing and Mucoadhesive Properties
The need to better understand the functions and mechanisms of action of GAGs has driven the development of both qualitative and quantitative analytical techniques. These include classical staining methods like alcian blue and toluidine blue, as well as separation techniques such as paper chromatography, thin-layer chromatography, gas chromatography, HPLC, and capillary electrophoresis. In addition, advanced methods such as the 1,9-dimethylmethylene blue assay, enzyme-linked immunosorbent assays (ELISA), and mass spectrometry have been developed to provide more precise and detailed analyses of GAGs [
225].
Despite promising in vitro results of bioadhesion tests, a major challenge lies in standardizing these tests, as variability in biological tissues and testing conditions can result in poor reproducibility and limit direct comparisons across studies [
226]. Mucoadhesion is commonly described as a two-stage process. In the contact stage, the formulation wets and makes intimate contact with the mucus layer; viscosity, surface energy, and initial polymer–mucin interactions (electrostatics, hydrogen bonding) dominate. The consolidation stage follows, in which polymer chains interpenetrate the mucus network and stronger interactions form (secondary bonding, ionic bridges, or covalent linkages), increasing the work of adhesion and resistance to shear [
227]. Relative to neutral matrices (e.g., HPMC/PVA), GAGs offer additional adhesion mechanisms. (i) Electrostatics and hydration: anionic HA/alginate and cationic chitosan provide high interfacial hydration and charge-mediated attraction to mucins; polymer–mucin rheological synergism (ΔG′ or viscosity gain) is frequently observed and correlates with longer residence [
228]; (ii) Covalent/coordination bonding: thiolated chitosans form disulfide bridges with mucin cysteines, and catechol-grafted GAGs enable wet adhesion via catechol oxidation/coordination, both increasing adhesive strength beyond hydrogen-bonding alone [
157]; (iii) Ionic crosslinking/bridging: alginate networks stabilized by Ca
2+ (including CaCO
3/GDL systems) resist dilution and can maintain contact under flow, a consolidation effect sensitive to calcium/phosphate balance. (iv) Bio-specific retention: HA–CD44 interactions add a receptor-mediated component to consolidation in inflamed or diseased oral tissues where CD44 is expressed or upregulated [
222]. Applied examples reflect these mechanisms: thiolated chitosans consistently show higher detachment forces than unmodified chitosan; catechol-modified chitosan/HA systems improve wet adhesion on oral models; and chondroitin-sulfate–based oral liquids exhibit polymer–mucin synergism and barrier effects on reconstructed epithelia [
229].
Both soft (mucosal) and hard (enamel and dentin) tissues in the oral cavity are rapidly coated by the acquired oral pellicle within minutes, beginning with an electron-dense protein layer followed by a more complex globular layer [
230].
This pellicle consists mainly of selectively adsorbed salivary proteins and peptides, but it also includes components from gingival crevicular fluid, blood, bacteria, mucosal cells, and dietary substances. It plays multiple protective roles: acting as a lubricant, shielding the dental surface, preventing decalcification, and providing antibacterial defense through enzymes such as lysozyme and peroxidases. However, it also contains molecules that facilitate bacterial adhesion—such as glycolipids, fibrinogen, and collagen—which support the attachment of early colonizers like
Streptococcus and
Actinomyces, initiating biofilm formation. Bacterial adhesins mediate this binding, and the final biofilm composition varies by location in the mouth (e.g., supragingival vs. subgingival) and between individuals. Nevertheless, studies indicate no major differences in pellicle structure or protein profile between caries-active and caries-inactive subjects [
231].
7.4. Stability, Physicochemical Characterization, and Biocompatibility
Stability assessment—including storage stability and drug–polymer compatibility—is essential for ensuring the practical viability of oral formulations. Techniques such as dynamic light scattering (DLS) provide critical data on particle size distribution and zeta potential, which are directly linked to colloidal stability, aggregation behavior, and mucoadhesive performance. Although many studies report favorable initial physicochemical profiles, long-term stability data under physiological and storage conditions are often underreported, potentially limiting scalability and clinical translation.
Physicochemical characterization of GAG-based MDDS should link material identity to performance. Functional groups and crosslink signatures are verified by FTIR/Raman, while molecular-weight distributions (and degradation fragments) are best quantified by SEC coupled to multi-angle light scattering (SEC-MALS) [
232,
233]. Viscoelastic behavior and viscosity are measured with rotational rheometry following pharmacopeial guidance (USP <912>) and, for mucoadhesive systems, complemented by mucin–polymer “rheological synergism” (ΔG′/viscosity gains) as a mechanistic proxy for adhesion. For nanoscale carriers (nanogels), dynamic light scattering provides hydrodynamic size and swelling behavior in relevant media [
234,
235]. Finally, chemistry-based safety tests include residual solvent quantification per ICH Q3C (R9), aligned to permitted daily exposures and validated analytical methods [
236,
237].
Capillary electrophoresis (CE) is highly sensitive and efficient, offering rapid separation and compatibility with multiple detection methods, such as UV spectroscopy, MS, NMR, and Laser-Induced Fluorescence Detection (LIF) [
238]. While CE-LIF is highly effective, it has traditionally required multiple enzymatic digestion and separation steps, complicating the workflow. Recent advancements in CE-LIF protocols have simplified the process, enabling higher throughput by allowing analysis of GAG-derived disaccharides in a single run [
239].
FACE, which uses derivatization with fluorophores such as 2-Aminoacridone (AMAC), offers high sensitivity and allows simultaneous analysis of multiple samples [
240]. FACE can detect disaccharides from extremely small sample volumes and has been validated as highly selective and accurate for chondroitin sulfate (CS) in biological matrices. It also enables concurrent separation of CS/dermatan sulfate (DS) and hyaluronan, making it a powerful tool for analyzing low-abundance sulfated disaccharides.
For GAG-based mucosal drug-delivery systems, biocompatibility should follow a risk-based ISO 10993-1 strategy for surface-contacting devices, selecting endpoints by contact type/duration (e.g., cytotoxicity, irritation, sensitization) [
241]. Cytotoxicity is typically shown by ISO 10993-5 [
241] and USP <87> in vitro reactivity assays; irritation/sensitization are addressed per the ISO 10993 framework [
241]. Because the target tissue is oral epithelium, reconstructed or cell-line models (TR146) are appropriate to confirm epithelial integrity (e.g., TEER recovery) and buccal tolerance [
130]. Chemistry-based safety complements biology: residual solvents are controlled per ICH Q3C (R8/R9) and elemental impurities per ICH Q3D (R2), with testing aligned to permitted daily exposures [
237,
242]. FDA’s ISO 10993 guidance and endpoint matrices can be used to justify the overall test plan and document acceptability for intraoral contact [
241].
By combining different analytical techniques—such as enzymatic depolymerization, chromatography, MS, CE, and FACE—researchers can achieve detailed qualitative and quantitative assessments of GAG structure and behavior, despite their inherent complexity and heterogeneity.
These techniques are frequently employed to characterize chemical structure, confirm drug loading, and detect degradation products. When combined with biological assays—such as cytotoxicity tests (methyl thiazolyl tetrazolium (MTT), lactate dehydrogenase (LDH)), hemolysis assays, and histocompatibility studies—they form a comprehensive toolkit for validating the safety and performance of GAG-based materials.
Nonetheless, there is a critical need for more advanced and standardized in vivo biocompatibility testing, particularly due to the complex degradation pathways that natural polymers may undergo in the gastrointestinal tract. While analytical methods for evaluating GAG-based oral drug delivery systems have significantly advanced, key challenges remain in harmonizing in vitro and in vivo results, standardizing bioadhesion and permeation assays, and expanding stability studies under realistic storage and usage conditions. A more integrated application of cutting-edge techniques—such as high-resolution MS, atomic force microscopy (AFM), and in situ imaging—could further deepen mechanistic understanding and accelerate the clinical translation of these promising biomaterials.
7.5. Microscale Imaging and Characterization Methods
Several analytical techniques have been employed to elucidate the bonding mechanisms between dental hard tissues, luting agents, and restorative materials. Among these, AFM, though widely used in materials science, remains underutilized in dental research despite its extensive capabilities. AFM offers atomic-level resolution with minimal sample preparation, making it well-suited for investigating dental substrates. It has been widely applied in studies characterizing enamel and dentin erosion. More recently, AFM nanoindentation has enabled detailed assessments of the mechanical properties and demineralization processes of enamel [
243].
Although AFM is most commonly used to obtain topographic images of surfaces, it includes dozens of modes—both basic and advanced—that can reveal additional properties of biomaterials. In dental research, these capabilities are rarely explored. Techniques such as phase-contrast imaging, force-distance curve analysis, nanomechanical mapping, and Kelvin Probe Force Microscopy (KPFM) allow for the exploration of topological, mechanical, and electrical properties of modified Y-TZP (yttria-stabilized tetragonal zirconia polycrystal) surfaces. These advanced AFM modes provide a deeper understanding of surface interactions, enabling accurate characterization of adhesive features [
244]. As such, AFM emerges as a vital interdisciplinary tool, bridging solid-state physics, microbiology, and dental materials science [
245].
In addition to AFM, other high-resolution imaging methods such as Scanning Electron Microscopy (SEM), Energy-Dispersive X-ray Spectroscopy (SEM-EDX), and Transmission Electron Microscopy (TEM) are widely used. SEM provides detailed visualization of the surface morphology of dental hard tissues and is particularly effective for analyzing enamel. Proper sample preparation—including drying, embedding, sectioning orientation, and acid etching—is essential to ensure optimal imaging results. These parameters are especially important when observing small specimens or multiple planes within a single sample [
246].
SEM-EDX, a combined imaging and elemental analysis technique, is commonly employed to examine enamel surface morphology and quantitatively assess the calcium-to-phosphorus (Ca/P) ratio—an important marker of enamel integrity. The crystalline structure and Ca/P ratio are essential for evaluating the effects of remineralizing agents. A recent study by Raj demonstrated that enamel demineralization in the control group (exposed to an acidic pH < 5.5) led to a decrease in the Ca/P ratio. Four remineralizing agents were tested in comparison, and an increased Ca/P ratio was associated with more effective enamel recovery. Group II demonstrated the highest mineralization efficacy after 21 days, followed by Groups I, III, and IV. SEM imaging at 1500× magnification revealed partial crystal recovery and re-establishment of the interprismatic enamel structure in Groups I and II, with Group II showing superior surface uniformity and smoothness [
247].
To reduce methodology reporting, each characterization was selected for its predictive value against an in-mouth barrier: oscillatory rheology (G′/G″, LVER, ΔG′ with mucin) predicts residence time and washout resistance; texture analysis (detachment work/force) quantifies adhesion under shear; and TR146/OME assays (TEER recovery, permeability, viability) balance permeation gains with epithelial safety. All rheology methods should follow compendial guidance (USP <911>/<912>, Ph. Eur. 2.2.8) and report geometry, temperature, and conditioning to minimize lab-to-lab variability [
25]. Where possible, rheological synergism (ΔG′ with mucin) and TA detachment should co-trend with in vitro residence under flow and clinical proxy outcomes (e.g., barrier/soothing effects in HA/CS liquids), providing a transparent chain from method to barrier mitigation to patient-relevant performance [
152].
Table 5 presents rheology studies of GAG systems for oral mucosa, reporting details and barrier relevance to reduce lab-to-lab variability [
152,
248,
249,
250,
251,
252].
9. Clinical Applications and Future Therapeutic Directions
9.1. Local Therapy: Candidiasis, Ulcers, Mucositis, and Oral Cancer
GAG-based systems have shown considerable promise in the local treatment of oral mucosal pathologies such as candidiasis, aphthous ulcers, chemotherapy-induced mucositis, and oral cancer. These applications leverage the mucoadhesive, anti-inflammatory, and tissue-healing properties of natural GAGs—particularly HA and chitosan—to prolong drug contact with the affected area and support tissue regeneration while minimizing systemic side effects [
44,
277].
In oral candidiasis, HA-enhanced nanoemulsions and chitosan–pectin polyelectrolyte films have been utilized to deliver antifungal agents such as miconazole and clotrimazole [
178,
190]. These GAG-containing formulations not only increase drug retention on the buccal mucosa but also improve drug permeation into the fungal biofilm, thereby enhancing antifungal efficacy [
178]. In vitro studies and early clinical observations suggest that chitosan itself may exhibit synergistic antifungal activity, attributed to its membrane-disrupting properties and its ability to reduce Candida adhesion [
44].
For aphthous ulcers and oral mucositis, HA-based mucoadhesive films and gels have demonstrated significant clinical benefit. A randomized trial comparing an HA/polyvinylpyrrolidone gel with placebo found accelerated healing and reduced pain in patients with orthodontic appliance-related ulcers [
44]. In chemotherapy-induced mucositis, high-molecular-weight HA has been shown to reduce epithelial apoptosis and inflammation both in vitro and in vivo, likely due to its antioxidant and cytoprotective effects on keratinocytes [
108]. Pediatric trials using HA sprays (e.g., Mucosamin
®) have also reported shortened mucositis duration and improved tolerability [
278].
The therapeutic role of GAG-based systems is expanding into the oncologic setting. In a 2024 preclinical study, an electrospun HA nanofiber membrane co-loaded with methotrexate and glycyrrhizin exhibited high mucoadhesion and sustained chemotherapeutic release at the tumor site [
279]. This dual-action platform promoted apoptosis of oral squamous carcinoma cells while simultaneously mitigating chemotherapy-associated mucosal damage via glycyrrhizin’s anti-inflammatory effects [
280]. The targeting capability of HA, via CD44 receptor interaction, further enhanced localization of drug release to malignant tissues [
281]. Such dual-functional systems highlight the translational value of GAGs in precision oncology, particularly for head and neck malignancies where localized therapy is highly desirable.
Overall, these findings demonstrate that GAG-based delivery platforms offer a non-invasive, biocompatible, and therapeutically synergistic approach for managing a wide spectrum of localized oral pathologies. As their clinical validation progresses, these systems are expected to supplement or replace traditional mouthwashes, gels, and lozenges, especially in chronic or recurrent cases.
9.2. Systemic Delivery: Hormones, Peptides, and Insulin
While the oral mucosa has traditionally been targeted for local therapy, it is increasingly being explored as a route for systemic drug delivery—particularly for biologics and small-molecule drugs with poor gastrointestinal stability or extensive first-pass metabolism [
76]. GAG-based delivery systems, especially those incorporating HA and CS, have demonstrated promising capabilities in overcoming key mucosal barriers and facilitating transmucosal absorption of hormones and peptides [
44].
Among the most studied examples is the buccal delivery of insulin. HA-modified nanoparticles have been used to encapsulate insulin in protective matrices that resist enzymatic degradation in the oral cavity. These nanoparticles adhere to the mucosal surface via mucoadhesive interactions and release insulin in a controlled manner, allowing for absorption through both paracellular and CD44-mediated transcellular pathways. In diabetic rat models, such systems achieved significant reductions in blood glucose levels and improved plasma insulin concentrations, with reported bioavailability reaching 12–15%—a marked improvement over unprotected oral insulin [
282].
Similar strategies have been applied to other peptide drugs, such as exenatide (a GLP-1 receptor agonist), calcitonin, and desmopressin. GAG-based nanocarriers facilitate their stabilization in the oral cavity and prolong contact time at the absorption site, allowing for effective transmucosal uptake. Co-formulation with permeation enhancers, such as bile salts or surfactants, has been shown to further increase transport efficiency without compromising mucosal integrity [
283].
In addition to peptides, hormone therapies—including estradiol and testosterone—have been formulated using GAG-based films and gels for buccal or sublingual administration. These systems offer discrete, non-invasive delivery options with steady plasma profiles, improved patient compliance, and reduced hepatic metabolism. HA and CS have been particularly favored in these applications due to their ability to form stable mucoadhesive matrices that conform to the mucosal surface and control drug release kinetics [
44].
Preclinical models and early-phase clinical trials suggest that GAG-functionalized nanocarriers may also hold potential for transmucosal vaccine and immunotherapy delivery. HA-coated nanoparticles carrying peptide antigens or nucleic acids have demonstrated mucosal uptake and immune activation in animal models, providing a needle-free platform for immunization against oral or systemic pathogens [
284].
Collectively, these developments highlight the growing potential of GAG-based platforms for systemic delivery via the oral mucosa. By enabling non-invasive administration of labile or poorly absorbed therapeutics, these systems may significantly expand the therapeutic repertoire of oral transmucosal delivery in endocrine, metabolic, and infectious disease management.
9.3. Personalization and Smart-Release Platforms
The integration of responsive and customizable technologies into glycosaminoglycan GAG-based systems has accelerated the shift toward personalized medicine in oral mucosal drug delivery. These platforms aim to adapt drug release in response to local physiological cues—such as pH, enzymatic activity, redox state, or hydration—while also offering design flexibility for patient-specific anatomy, dosage, or treatment duration [
44,
182].
Stimuli-responsive GAG hydrogels and nanogels represent a cornerstone of this approach. For example, thiolated hyaluronic acid (HA–SH) formulations form disulfide linkages with mucins, enhancing mucoadhesion and enabling sustained drug release that is responsive to oxidative degradation in inflamed tissues. These systems have been particularly useful in conditions such as oral ulcers and mucositis, where oxidative stress contributes to pathogenesis and healing dynamics [
187].
pH-sensitive platforms have also been developed to release drugs preferentially in the acidic or alkaline microenvironments associated with infection or malignancy. In one study, HA hydrogels incorporating pH-labile crosslinks remained stable at neutral pH but rapidly disassembled in mildly alkaline environments, as found in dental plaque or infected wounds—releasing both antimicrobial agents and anti-inflammatory ions (e.g., Zr
4+) on demand [
264].
In parallel, 3D printing technologies are enabling the fabrication of anatomically tailored mucoadhesive devices. Multi-layer scaffolds incorporating HA and other GAGs can be printed to match lesion shape and depth, optimizing surface contact and controlling drug release gradients. A 3D-printed HA-gelatin-alginate patch was tested in several studies with distinct adhesive and drug-loaded layers that released corticosteroids over four days, offering a proof-of-concept for lesion-specific treatment in chronic oral conditions [
285,
286,
287].
Moreover, multifunctional GAG-based formulations are being designed to address complex pathologies that involve both microbial dysbiosis and inflammation. For instance, a eutectogel composed of HA and xanthan gum was recently formulated with ibuprofen and antimicrobial agents to treat oral lichen planus. This system combined prolonged release (up to 24 h) with broad-spectrum antibacterial activity, reducing both inflammation and pathogen load using a lower drug dose than conventional gels [
288].
The development of these personalized and responsive systems aligns closely with the broader trend toward precision therapy. GAG-based carriers can be tuned in terms of molecular weight, crosslink density, and surface functionalization to match patient-specific mucosal conditions or pharmacokinetic requirements. In the future, integration with salivary diagnostics or microbiosensor feedback loops may allow real-time control over drug release, further individualizing care.
In summary, personalization and smart-release features are transforming GAG-based delivery platforms from passive carriers into interactive therapeutic systems. These innovations promise to enhance clinical outcomes, reduce dosing frequency, and improve patient adherence in the management of both acute and chronic oral diseases.
9.4. Ongoing Clinical Trials and Translational Challenges
The past five years have witnessed encouraging progress in the clinical development of GAG-based drug delivery systems for the oral mucosa. While several formulations have advanced from laboratory prototypes to human trials, the path toward widespread clinical adoption is still marked by regulatory, manufacturing, and patient-centered challenges.
Multiple clinical trials have evaluated the therapeutic efficacy of HA-based gels and sprays in oral mucositis, aphthous ulcers, and chemotherapy-induced lesions. For instance, a completed Phase IV pediatric study (NCT05818007) assessed a topical HA formulation for oral mucositis in children undergoing chemotherapy. The results suggested modest but statistically significant improvements in lesion healing and patient comfort when used alongside standard oral care [
288]. Similarly, HA mouthwashes and bioadhesive gels have been tested in adults with radiation-induced mucositis, showing reductions in pain severity and lesion duration [
44].
More advanced GAG-conjugated systems, including HA–drug complexes and HA-coated nanoparticles, are under investigation for oncologic and immunologic applications. ONCOFID-P, a paclitaxel–HA conjugate, has reached Phase III evaluation for localized chemotherapy, although its initial application has been in bladder cancer. However, its design principles—exploiting CD44 targeting and mucosal retention—are highly relevant for oral squamous cell carcinoma and may inform future intraoral formulations [
289].
Despite these advances, several translational hurdles remain. First, natural variability in GAG source materials—such as differences in molecular weight, degree of acetylation, and purity—can introduce batch-to-batch inconsistencies, complicating manufacturing and regulatory approval. Moreover, many GAGs (e.g., HA and CS) are hygroscopic, posing stability concerns during storage, particularly in humid environments. To address this, formulations must incorporate protective packaging or use stabilizing additives, such as sugars, amino acids, or crosslinking agents [
43].
Scalability is another constraint. Advanced fabrication methods—such as freeze-drying, layer-by-layer deposition, or 3D bioprinting—often require specialized equipment and quality control protocols that are not yet standardized across pharmaceutical manufacturing pipelines. This can lead to high production costs and limit commercial viability unless these processes are simplified or automated [
1].
Patient compliance and sensory acceptability also warrant close attention. Mucoadhesive patches and gels must be discreet, palatable, and non-irritating. Overly strong adhesion may cause discomfort or impede natural oral functions, while insufficient adhesion risks premature dislodgement. Moreover, some natural GAGs may trap food debris or interfere with the oral microbiome if used chronically, raising concerns about long-term mucosal compatibility [
99].
From a regulatory perspective, there remains ambiguity around whether GAG-based systems are classified as drugs, devices, or combination products. While some HA oral formulations are currently marketed as Class I medical devices or nutraceuticals, more advanced drug-loaded systems will require robust clinical trials demonstrating superiority over standard therapies. This includes head-to-head comparisons with existing gels, rinses, or systemic medications in terms of efficacy, safety, and patient-reported outcomes [
36,
290,
291,
292,
293,
294,
295] (
Table 6).
Finally, health economic considerations are increasingly important. High-purity GAGs and sophisticated delivery platforms can increase treatment costs, which may limit accessibility unless reimbursement strategies or cost-effective production methods are developed.
10. Conclusions
Glycosaminoglycan-based (GAG) platforms have progressed from ancillary excipients to tunable matrices that extend residence, regulate interfacial hydration/charge, and enable controlled, site-directed release on the oral mucosa. Hyaluronan, chitosan (including thiolated derivatives), and alginate now underpin thin films, wafers, hydrogels, and electrospun scaffolds with reproducible gains in mucoadhesion and local pharmacodynamic exposure. Nevertheless, evidence for the most ambitious functions—multi-drug sequencing, receptor-mediated targeting, and light-triggered setting—remains predominantly preclinical, and superiority to standard care has not yet been demonstrated in controlled human studies.
Translation is limited by non-harmonized mucoadhesion/permeation assays and weak in vitro–in vivo correlation; polymer heterogeneity, sterilization-induced molecular-weight drift, and long-term stability; combination-product classification and PMOA justification; and patient-centric usability. Manufacturing risks include content uniformity across large sheets, residual solvents, and sterilization that preserves mechanics and adhesion, necessitating QbD/DoE and inline QA.
Near-term opportunities (12–24 months) include single-drug HA or chitosan films/wafers for clearly defined lesions, xerostomia coatings guided by mucin-synergism metrics, and pilot randomized trials built on standardized panels (ΔG′ with mucin, wet-peel/tensile, TEER-recovery). Longer-term goals (≥3–5 years) involve clinically validated multi-drug and HA–CD44-targeted systems, safe triggerable formats, and selected systemic delivery once human PK/PD is established. Priorities are interlaboratory ring trials, explicit IVIVC development, head-to-head clinical testing versus standard care, post-sterilization re-qualification of properties, and early regulatory and health-economic planning.