- Review
Cationic Gemini Surfactants in the Oil Industry: Applications in Extraction, Transportation and Refinery Products
- Bogumił Brycki,
- Adrianna Szulc and
- Justyna Brycka
- + 1 author
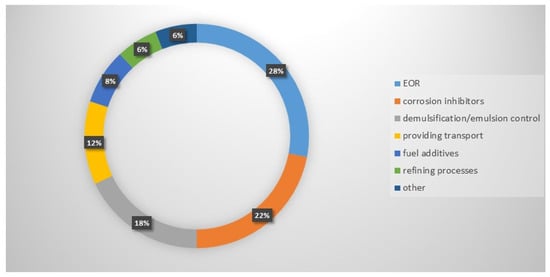
The petroleum industry faces intensifying challenges related to the depletion of easily accessible reservoirs and the growing energy demand, necessitating the adoption of advanced chemical agents that can operate under extreme conditions. Cationic gemini surfactants, characterized by their unique dimeric architecture consisting of two hydrophilic head groups and two hydrophobic tails, have emerged as superior alternatives to conventional monomeric surfactants due to their enhanced interfacial activity and physicochemical resilience. This review provides a comprehensive analysis of the literature concerning the molecular structure, synthesis, and functional applications of cationic gemini surfactants across the entire oil value chain, from extraction to refining. The analysis reveals that gemini surfactants exhibit critical micelle concentrations significantly lower than their monomeric analogs and maintain stability in high-temperature and high-salinity environments. They demonstrate exceptional efficacy in enhanced oil recovery through ultra-low interfacial tension reduction and wettability alteration, while simultaneously serving as effective drag reducers, wax inhibitors, and dual-action biocidal corrosion inhibitors in transportation pipelines. Cationic gemini surfactants represent a transformative class of multifunctional materials for the oil industry.
27 December 2025