1. Introduction
Prodrug strategies have emerged as a vital aspect of drug discovery and development, frequently employed to address deficiencies in drug properties by improving solubility, permeability, stability, taste, and gastrointestinal (GI) tolerance [
1,
2,
3,
4]. Prodrugs themselves are pharmacologically inactive but are converted into the active parent drugs in vivo through enzymatic and/or chemical reactions. Notably, nearly 10% of all FDA-approved drugs are prodrugs. Over the past decade, the FDA has approved approximately 50 prodrugs, accounting for 13% of all approved small-molecule new chemical entities [
5].
Among various prodrug strategies, ester prodrugs are particularly notable for their ability to modify the pharmacokinetic (PK) and pharmacodynamic properties of parent drugs by introducing ester functional groups [
6]. Ester prodrugs commonly enhance the lipophilicity of parent drugs by masking polar functionalities, such as alcohol or carboxylic acid groups, converting them into esters and thereby improving membrane permeability. Examples of ester prodrugs include simple esters, amino acid esters, sugar esters, lipid esters, polymeric esters, thioesters, and phosphoesters. These approaches are widely used to modulate the solubility and permeability of parent drugs [
2,
4]. However, achieving a balance between solubility and permeability can be challenging. While masking polar groups of the parent drug increases lipophilicity, it can also reduce solubility. Additionally, ester prodrugs are often susceptible to aqueous and enzymatic hydrolysis in the GI tract, especially in cases where hydrolysis is catalyzed by varying pH conditions within the GI tract. Esterases, which catalyze the hydrolysis of esters into carboxylic acids and alcohols, are responsible for breaking down a wide range of chemical bonds, including esters, thioesters, phosphoesters, amides, and epoxides [
6,
7]. The stability of ester prodrugs in the GI tract is therefore crucial to maximize target-site exposure of the parent drugs [
4]. Whether intended to enhance solubility or permeability, oral ester prodrugs must possess sufficient stability in the GI tract and enterocytes to avoid premature hydrolysis, enabling them to reach the bloodstream and liver where they are converted to their active forms. Ester prodrugs can enhance oral bioavailability only if they remain stable within the GI tract. Despite considerable efforts to improve their stability, most solutions have primarily focused on chemical modifications to the structures of ester prodrugs. Recent advances in nanoparticle technology have shown promise in protecting ester prodrugs from aqueous environments by encapsulating them within nanoparticles [
8]. However, beyond nanoparticle encapsulation, formulation-based strategies have been largely underexplored as potential solutions to these challenges.
Recently, we investigated and demonstrated a novel class of lipid-based drug delivery system, termed binary lipid systems (BLS). These systems consist of a single lipid and a single water-soluble surfactant, which together form stable microemulsions [
9]. We developed and explored prototypes of BLS using various lipids and surfactants. To assess the potential of BLS for oral solid dosage forms, we also investigated solid microemulsions, in which the BLS is absorbed onto solid carriers to produce solid granules that form stable microemulsions upon contact with aqueous media. In this study, we evaluated the application of BLS in solid granules to improve the oral absorption of ester prodrugs and studied the impact of the compositions of BLS on oral absorption. Conventional lipid-based formulations, such as emulsions, microemulsions, and lipid nanoparticles, require the drug to be fully solubilized in excipients, which limits drug loading capacity [
10,
11]. Additionally, these formulations are typically prepared as liquid dosage forms, which pose stability challenges [
12]. To address these issues, we explored a novel approach of creating solid drug granules by coating drug crystals with BLS, thereby resolving the challenges of low drug loading and stability. Lipids have diverse metabolic pathways and functional properties. For instance, long-chain triglycerides are digested by enzymes in the stomach and small intestine into mono- and diglycerides, stimulating lipoprotein formation and facilitating lymphatic transport. In contrast, medium-chain monoglycerides are either directly transported to systemic circulation via the portal blood or further digested in the small intestine by carboxylesterases (CE) that are esterases to hydrolyze carboxylic esters [
13]. Additionally, lipids have demonstrated potential to enhance drug permeability [
14,
15]. Based on these properties, we hypothesize that selecting appropriate lipids for the BLS can protect ester prodrugs from degradation in the GI tract and enhance their solubility and permeability, ultimately leading to improved oral absorption.
The objective of this study is to explore the application of BLS for enhancing the oral absorption of ester prodrugs by coating drug crystals with BLS and study the impact of the compositions of BLS on oral absorption. Olmesartan medoxomil (OLM), a selective angiotensin II receptor blocker approved for the treatment of hypertension [
16], was chosen as the model drug. OLM is a methyl ester prodrug of olmesartan (OL). OL is di-anionic due to its carboxyl (pKa 3.7) and tetrazole (pKa 4.8) groups, leading to complete ionization in the GI tract, which makes OL poorly permeable. To mask these acidic groups and improve permeability, a prodrug approach has been used for OL by esterifying the carboxyl group into a mono-anionic methyl ester, resulting in OLM [
17]. Once absorbed, OLM is rapidly and completely converted to OL in the blood and liver before entering systemic circulation [
18]. However, this increase in lipophilicity simultaneously reduces solubility, making OLM poorly soluble in water (~10 µg/mL). Additionally, OLM undergoes hydrolysis in the GI tract, converting it into the impermeable parent drug OL through unfavorable ester bond cleavage by water and CE [
19,
20]. OLM also experiences P-glycoprotein-mediated efflux, further complicating its absorption [
21]. These challenges contribute to OLM’s low oral bioavailability (~26%) [
22], making it a suitable model drug to test the hypothesis of this study. OLM solid granules were prepared using BLS with different lipids and evaluated through in vitro two-step dissolution studies and in vivo PK and tissue distribution studies. To examine the protective effect of BLS against CE-mediated hydrolysis, CE was added to the dissolution medium. Six OLM formulations were developed and compared to assess their ability to enhance oral absorption and to elucidate the mechanisms by which BLS provides protection for OLM.
2. Methods and Materials
2.1. Materials
Migloyl 812 was generously provided by Cremer (Eatontown, NJ, USA). Capmul MCM C8 and propylene glycol monocaprylate (Capmul PG-8) were kindly supplied by Abitec (Columbus, OH, USA). Aeropearl 300 and Avicel 105 were obtained as gifts from Evonik (Parsippany, NJ, USA). D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) was provided as a gift by Antares (Saint Charles, IL, USA). OLM was procured from TCI America (Portland, OR, USA), and OL was sourced from Santa Cruz Biotechnology (Dallas, TX, USA). Acetonitrile and methanol were purchased from Fisher Scientific (Pittsburgh, PA, USA), while phosphoric acid (H3PO4) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Esterase from porcine liver, a source of CE, was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animals
Sprague-Dawley rats (male, 250–300 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). All animal experiments were performed in compliance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of North Texas Health Science Center. The rats were housed in pairs under a 12 h light/dark cycle with free access to food and water for one week prior to the experiments.
2.3. Selection of Lipid and Surfactant for Construction of Binary Lipid Systems
TPGS, a surfactant and a synthetic derivative of vitamin E, was selected as the surfactant in the BLS. Pharmaceutical lipids including glycerides such as Capmul MCM C8 and Miglyol 812 and propylene glycol such as Capmul PG-8, which are approved by the FDA, were chosen to form the BLS with TPGS. The chemical compositions of lipids used in the study are shown in
Table 1.
2.4. HPLC Method for OLM Measurement
An HPLC method to simultaneously measure both OL and OLM was conducted as previously reported [
23]. Briefly, a Waters HPLC system equipped with an Eclipse XDB C18 column (3.5 µm, 4.6 × 100 mm; Agilent, Santa Clara, CA, USA) was used. The flow rate was set at 1.0 mL/min, and the mobile phase consisted of solvent A (pure methanol) and solvent B (0.1% H
3PO
4 in water). The injection volume was 20 µL. A gradient elution was employed with the following program: solvent A was maintained at 50% for 2 min, increased to 60% by 4 min, returned to 50% at 6.1 min, and held at 50% until 8.5 min. For sample preparation, a mixture of solvent A and solvent B in a 1:1 (
v/
v) ratio was used as the mobile phase for sample dilution as described below.
2.5. Preparation of OLM Formulations
The compositions of OLM formulations are shown in
Table 2. Briefly, OLM, lipid and TPGS were mixed at 37 °C for 15 min with stirring, and then Aeroperl 300 was added and mixed. After cooling to room temperature, OLM granules were formed. OLM was directly mixed with Avicel 105 to prepare OLM powder. Drug loading was calculated as (OLM weight)/(total formulation weight) × 100%.
2.6. Particle Size Measurement of Reconstituted OLM Formulations
To evaluate particles generated from OLM formulations, 1 mL MilliQ water was added into 10 mg OLM granules or 5 mg OLM MCM-TPGS Liquid. After mixed and centrifugation, particle size and size distribution in the supernatants were measured by dynamic light scattering using a particle analyzer (Malvern Zetasizer Ultra Particle Analyzer, Malvern Panalytical, Westborough, MA, USA). The polydispersity index (PI) was used to indicate size distribution.
2.7. Determination of Physical State of OLM in OLM Formulations
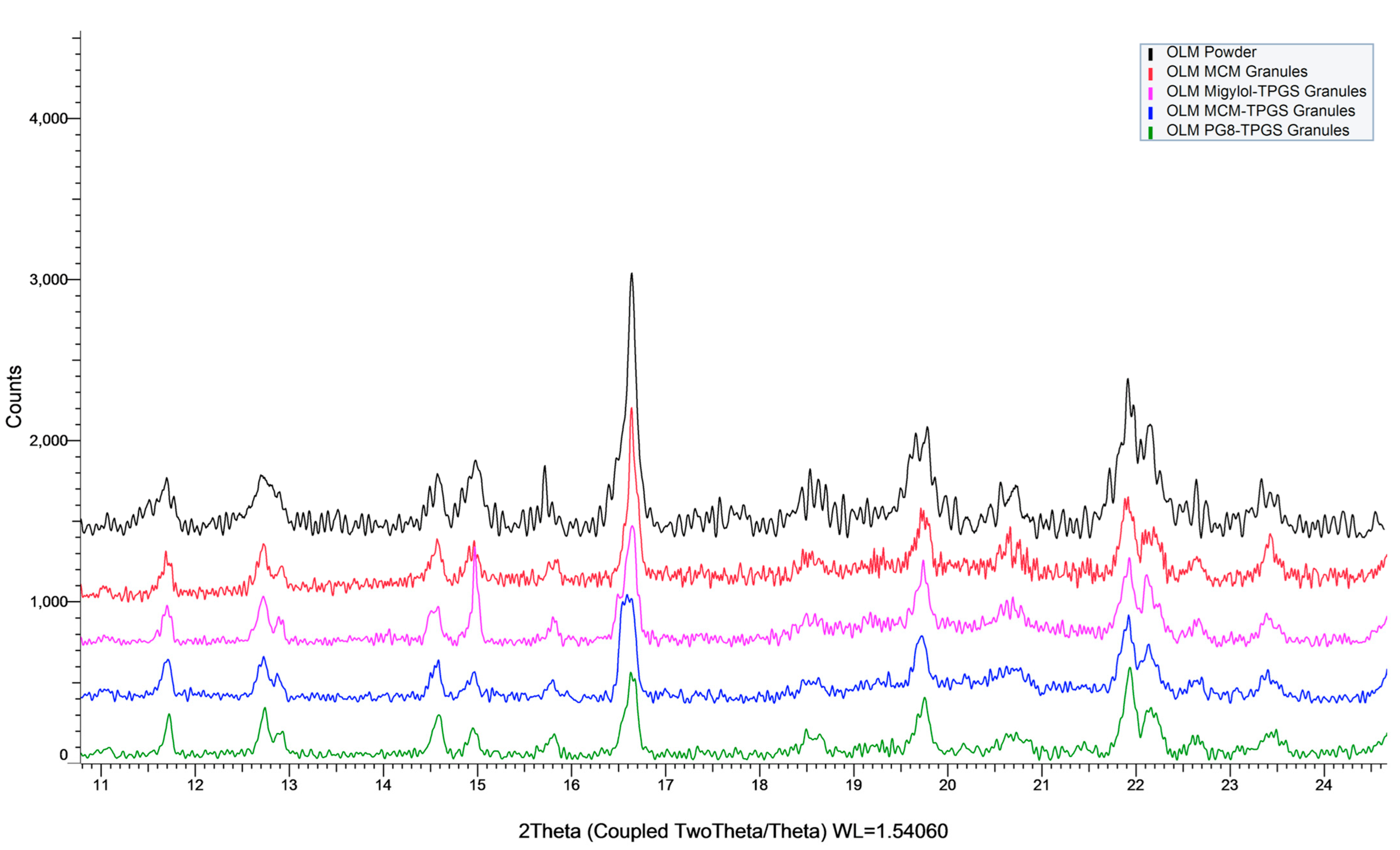
The physical state of OLM within OLM formulations was evaluated visual observation during the formulation preparation and measured by powder X-ray diffraction (XRD). XRD was performed using a Bruker D8 Advance (Bruker, Karlsruhe, Germany) to determine the physical state of OLM in the formulations. The measurements were conducted over a 2θ range of 8–25° with a scan rate of 1.0 s per step.
2.8. Two-Step Dissolutions of OLM Formulations in the Absence and Presence of an Esterase
Two two-step dissolution studies were performed as previously reported with modification [
24] to evaluate the release of OLM from its formulation and elucidate the mechanisms underlying absorption enhancement. Since the pH conditions in the human GI tract differ from those in rats, two sets of dissolution media were employed. The first set consisted of simulated gastric fluid (SGF, pH 1.2) followed by simulated intestinal fluid (SIF, pH 6.8), mimicking the pH conditions of the human stomach and intestine [
24]. The second set used SGF at pH 3.5 and followed by SIF at pH 6 to replicate the GI environment in rats. Additionally, to assess the impact of enzyme-mediated hydrolysis on OLM, dissolution studies were conducted both in the absence and presence of CE, an esterase responsible for hydrolyzing OLM as well as mono- and di-glycerides in the small intestine and cells [
13,
19]. Because CE is present in the small intestine, CE was added into the second step of dissolution in pH 6.8 (human) or pH 6 (rat).
For the dissolution studies conducted in the absence of CE, the procedures were performed to mimic human and rat GI conditions. To mimic the human GI conditions, OLM formulation (50 mg) was added into 4.5 mL SGF at pH 1.2 at 37 °C stirred at 100 rpm. Samples of 200 µL were collected at 15, 30, 45, and 60 min, and replaced with fresh medium after each collection. At 60 min, 4.5 mL of KH2PO4 (99.9 mM) and 250 µL NaOH (2M) were added to adjust the medium to SIF at pH 6.8. At 65, 90, 120, 150, and 180 min, 200 µL samples were withdrawn and replaced with fresh medium. To mimic the rat GI conditions, OLM formulation (50 mg) was added into 5 mL SGF at pH 3.5 at 37 °C stirred at 100 rpm. At 15, 30, 45, and 60 min, 200 µL samples were collected and replaced with fresh medium after each collection. At 60 min, 150 µL NaOH (2M) were added into to adjust the medium to SIF at pH 6. At 65, 90, 120, 150, and 180 min, 200 µL samples were withdrawn and replaced with fresh medium. For both conditions, collected samples were centrifuged at 15,000 rpm for 10 min, and the supernatants were diluted with the HPLC mobile phase at a 1:10 ratio. The diluted samples were analyzed using HPLC.
For the dissolution studies conducted in the presence of CE, the same procedure was followed with modification. At 65 min, after sample collections, 10 mg of CE were added to produce a final enzyme concentration of 2 mg/mL. Then samples were collected at 75, 90, 120, 150, and 180 min. The collected samples were centrifuged at 15,000 rpm for 10 min. The supernatants were then mixed with methanol at a 1:1 ratio (v/v) and then centrifuged again to remove precipitated CE. The final supernatants were diluted with the HPLC mobile phase at a 1:10 ratio (v/v) before analysis using HPLC. All experiments were performed in triplicate.
2.9. Pharmacokinetics of OLM Formulations in Rats
PK of OLM formulations were evaluated in male Sprague-Dawley rats (n = 4–6 per group). The rats were randomly assigned to groups and administered OLM formulations by oral gavage at a dose of 2 mg/kg OLM. Prior to dosing, OLM formulations were mixed with water to create suspensions. Blood samples were collected at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, and 24 h post-dosing in heparin-coated tubes and immediately centrifuged at 4000 rpm for 5 min at 4 °C to separate plasma. Plasma samples were stored at −80 °C until analysis.
Because OLM is rapidly and completely converted to OL in the body and only OL is detectable in the body [
18], OL concentrations in plasma and tissue samples were measured using a validated LC-MS method. Plasma samples were mixed with paclitaxel (internal standard) and extracted with a methanol-acetonitrile solution (1:3,
v/
v) containing 0.1% formic acid. The mixture was shaken at room temperature for 30 min, followed by centrifugation at 15,000 rpm for 10 min. The supernatants were analyzed using an LC-MS/MS system (Shimadzu LCMS-8060 triple quadrupole mass spectrometer). The LC system included pumps A and B (LC-30AD) and an autosampler (SIL-30AC, Shimadzu Scientific Instruments, Tokyo, Japan). The analysis was performed in positive electrospray ionization mode (+ESI) with multiple reaction monitoring (MRM). Instrument parameters were optimized as follows: interface voltage at 4.0 kV, interface temperature at 300 °C, DL temperature at 300 °C, heating block temperature at 400 °C, drying gas (N
2) at 10 L/min, nebulizing gas (N
2) at 3 L/min, heating gas (air) at 10 L/min, and collision-induced dissociation (CID) gas (Ar) at 230 psi. Transitions for OLM (906.5+ > 743.4+) and the internal standard (876.4+ > 308.2+) were selected based on method optimization, ensuring optimal MRM responses. Data were processed using Shimadzu LabSolutions software (v5.99). The LC mobile phases consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The gradient program progressed from 10% to 98% B over 2 min, held at 98% B for 1.2 min, and equilibrated at 10% B for 1.3 min. The flow rate was set to 0.5 mL/min with an injection volume of 5 µL. The LC-MS method was validated for linearity, precision, accuracy, carryover, and specificity as previously described [
25].
PK parameters were evaluated using non-compartmental analysis conducted with Phoenix WinNonlin software (version 8.40, Princeton, NJ, USA). The parameters included peak plasma concentration (Cmax), terminal elimination half-life (T1/2), time to peak concentration (Tmax), area under the plasma concentration-time curve from time 0 to the last measurable concentration (AUClast), area under the plasma concentration-time curve from time 0 to infinity (AUCinf), apparent volume of distribution (Vz/F), and apparent total body clearance (CL/F).
2.10. Tissue Distribution of OLM Formulations in Rats
The tissue distribution of OLM formulations was assessed in male Sprague-Dawley rats (n = 4 per group) using a validated LC-MS method. Male rats (250–300 g) were randomly assigned to groups and administered OLM formulations at a dose of 2 mg/kg. After 2 h, the rats were sacrificed, and tissues including plasma, lung, liver, kidney, brain, heart, spleen, and mesenteric lymph nodes were collected. Tissue samples were stored at −80 °C until analysis. The collected tissues were weighed and homogenized in a methanol-water mixture (70:30, v/v) containing 0.1% formic acid. Homogenates were processed using protein precipitation and extraction methods as described above. The OL concentrations in the tissue samples were quantified using the validated LC-MS method described above.
2.11. Statistical Analysis
Data are presented as mean ± standard deviation (SD). Comparisons between two groups were made using an unpaired Student’s t-test. Statistical comparisons among the six groups were performed by one-way analysis of variance (ANOVA) using Graphpad Prism (version 10, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
4. Discussion
Various OLM formulations incorporating lipids and surfactants have been studied. However, due to OLM’s low solubility in these excipients, previous formulations were limited by low drug loading, such as 2% in OLM micelles [
28] and 0.1% in solid lipid nanoparticles [
29]. Additionally, these formulations were either finished as liquids or lyophilized powders, often requiring complex preparation processes. Although OLM has a LogP of 4.31, it has low solubility in lipids and surfactants. While lipid- and surfactant-based formulation strategies are widely employed to enhance the oral absorption of water-insoluble drugs, conventional approaches such as emulsions, microemulsions, micelles, and nanoparticles are unsuitable for OLM due to these solubility limitations. In this study, we demonstrated a novel application of lipid and surfactant in a BLS to coat OLM crystals, creating solid granules with a 10% drug loading. This approach overcame the challenges of low drug loading and formulation stability. Remarkably, even though OLM remained in crystalline form, OLM MCM-TPGS solid granules significantly enhanced oral absorption, showcasing the effectiveness of this novel formulation strategy. With this approach, solubility is no longer a limiting factor when selecting lipids and surfactants, representing a significant advancement in formulation technologies, and expanding the potential applications of lipids and surfactants for water-insoluble drugs.
Ester prodrugs are commonly used to enhance solubility, permeability, and drug transport [
4,
30]. However, the primary criterion for these strategies to be effective is stability, which is particularly critical for oral ester prodrugs as they encounter varying pH conditions and enzymatic degradation in the GI tract. To reach systemic circulation, oral ester prodrugs must remain stable within the GI tract. OLM is a typical medoxomil ester prodrug, where the carboxylic group of OL is masked by a medoxomil ester to enhance permeability. Ideally, OLM remains intact in the GI tract and, after absorption, is converted to OL in the blood and tissues. However, OLM undergoes hydrolysis by water and esterases in the GI tract (
Figure 2 and
Figure 3), leading to the premature conversion of OLM to OL, loss of permeability, and consequently, low bioavailability. Because OLM exhibits pH-dependent solubility and undergoes enzymatic hydrolysis, a two-step dissolution process with esterase more accurately mimics the physiological conditions for OLM. However, most previous studies employed a one-step dissolution method, either at pH 1.2 or pH 6.8, with very few incorporating esterase into the dissolution media [
21,
28,
29]. In this study, the addition of CE to the dissolution media effectively distinguished the differences among the OLM formulations (
Figure 2C and
Figure 3C), whereas all formulations showed similar release profiles in the absence of CE (
Figure 2A and
Figure 3A). Therefore, incorporating esterase in a two-step dissolution study is essential for accurately evaluating the dissolution behavior of ester drugs and comparing different formulations. The data also suggest that OLM may be subject to a food effect, as it achieved 100% release at pH 1.2 (fasted) (
Figure 2A) but less than 3% release at pH 3.5 (fed) (
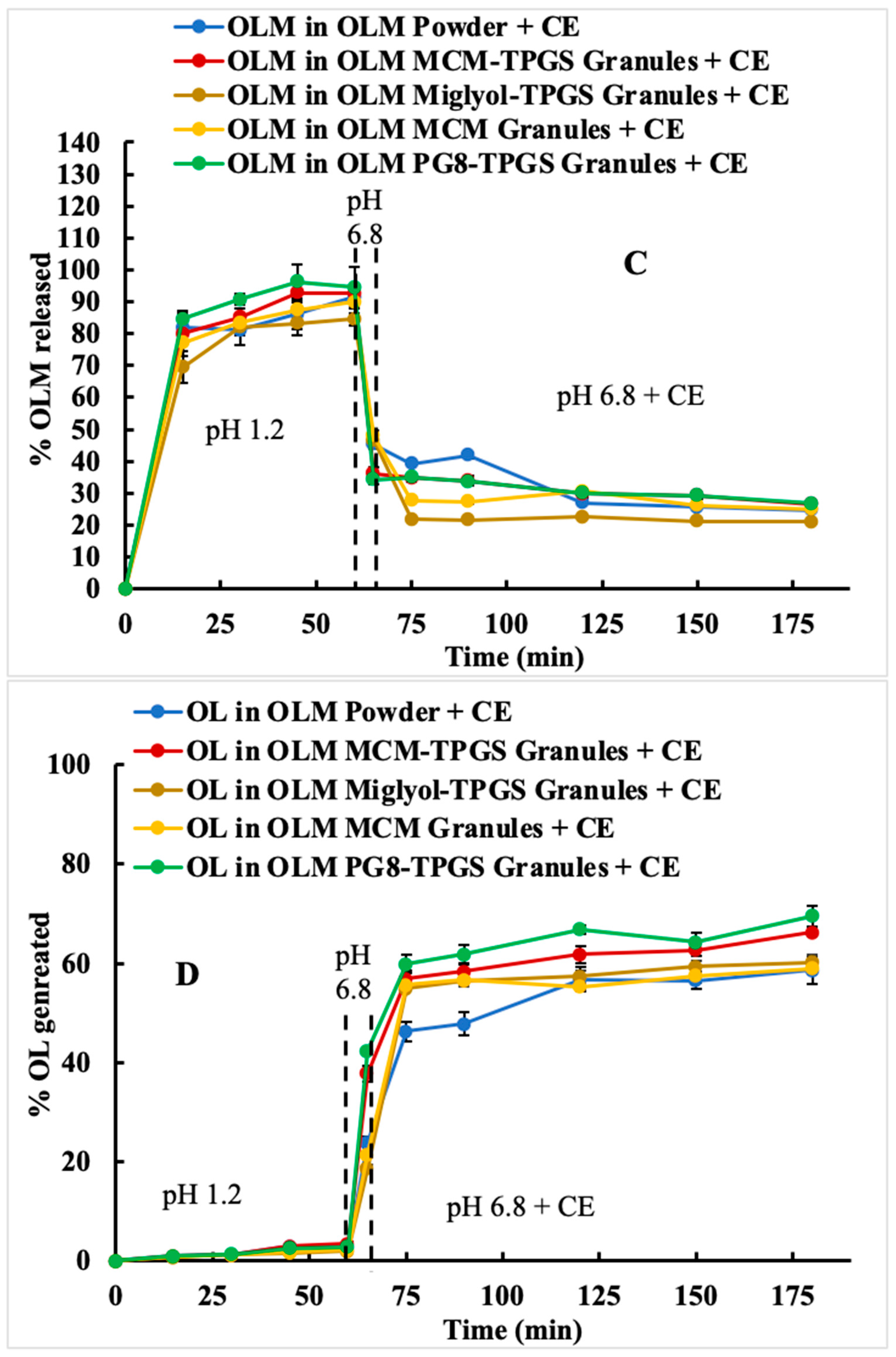
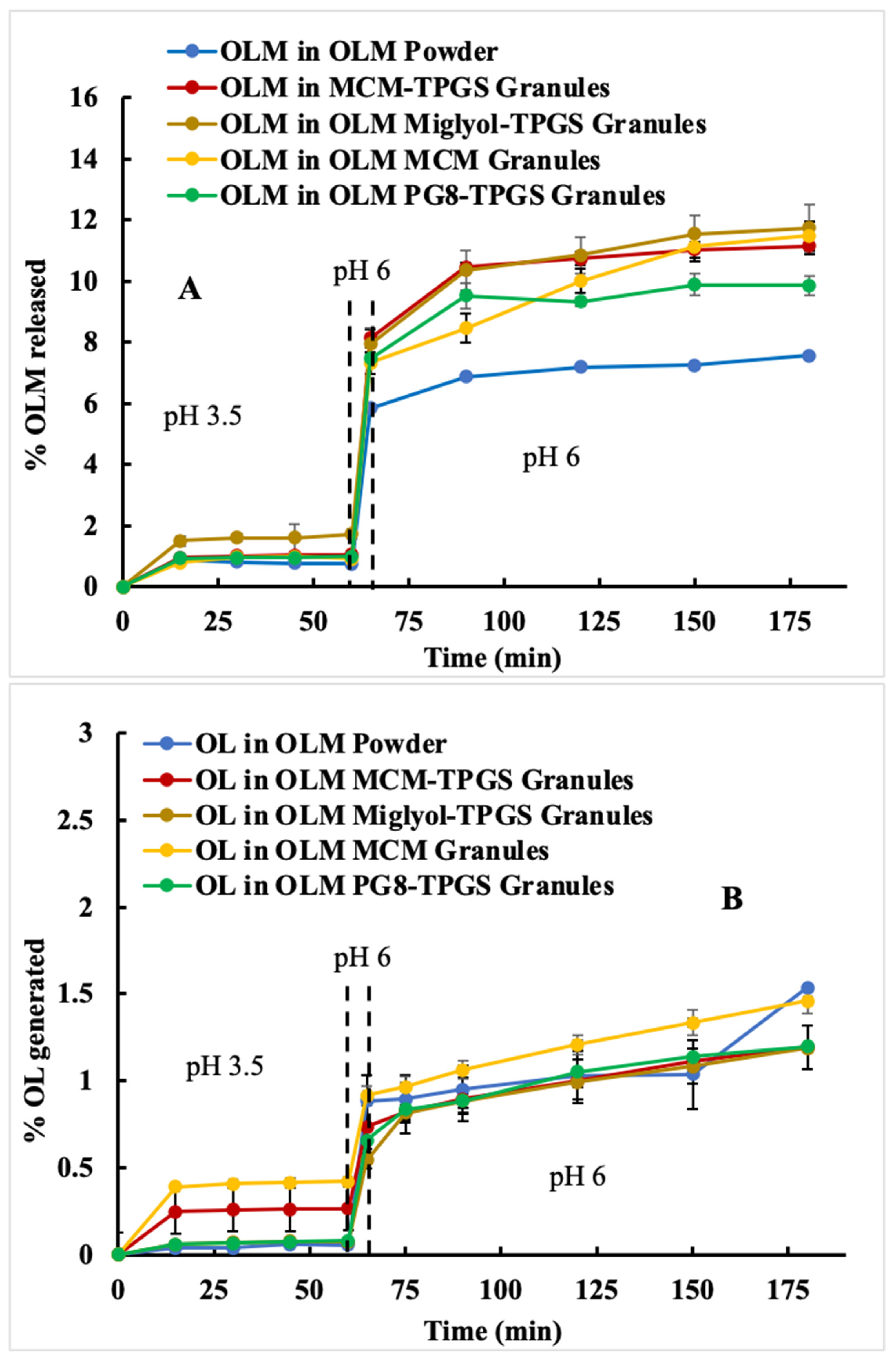
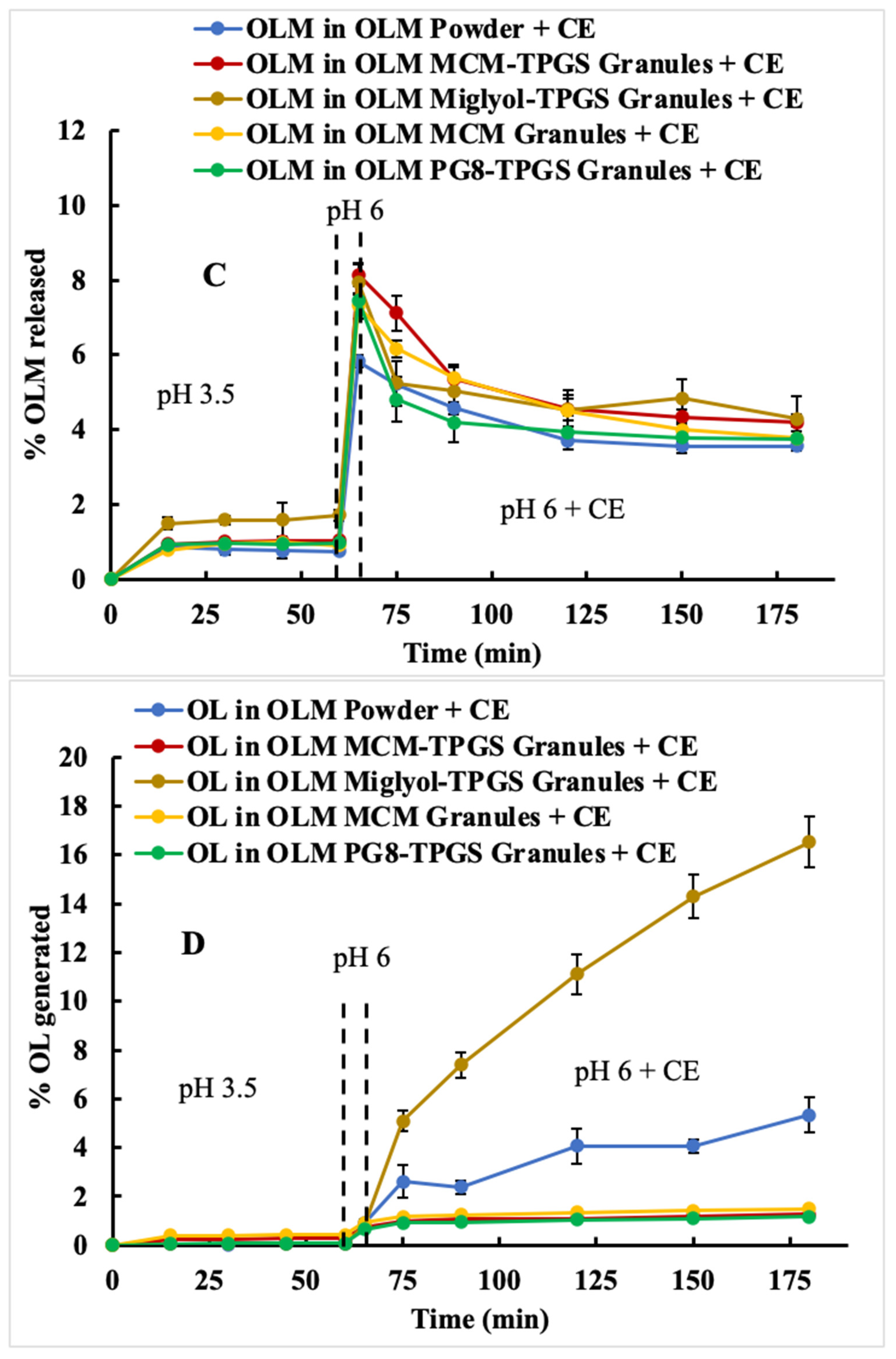
Figure 3A). However, food is not currently considered a significant factor in the administration of OLM for patients. Based on the two-step dissolution data, the potential food effect may be another contributing factor to OLM’s low bioavailability.
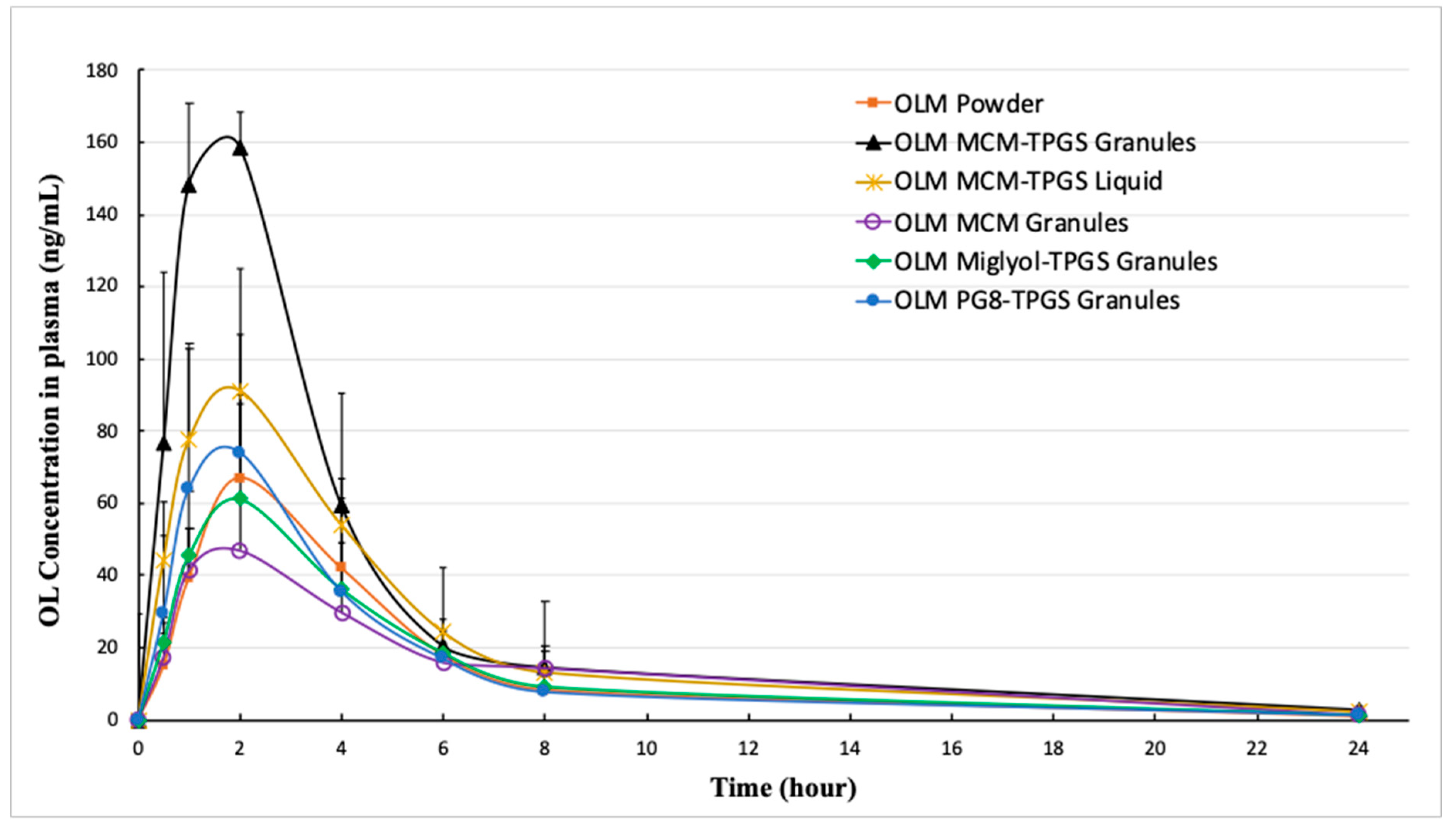
We designed three different OLM granules using various lipids. Among these, OLM MCM-TPGS granules demonstrated our hypothesis that coating OLM crystals with a BLS increases OLM solubility and protects it from aqueous and CE-mediated degradation. OLM MCM-TPGS granules significantly increased drug release and protected OLM from CE-mediated degradation in two-step dissolution studies (
Figure 2C and
Figure 3C). PK studies confirmed that OLM MCM-TPGS granules enhanced oral absorption by 145% compared to OLM powder (
Table 3). OLM has been solubilized in self-microemulsifying drug delivery systems (SMEDDS) and solid lipid nanoparticles, with relative bioavailability increases of 171% [
21] and 232% [
29], respectively, compared to OLM powder. In comparison, the relative bioavailability of OLM MCM-TPGS granules was 245% relative to OLM powder. Notably, although OLM remained in its crystalline form in the MCM-TPGS granules, the granules achieved higher bioavailability than the previously reported formulations where OLM was solubilized. This highlights the effectiveness of this novel strategy in enhancing the oral absorption of ester prodrugs.
Detailed analysis of the effects of lipid composition, TPGS, and the solid granule format revealed the importance of the BLS composition and solid form. OLM MCM granules failed to protect OLM from CE-mediated degradation (
Figure 2C and
Figure 3C) and did not enhance oral absorption (
Table 3). Similarly, substituting Capmul MCM C8 with Miglyol 812 or Capmul PG-8 did not improve oral absorption (
Table 3). Moreover, the liquid BLS formulation without Aeroperl 300, composed of Capmul MCM C8 and TPGS, also failed to enhance oral absorption (
Table 3). Capmul MCM C8, composed of mono- and diglycerides, directly enters the bloodstream or undergoes minor digestion to free fatty acids by esterases in the small intestine. In contrast, Miglyol 812, a triglyceride, is extensively digested in the small intestine by esterases into diglycerides, monoglycerides, and eventually free fatty acids. Thus, Capmul MCM C8 is more resistant to CE-mediated degradation compared to Miglyol 812. Capmul PG-8, a propylene glycol derivative, is absorbed through the GI tract and metabolized in the liver. In two-step dissolution studies (
Figure 3C), both Capmul MCM C8 and Capmul PG-8 demonstrated protection against CE-mediated hydrolysis. However, Miglyol 812 was rapidly digested by CE, causing the BLS coating composed of Miglyol 812 and TPGS to break down quickly, providing no protective effect (
Figure 3C,D). Although OLM PG8-TPGS LSG and OLM MCM-TPGS LSG showed similar release profiles (
Figure 3A,C), Capmul MCM C8 exhibited higher lipophilicity and has potential to increase permeability [
31], thereby leading to 2-fold increase in oral absorption (
Table 3). An in vitro-in vivo correlation (IVIVC) could not be established for OLM LSG, consistent with prior reports that IVIVC for lipid-based formulations is difficult due to their complex, digestion-dependent absorption [
32]. We also observed potential food effects for OLM, which further complicate IVIVC. To better evaluate IVIVC for OLM LSG, future dissolution testing should incorporate lipases and relevant food components in the dissolution medium. It is known that long-chain triglycerides or fatty acids increase lymphatic drug uptake through chylomicron formation, whereas median-chain glycerides are rapidly hydrolyzed and facilitate drug absorption directly into the blood circulation [
33,
34]. Capmul MCM C8 is a median-chain glyceride. As shown in our results, OLM MCM-TPGS granules increased plasma and tissue uptake (
Figure 5) but did not increase the lymphatic uptake. This indicates that the oral bioavailability improvement achieved with OLM MCM-TPGS granules primarily arises from enhanced dissolution and protection against both aqueous and CE-mediated degradation. The proposed mechanisms are illustrated in
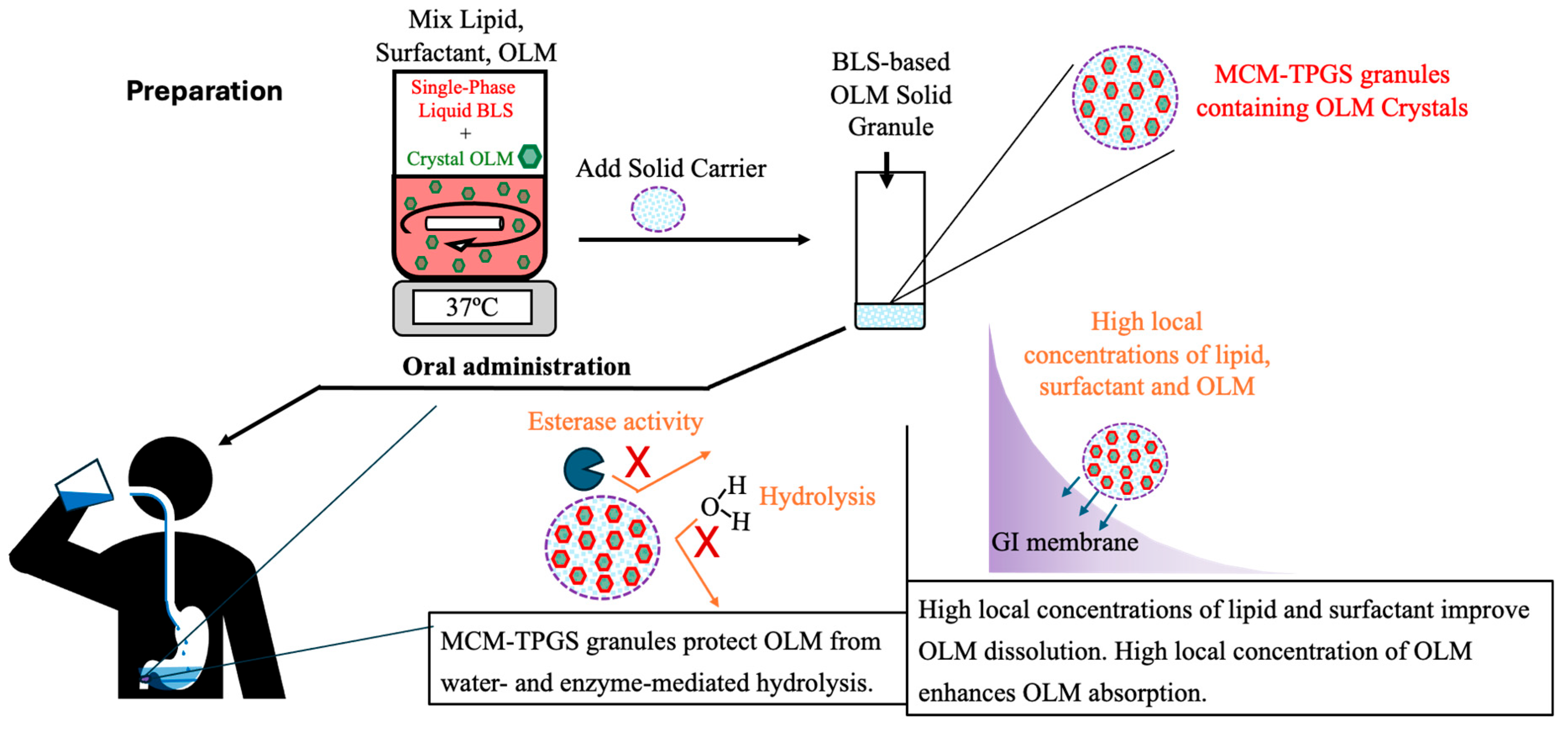
Figure 6. After oral administration, the coating of BLS protects OLM from water- and enzyme-mediated hydrolysis. When BLS-based OLM granules reach the GI membrane, MCM C8 and TPGS are released from the granules, generating high local concentrations of MCM C8 and TPGS that enhance OLM dissolution. Because OLM is released in a specific site, its local concentration increases, thereby facilitating OLM absorption.
OLM represents the first ester prodrug we have investigated using this coating strategy to improve oral absorption of crystalline drug forms. By formulating drug crystals into solid granules, this approach effectively addresses two challenges of lipid-based formulations such as low drug loading and poor stability. Importantly, the BLS-based granules use FDA-approved excipients, and the manufacturing process is simple, scalable and compatible with conventional pharmaceutical equipment (
Figure 6). Given that many marketed ester prodrugs have poor bioavailability due to premature hydrolysis, the strategy presented here offers a generalizable formulation-based solution to prevent both water- and enzyme-mediated degradation while simultaneously improving solubility. Rather than modifying the chemical structures of ester prodrugs, this approach leverages formulation design to overcome these limitations. Thus, BLS-based formulation technology shows strong promise for advancing clinical use of ester prodrugs, potentially enabling more effective and reliable oral therapies.