Conformational Evolution of Bicalutamide in Chloroform: A Comprehensive NMR Study
Abstract
1. Introduction
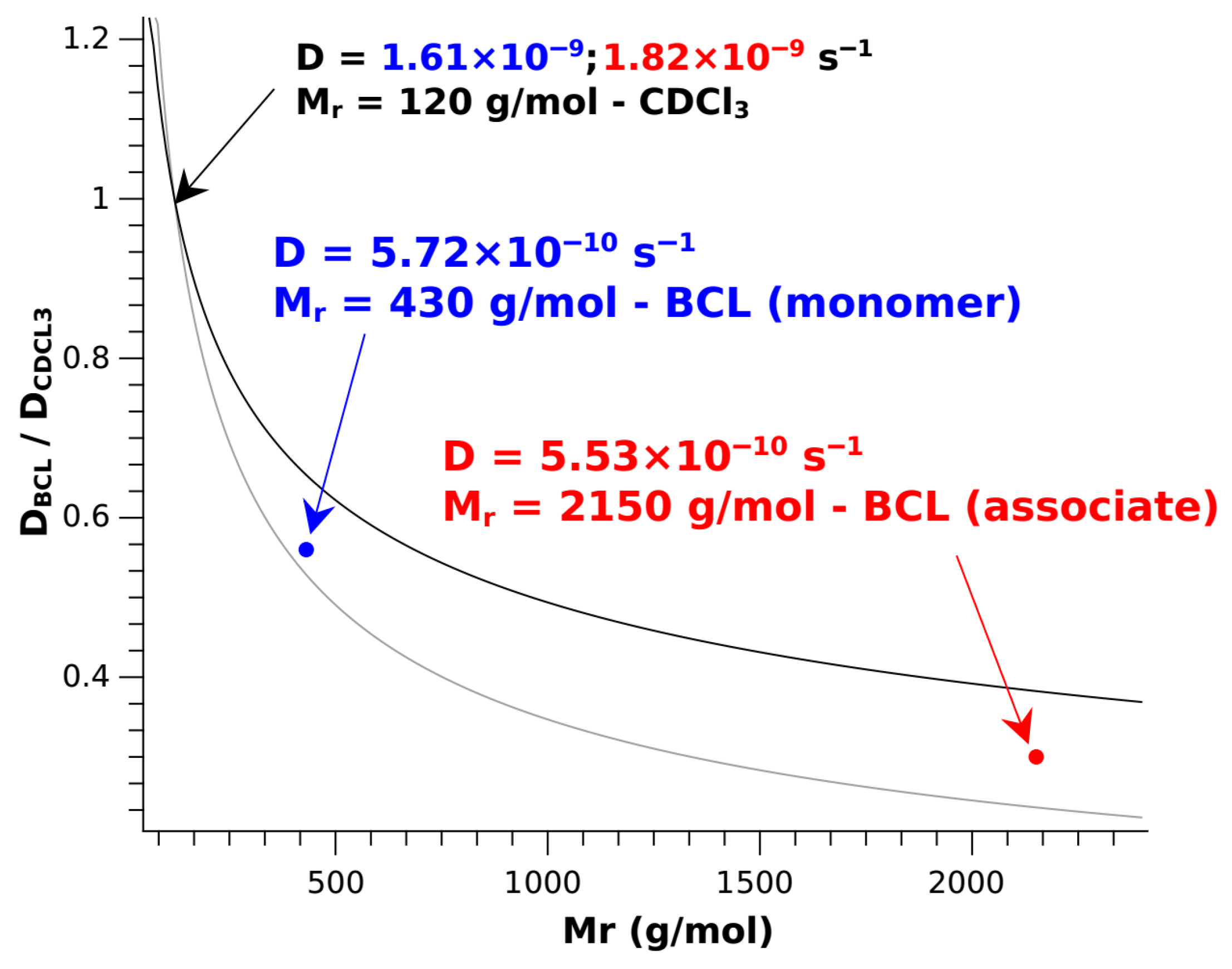
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, R.; Qin, X.; Lin, J.; Wang, L.; Wu, S.; Gong, J. Discovery and Selective Nucleation of Polymorphs of Flexible GABA Molecules: Solvation Effects and Conformational Rearrangement. Cryst. Growth Des. 2024, 24, 8505–8515. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Huang, X.; Li, X.; Zong, S.; Wang, N.; Hao, H. Conformational Selectivity and Evolution Affected by the Desolvation Process. Cryst. Growth Des. 2022, 22, 1283–1291, Erratum in Cryst. Growth Des. 2023, 23, 2011–2012 https://doi.org/10.1021/ACS.CGD.3C00061. [Google Scholar] [CrossRef]
- Tian, B.; Hao, H.; Huang, X.; Wang, T.; Wang, J.; Yang, J.; Li, X.; Li, W.; Zhou, L.; Wang, N. Solvent Effect on Molecular Conformational Evolution and Polymorphic Manipulation of Cimetidine. Cryst. Growth Des. 2023, 23, 7266–7275. [Google Scholar] [CrossRef]
- Shi, P.; Xu, S.; Ma, Y.; Tang, W.; Zhang, F.; Wang, J.; Gong, J. Probing the Structural Pathway of Conformational Polymorph Nucleation by Comparing a Series of a,x-Alkanedicarboxylic Acids. IUCrJ 2020, 7, 422–433. [Google Scholar] [CrossRef]
- Tiruveedi, V.; Prasad, S.R.; Swain, D.; Nechipadappu, S.K.; Balasubramanian, S. Third Polymorph and Salt Complexes of Teriflunomide-A Multiple Sclerosis Drug. J. Mol. Struct. 2025, 1347, 143371. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Shi, P.; Han, Y.; Zhu, Z.; Gong, J. Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique. Crystals 2023, 13, 1206. [Google Scholar] [CrossRef]
- Nangia, A. Conformational Polymorphism in Organic Crystals. Acc. Chem. Res. 2008, 41, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Dyba, A.J.; Janczak, J.; Morritt, A.; Fábián, L.; Karolewicz, B.; Khimyak, Y.Z.; Braun, D.E.; Nartowski, K.P. Directing Crystallization Outcomes of Conformationally Flexible Molecules: Polymorphs, Solvates, and Desolvation Pathways of Fluconazole. Mol. Pharm. 2022, 19, 456–471. [Google Scholar] [CrossRef]
- Wu, D.; Li, S.; Feng, Y.; Chen, C.; Fu, L.; Chen, G.; Wang, N.; Wang, T.; Huang, X.; Hao, H. Unveiling the Conformational Landscape of Tizanidine and Its Effects on Selective Nucleation: Enabling Controlled Manipulation of Polymorphs and Solvates. Sep. Purif. Technol. 2025, 354, 128827. [Google Scholar] [CrossRef]
- Zeglinski, J.; Kuhs, M.; Khamar, D.; Hegarty, A.C.; Devi, R.K.; Rasmuson, Å.C. Crystal Nucleation of Tolbutamide in Solution: Relationship to Solvent, Solute Conformation, and Solution Structure. Chem.—A Eur. J. 2018, 24, 4916–4926. [Google Scholar] [CrossRef]
- Pons Siepermann, C.A.; Myerson, A.S. Inhibition of Nucleation Using a Dilute, Weakly Hydrogen-Bonding Molecular Additive. Cryst. Growth Des. 2018, 18, 3584–3595. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-Nucleation Clusters as Solute Precursors in Crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Grünwald, M. Pre-Nucleation Clusters Predict Crystal Structures in Models of Chiral Molecules. J. Am. Chem. Soc. 2021, 143, 21580–21593. [Google Scholar] [CrossRef]
- Dighe, A.V.; Coliaie, P.; Podupu, P.K.R.; Singh, M.R. Selective Desolvation in Two-Step Nucleation Mechanism Steers Crystal Structure Formation. Nanoscale 2022, 14, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Oparin, R.D.; Krestyaninov, M.A.; Ivlev, D.V.; Kiselev, M.G. Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in ScCO2. Materials 2023, 16, 1403. [Google Scholar] [CrossRef] [PubMed]
- Khodov, I.A.; Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Kiselev, M.G. Pressure Effect on Lidocaine Conformational Equilibria in ScCO2: A Study by 2D NOESY. J. Mol. Liq. 2022, 367, 120525. [Google Scholar] [CrossRef]
- Koperwas, K.; Tu, W.; Affouard, F.; Adrjanowicz, K.; Kaskosz, F.; Paluch, M. Pressure Dependence of the Crystallization Rate for the S-Enantiomer and a Racemic Mixture of Ibuprofen. Cryst. Growth Des. 2021, 21, 7075–7086. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.E.; Cole, J.C.; Cruz-Cabeza, A.J. Conformational Change in Molecular Crystals: Impact of Solvate Formation and Importance of Conformational Free Energies. Cryst. Growth Des. 2021, 21, 6924–6936. [Google Scholar] [CrossRef]
- Cordova, I.W.; Teixeira, G.; Ribeiro-Claro, P.J.A.; Abranches, D.O.; Pinho, S.P.; Ferreira, O.; Coutinho, J.A.P. Using Molecular Conformers in COSMO-RS to Predict Drug Solubility in Mixed Solvents. Ind. Eng. Chem. Res. 2024, 63, 9565–9575. [Google Scholar] [CrossRef]
- Szczurek, J.; Rams-Baron, M.; Knapik-Kowalczuk, J.; Antosik, A.; Szafraniec, J.; Jamróz, W.; Dulski, M.; Jachowicz, R.; Paluch, M. Molecular Dynamics, Recrystallization Behavior, and Water Solubility of the Amorphous Anticancer Agent Bicalutamide and Its Polyvinylpyrrolidone Mixtures. Mol. Pharm. 2017, 14, 1071–1081. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular Disorder of Bicalutamide—Amorphous Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Blokhina, S.V.; Manin, N.G.; Volkova, T.V.; Tkachev, V.V. Polymorphism and Solvatomorphism of Bicalutamide: Thermophysical Study and Solubility. J. Therm. Anal. Calorim. 2013, 111, 655–662. [Google Scholar] [CrossRef]
- Vega, D.R.; Polla, G.; Martinez, A.; Mendioroz, E.; Reinoso, M. Conformational Polymorphism in Bicalutamide. Int. J. Pharm. 2007, 328, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bis, J.A.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Hydroxyl···Pyridine Hydrogen Bonds in Cocrystals That Contain a Cyano Acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar] [CrossRef]
- Bohl, C.E.; Gao, W.; Miller, D.D.; Bell, C.E.; Dalton, J.T. Structural Basis for Antagonism and Resistance of Bicalutamide in Prostate Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.R.; Gu, J.M. N-[4-Cyano-3-(Trifluoromethyl)Phenyl]-3-(4-Fluorophenylsulfonyl) -2-Hydroxy-2-Methylpropionamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o3897–o3898. [Google Scholar] [CrossRef]
- Belov, K.V.; Khodov, I.A. Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review. Molecules 2025, 30, 3793. [Google Scholar] [CrossRef]
- Borrego-Munõz, P.; Becerra, L.D.; Ospina, F.; Coy-Barrera, E.; Quiroga, D. Synthesis (Z) vs (E) Selectivity, Antifungal Activity against Fusarium Oxysporum, and Structure-Based Virtual Screening of Novel Schiff Bases Derived from l-Tryptophan. ACS Omega 2022, 7, 24714–24726. [Google Scholar] [CrossRef] [PubMed]
- Ulutürk, M.; Karabacak Atay, Ç.; Tilki, T.; Dede, B. Structural Properties, Molecular Modeling Studies and Antioxidant Activities of Novel Benzoic Acid-Based Azo Molecules: A Combined in Vitro and in Silico Study. J. Mol. Struct. 2025, 1344, 142990. [Google Scholar] [CrossRef]
- Belov, K.V.; Khodov, I.A. NOESY Investigation of Bicalutamide Conformational Changes in DMSO: Role of Solution Concentration in Polymorph Formation. J. Mol. Liq. 2025, 422, 126896. [Google Scholar] [CrossRef]
- Yamasaki, R.; Tanatani, A.; Azumaya, I.; Masu, H.; Yamaguchi, K.; Kagechika, H. Solvent-Dependent Conformational Switching of N-Phenylhydroxamic Acid and Its Application in Crystal Engineering. Cryst. Growth Des. 2006, 6, 2007–2010. [Google Scholar] [CrossRef]
- Mololina, A.A.; Sobornova, V.V.; Belov, K.V.; Krestyaninov, M.A.; Khodov, I.A. Role of Non-Covalent Interactions in the Conformational Stability of Bicalutamide in Different Solvent Environments: Insights from Quantum-Chemical Calculations and NMR Spectroscopy. J. Mol. Liq. 2025, 423, 126921. [Google Scholar] [CrossRef]
- Pronina, Y.A.; Stepakov, A.V.; Berezhnaya, E.V.; Boitsov, V.M.; Baichurin, R.I.; Selivanov, S.I. The Applicability of NMR Spectroscopy in the Determination of Structures Containing a Fragment of Spiro[1-Azabicyclo[3.3.0]Octane]. Appl. Magn. Reson. 2025, 56, 937–953. [Google Scholar] [CrossRef]
- Klochkov, V.V.; Karatayeva, F.K.; Shaikhutdinov, R.A.; Khairutdinov, B.I.; Molins, M.A.; Pons, M. Separation of Cross-Relaxation and Exchange in Two-Site Spin Systems without Resolved Couplings. Appl. Magn. Reson. 2002, 22, 431–438. [Google Scholar] [CrossRef]
- Gadiev, T.A.; Khairutdinov, B.I.; Shaikhutdinov, R.A.; Karatayeva, F.K.; Aganov, A.V.; Klochkov, V.V. Spatial Structure of Dimeric Capsules of Tetraurea Calix[4]Arenes in Solutions According to 2-D NMR (NOESY) Spectroscopy. Appl. Magn. Reson. 2003, 25, 347–352. [Google Scholar] [CrossRef]
- Butts, C.P.; Jones, C.R.; Towers, E.C.; Flynn, J.L.; Appleby, L.; Barron, N.J.; Barrona, N.J. Interproton Distance Determinations by NOE—Surprising Accuracy and Precision in a Rigid Organic Molecule. Org. Biomol. Chem. 2011, 9, 177–184. [Google Scholar] [CrossRef]
- Belov, K.V.; Batista de Carvalho, L.A.E.; Dyshin, A.A.; Efimov, S.V.; Khodov, I.A. The Role of Hidden Conformers in Determination of Conformational Preferences of Mefenamic Acid by NOESY Spectroscopy. Pharmaceutics 2022, 14, 2276. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Krestyaninov, M.A.; Sobornova, V.V.; Dyshin, A.A.; Kiselev, M.G. Does DMSO Affect the Conformational Changes of Drug Molecules in Supercritical CO2 Media? J. Mol. Liq. 2023, 384, 122230. [Google Scholar] [CrossRef]
- Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Efimov, S.V.; Khodov, I.A.; Kiselev, M.G. Conformational Preferences of Tolfenamic Acid in DMSO-CO2 Solvent System by 2D NOESY. J. Mol. Liq. 2022, 367, 120481. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D. The Interaction of Small Molecules with Phospholipid Membranes Studied by 1H NOESY NMR under Magic-Angle Spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Flasche, W.; Cismas, C.; Rost, M.; Herrmann, A.; Liebscher, J.; Huster, D. Design and Application of Lipophilic Nucleosides as Building Blocks to Obtain Highly Functional Biological Surfaces. J. Phys. Chem. B 2004, 108, 16279–16287. [Google Scholar] [CrossRef]
- Ma, T.; Chen, S.; Zhuang, Y.; Xiao, F.; Wei, J.; Wan, Z.; Qin, M.; Sun, T.; Chen, J. Experimental Crystal Structure, Conformational and Spectroscopic Studies of Allisartan Isoproxil Accomplished with DFT Calculations. J. Mol. Struct. 2025, 1347, 143289. [Google Scholar] [CrossRef]
- Dewis, L.; Crouch, R.; Russell, D.; Butts, C. Improving the Accuracy of 1H–19F Internuclear Distance Measurement Using 2D 1H–19F HOESY. Magn. Reson. Chem. 2019, 57, 1143–1149. [Google Scholar] [CrossRef]
- Yemloul, M.; Bouguet-Bonnet, S.; Ba, L.A.C.; Kirsch, G.; Canet, D. Selective HOESY Experiments for Stereochemical Determinations. Magn. Reson. Chem. 2008, 46, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Krishna, N.R. Influence of Conformational Exchange on the 2D NOESY Spectra of Biomolecules Existing in Multiple Conformations. J. Magn. Reson. 1992, 98, 36–48. [Google Scholar] [CrossRef]
- Butts, C.P.; Jones, C.R.; Song, Z.; Simpson, T.J. Accurate NOE-Distance Determination Enables the Stereochemical Assignment of a Flexible Molecule—Arugosin C. Chem. Commun. 2012, 48, 9023–9025. [Google Scholar] [CrossRef]
- Novikova, D.; Al Mustafa, A.; Grigoreva, T.; Vorona, S.; Selivanov, S.; Tribulovich, V. NMR-Verified Dearomatization of 5,7-Substituted Pyrazolo[1,5-a]Pyrimidines. Molecules 2023, 28, 6584. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Krishnamurthy, K. Revisiting the Initial Rate Approximation in Kinetic NOE Measurements. J. Magn. Reson. 2006, 182, 173–177. [Google Scholar] [CrossRef]
- Borgias, B.A.; Gochin, M.; Kerwood, D.J.; James, T.L. Relaxation Matrix Analysis of 2D NMR Data. Prog. Nucl. Magn. Reson. Spectrosc. 1990, 22, 83–100. [Google Scholar] [CrossRef]
- Bame, J.; Hoeck, C.; Carrington, M.J.; Butts, C.P.; Jäger, C.M.; Croft, A.K. Improved NOE Fitting for Flexible Molecules Based on Molecular Mechanics Data—A Case Study with S-Adenosylmethionine. Phys. Chem. Chem. Phys. 2018, 20, 7523–7531. [Google Scholar] [CrossRef]
- Macur, S.; Farmer, B.T.; Brown, L.R. An Improved Method for the Determination of Cross-Relaxation Rates from NOE Data. J. Magn. Reson. 1986, 70, 493–499. [Google Scholar] [CrossRef]
- Esposito, G.; Pastore, A. An Alternative Method for Distance Evaluation from NOESY Spectra. J. Magn. Reson. 1988, 76, 331–336. [Google Scholar] [CrossRef]
- Klochkov, V.V.; Khairutdinov, B.I.; Shaikhutdinov, R.A.; Findaisen, M.; Berger, S. Geometric Structure of 2-Phenyl-1,3-Dithia-5,6-Benzocycloheptene 1-Oxide. Russ. J. Gen. Chem. 2001, 71, 1266–1268. [Google Scholar] [CrossRef]
- Efimov, S.V.; Khodov, I.A.; Ratkova, E.L.; Kiselev, M.G.; Berger, S.; Klochkov, V.V. Detailed NOESY/T-ROESY Analysis as an Effective Method for Eliminating Spin Diffusion from 2D NOE Spectra of Small Flexible Molecules. J. Mol. Struct. 2016, 1104, 63–69. [Google Scholar] [CrossRef]
- Kolmer, A.; Edwards, L.J.; Kuprov, I.; Thiele, C.M. Conformational Analysis of Small Organic Molecules Using NOE and RDC Data: A Discussion of Strychnine and α-Methylene-γ-Butyrolactone. J. Magn. Reson. 2015, 261, 101–109. [Google Scholar] [CrossRef]
- Butts, C.P.; Jones, C.R.; Harvey, J.N. High Precision NOEs as a Probe for Low Level Conformers—A Second Conformation of Strychnine. Chem. Commun. 2011, 47, 1193–1195. [Google Scholar] [CrossRef]
- Sternberg, U.; Witter, R. Structures Controlled by Entropy: The Flexibility of Strychnine as Example. Molecules 2022, 27, 7987. [Google Scholar] [CrossRef] [PubMed]
- Pronina, Y.A.; Stepakov, A.V.; Popova, E.A.; Boitsov, V.M.; Baichurin, R.I.; Selivanov, S.I. Diastereomers of Cyclopropa[a]Pyrrolizine Spiro-Fused with a Benzo[4,5]Imidazo[1,2-a]Indole Fragment: Structure Determinations Using NMR Methods. Appl. Magn. Reson. 2023, 54, 999–1014. [Google Scholar] [CrossRef]
- Khodov, I.; Dyshin, A.; Efimov, S.; Ivlev, D.; Kiselev, M. High-Pressure NMR Spectroscopy in Studies of the Conformational Composition of Small Molecules in Supercritical Carbon Dioxide. J. Mol. Liq. 2020, 309, 113113. [Google Scholar] [CrossRef]
- Bauer, C.J.; Frenkiel, T.A.; Lane, A.N. A Comparison of the ROESY and NOESY Experiments for Large Molecules, with Application to Nucleic Acids. J. Magn. Reson. 1990, 87, 144–152. [Google Scholar] [CrossRef]
- Rakhmatullin, I.Z.; Galiullina, L.F.; Balandina, A.A.; Garipov, M.R.; Strelnik, A.D.; Galiullina, A.S.; Shtyrlin, Y.G.; Klochkov, V.V. Stereodynamics of Some Pyridoxine Derivatives. Magn. Reson. Chem. 2017, 55, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatullin, I.Z.; Galiullina, L.F.; Garipov, M.R.; Strel’Nik, A.D.; Shtyrlin, Y.G.; Klochkov, V.V. Dynamic NMR Study of Cyclic Derivatives of Pyridoxine. Magn. Reson. Chem. 2014, 52, 769–778. [Google Scholar] [CrossRef]
- Jones, C.R.; Greenhalgh, M.D.; Bame, J.R.; Simpson, T.J.; Cox, R.J.; Marshall, J.W.; Butts, C.P. Subtle Temperature-Induced Changes in Small Molecule Conformer Dynamics—Observed and Quantified by NOE Spectroscopy. Chem. Commun. 2016, 52, 2920–2923. [Google Scholar] [CrossRef]
- Oliva, A.I.; Gómez, K.; González, G.; Ballester, P. Diffusion-Ordered Spectroscopy (1H-DOSY) of Zn-Porphyrin Assemblies Induced by Coordination with DABCO. New J. Chem. 2008, 32, 2159–2163. [Google Scholar] [CrossRef]
- Efimov, S.V.; Zgadzay, Y.O.; Tarasova, N.B.; Klochkov, V.V. Evidence of Oligomerization of Bovine Insulin in Solution given by NMR. Eur. Biophys. J. 2018, 47, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.J.E.J.; Berger, S. HR-DOSY as a New Tool for the Study of Chemical Exchange Phenomena. Magn. Reson. Chem. 2002, 40, S122–S127. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Maltceva, O.V.; Lazovskiy, D.A.; Khodov, I.A.; Borovkov, V.; Mamardashvili, N.Z.; Koifman, O.I. Medium Viscosity Effect on Fluorescent Properties of Sn(IV)-Tetra(4-Sulfonatophenyl)Porphyrin Complexes in Buffer Solutions. J. Mol. Liq. 2019, 277, 1047–1053. [Google Scholar] [CrossRef]
- Volostnykh, M.V.; Kirakosyan, G.A.; Sinelshchikova, A.A.; Loboda, P.A.; Dorovatovskii, P.V.; Mikhaylov, M.A.; Tsivadze, A.Y.; Sokolov, M.N.; Gorbunova, Y.G. Supramolecular Hybrids Based on Ru(II) Porphyrin and Octahedral Mo(II) Iodide Cluster. Dalt. Trans. 2023, 52, 5354–5365. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, A.R.; Kuchel, P.W.; Lennon, A.J.; Chapman, B.E. NMR Diffusion Measurements to Characterise Membrane Transport and Solute Binding. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 30, 39–68. [Google Scholar] [CrossRef]
- Holz, M.; Mao, X.A.; Seiferling, D.; Sacco, A. Experimental Study of Dynamic Isotope Effects in Molecular Liquids: Detection of Translation-Rotation Coupling. J. Chem. Phys. 1996, 104, 669–679. [Google Scholar] [CrossRef]
- Dhaked, D.K.; Jain, V.; Kasetti, Y.; Bharatam, P.V. Conformational Polymorphism in Bicalutamide: A Quantum Chemical Study. Struct. Chem. 2012, 23, 1857–1866. [Google Scholar] [CrossRef]
- Sobornova, V.V.; Belov, K.V.; Krestyaninov, M.A.; Khodov, I.A. Influence of Solvent Polarity on the Conformer Ratio of Bicalutamide in Saturated Solutions: Insights from NOESY NMR Analysis and Quantum-Chemical Calculations. Int. J. Mol. Sci. 2024, 25, 8254. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedita—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
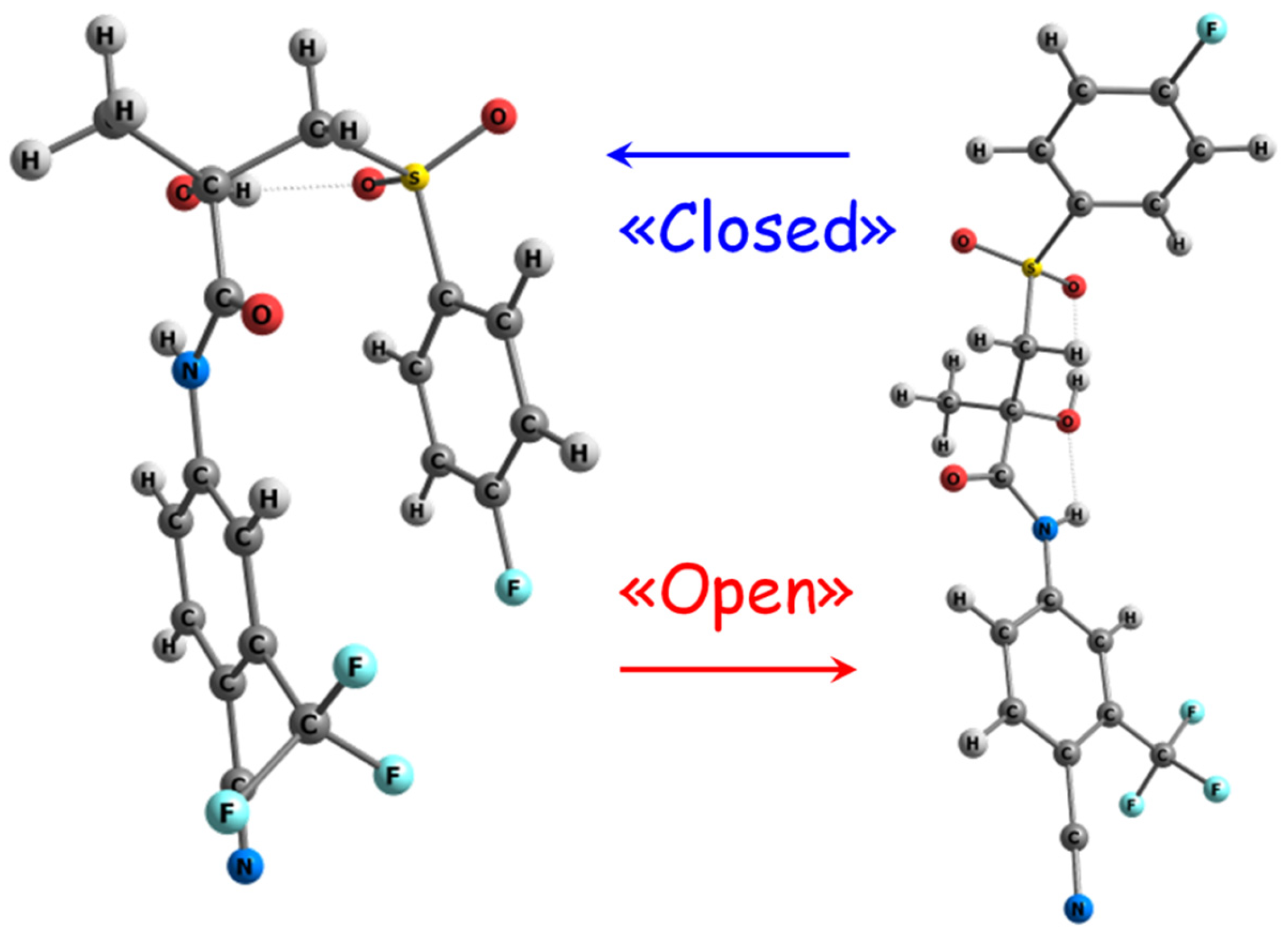

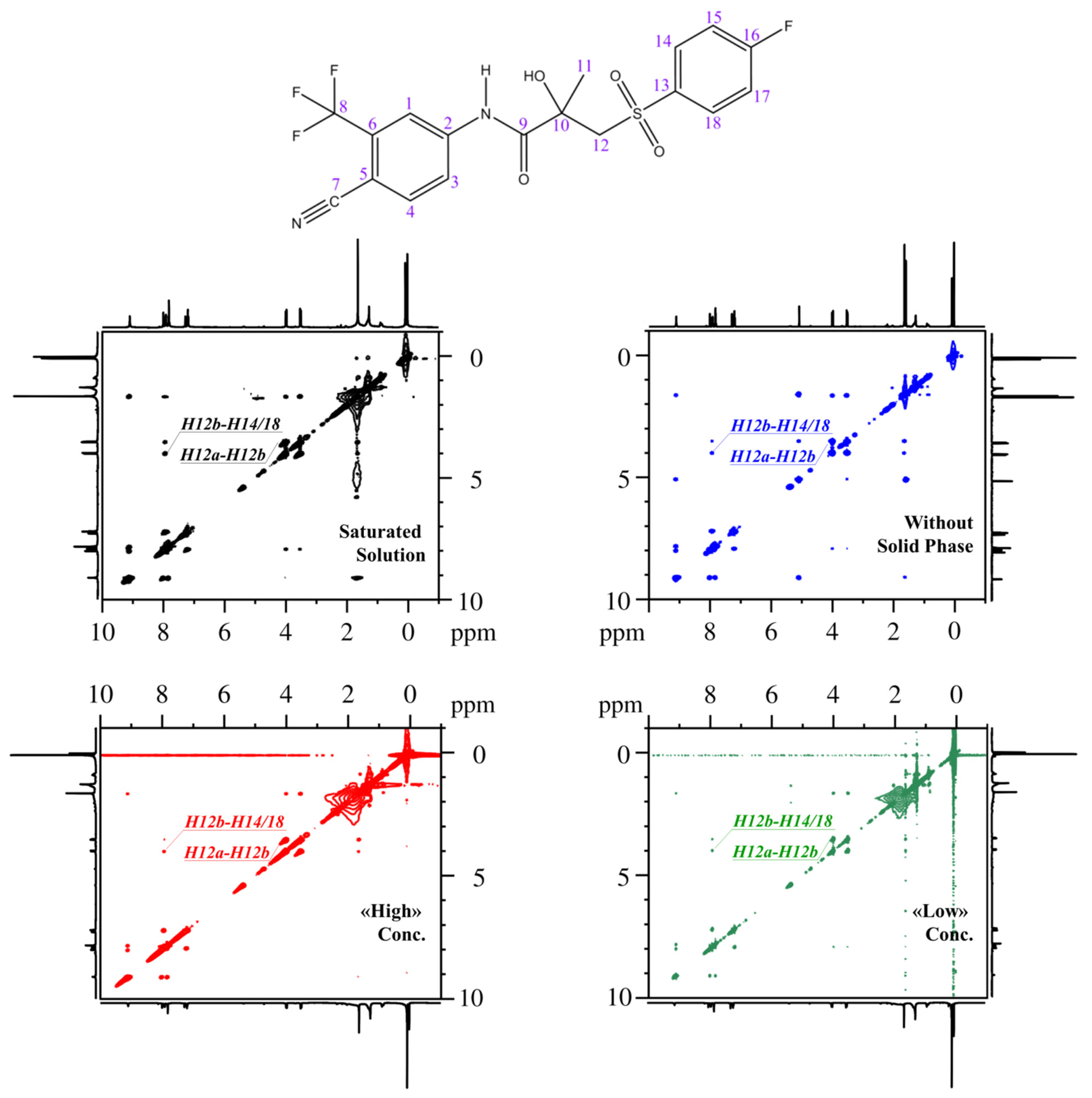
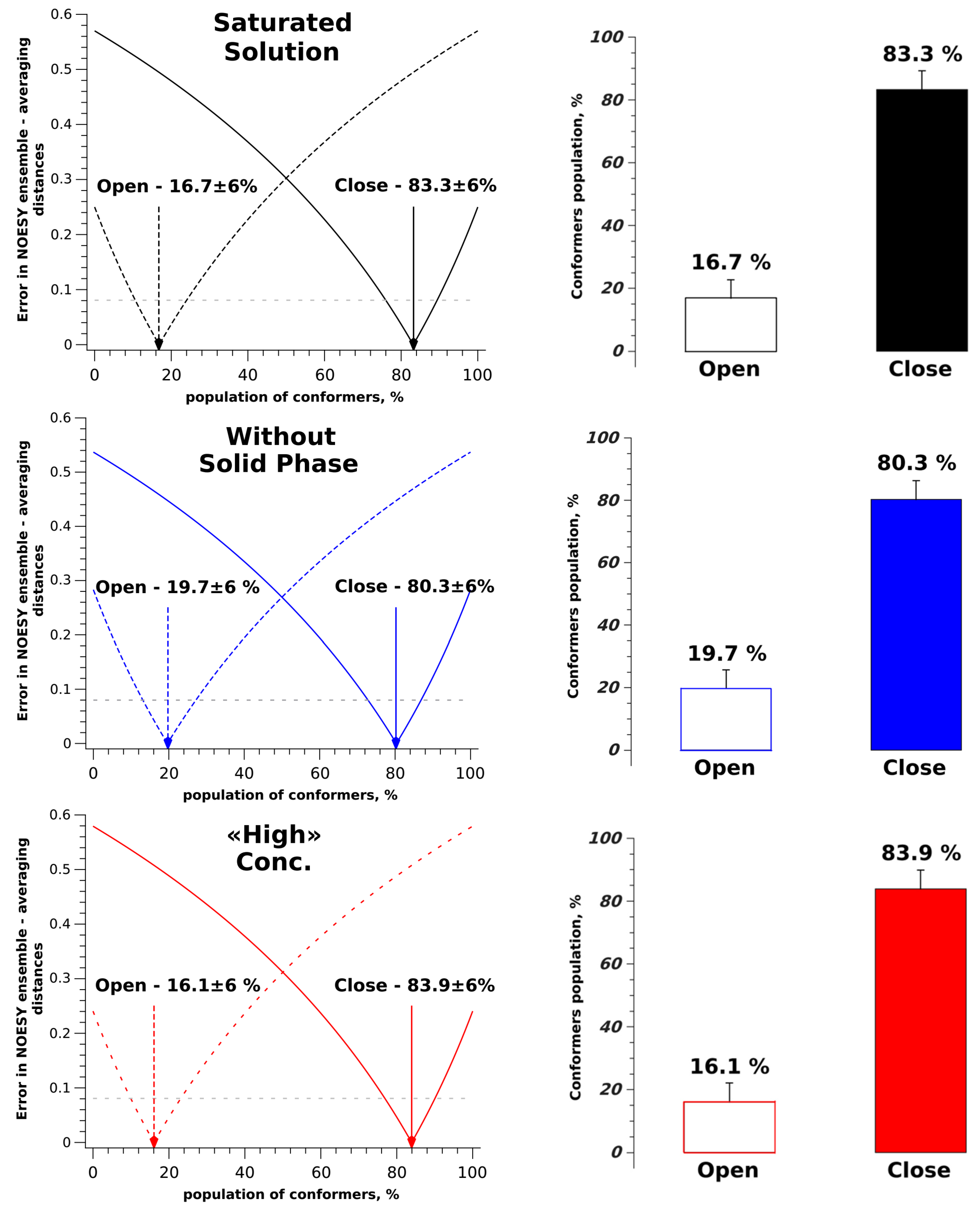
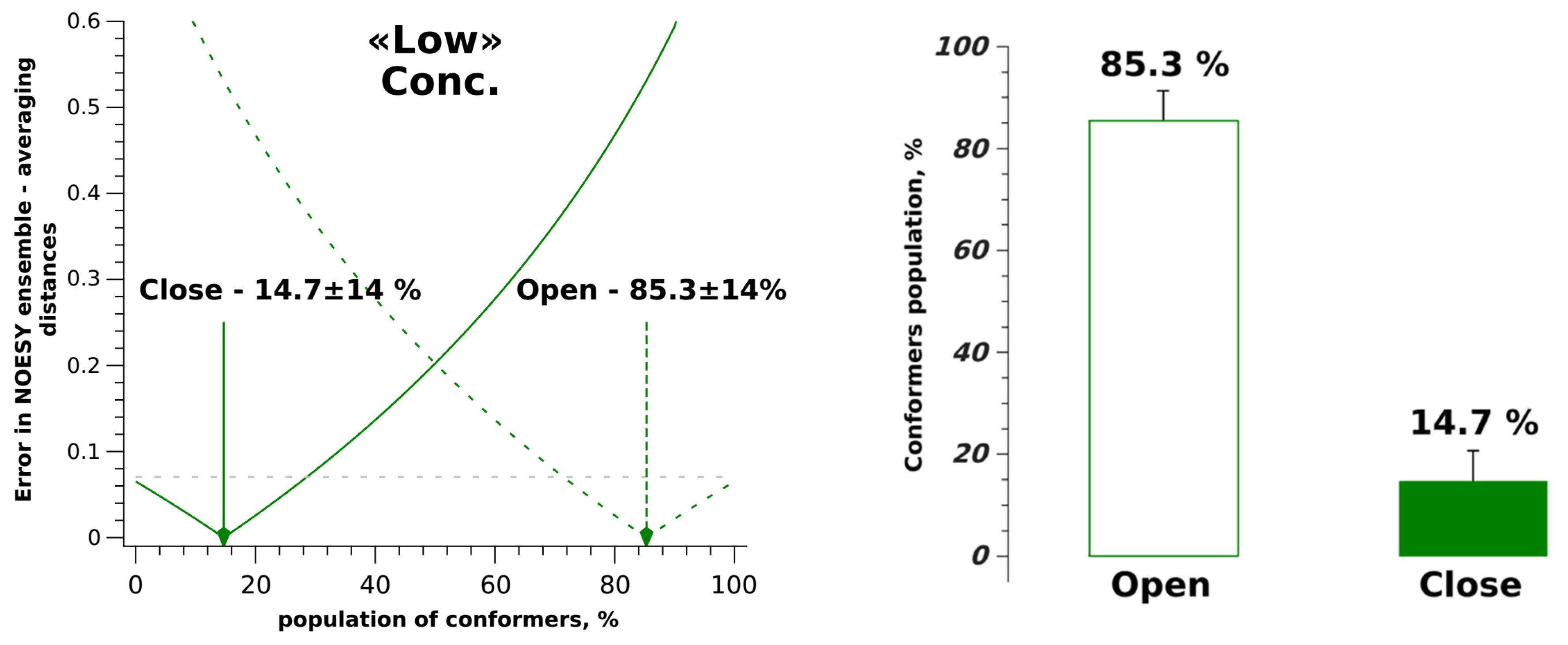
| System | σexp×10−3, s−1 | σ0×10−1, s−1 | rexp, Å | rClose, Å | rOpen, Å | r0, Å |
|---|---|---|---|---|---|---|
| Saturated solution | 1.87 ± 0.21 | 2.52 ± 0.99 | 4.03 ± 0.08 | 4.28 ± 0.18 | 3.46 ± 0.49 | 1.78 ± 0.01 |
| Without the solid phase | 1.96 ± 0.11 | 2.51 ± 0.11 | 3.99 ± 0.08 | |||
| «High» concentration | 1.85 ± 0.23 | 2.47 ± 0.14 | 4.04 ± 0.08 | |||
| «Low» concentration | 4.16 ± 0.37 | 2.51 ± 0.16 | 3.53 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, K.V.; Khodov, I.A. Conformational Evolution of Bicalutamide in Chloroform: A Comprehensive NMR Study. Molecules 2025, 30, 4479. https://doi.org/10.3390/molecules30224479
Belov KV, Khodov IA. Conformational Evolution of Bicalutamide in Chloroform: A Comprehensive NMR Study. Molecules. 2025; 30(22):4479. https://doi.org/10.3390/molecules30224479
Chicago/Turabian StyleBelov, Konstantin V., and Ilya A. Khodov. 2025. "Conformational Evolution of Bicalutamide in Chloroform: A Comprehensive NMR Study" Molecules 30, no. 22: 4479. https://doi.org/10.3390/molecules30224479
APA StyleBelov, K. V., & Khodov, I. A. (2025). Conformational Evolution of Bicalutamide in Chloroform: A Comprehensive NMR Study. Molecules, 30(22), 4479. https://doi.org/10.3390/molecules30224479








