Novel Insights in Renal Transplantation
A project collection of Journal of Clinical Medicine (ISSN 2077-0383). This project collection belongs to the section "Nephrology & Urology".
Papers displayed on this page all arise from the same project. Editorial decisions were made independently of project staff and handled by the Editor-in-Chief or qualified Editorial Board members.
Submission Status:
Closed
|
Viewed by 38580
Share This Project Collection
Editors
 Prof. Dr. Klemens Budde
Prof. Dr. Klemens Budde
 Prof. Dr. Klemens Budde
Prof. Dr. Klemens Budde
E-Mail
Website
Collection Editor
Department of Nephrology and Intensive Medical Care, Charité – Universitätsmedizin Berlin, Corporate Member of Freie, Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, 10117 Berlin, Germany
Interests: kidney transplantation; immunosuppression; antibody-mediated rejection; eHealth; rare kidney diseases
Special Issues, Collections and Topics in MDPI journals
 Dr. Michael Duerr
Dr. Michael Duerr
 Dr. Michael Duerr
Dr. Michael Duerr
E-Mail
Website
Collection Editor
Department of Nephrology and Intensive Medical Care, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, 10117 Berlin, Germany
Interests: immune tolerance; transplantation; HLA; autoantibodies; kidney transplantation; Hepatitis C Virus; glomerulopathy; graft rejection
Project Overview
Dear Colleagues,
Kidney transplantation is the best option with excellent short-term outcomes for patients with the need of renal replacement therapy. Still several unmet medical needs are existing to improve transplantation outcome. One of them is scarcity of organs that lead on one side to prolonged waiting time and associated higher mortality rates and on the other to worse results, when transplant candidates finally getting transplanted. Therefore, we need to find ways to enlarge the possible donor pool to increase utilization, which leads to higher transplantation rates. After transplantation, we still have the need for less toxic immunsuppressive regimens that have the potential to conserve renal transplant function, over the long term period. Beside less toxic regimens we should try to find ways to individualize therapies by analyzing immunological response to different therapies to prevent episodes of over or under-immunsuppression, both jeopardizing transplant and patient survival. Finally, strategies in the long-term follow-up that may detect non-adherence are desperately needed to prevent especially the development of donor specific antibodies and resulting graft loss.
In this collection we invite investigators to submit studies, that address novel approaches and unmet medical need in kidney transplant medicine.
Prof. Dr. Klemens Budde
Dr. Michael Duerr
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Clinical Medicine is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- renal transplantation
- CNI toxicity
- transplant rejection
- CNI-free therapies
- graft loss
- optimizing donor organ pool
- organ preconditioning
- adherence
- HLA-antibodies
Published Papers (10 papers)
Open AccessArticle
Assessment of Renal Transplant Perfusion by Contrast-Enhanced Ultrasound after Switch from Calcineurin Inhibitor to Belatacept: A Pilot Study
by
Bilgin Osmanodja, Frédéric Muench, Alexander Holderied, Klemens Budde, Thomas Fischer and Markus Herbert Lerchbaumer
Cited by 1 | Viewed by 2476
Abstract
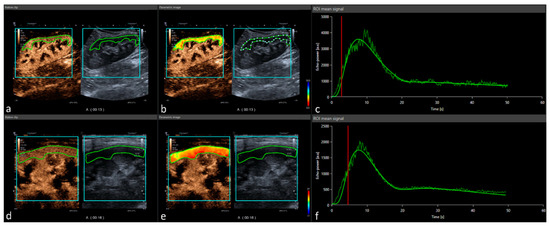
Calcineurin inhibitors (CNIs) have improved short-term kidney allograft survival but are nephrotoxic and vasoconstrictive. Vasoconstriction is potentially reversible after switching from CNIs to belatacept. The kidney allograft shows optimal requirements for dynamic perfusion imaging using contrast-enhanced ultrasound (CEUS). We performed standardized CEUS in
[...] Read more.
Calcineurin inhibitors (CNIs) have improved short-term kidney allograft survival but are nephrotoxic and vasoconstrictive. Vasoconstriction is potentially reversible after switching from CNIs to belatacept. The kidney allograft shows optimal requirements for dynamic perfusion imaging using contrast-enhanced ultrasound (CEUS). We performed standardized CEUS in patients after switching from CNIs to belatacept for clinical indication to study the suitability of CEUS, in order to assess the effects of CNI cessation on kidney allograft perfusion. Eleven kidney transplant patients were enrolled from February 2020 until November 2020. Demographic, clinical, and laboratory parameters, as well as perfusion imaging, were assessed at baseline and 6 months after switching immunosuppression. Quantification of perfusion imaging on CEUS was performed using a post-processing software tool on uncompressed DICOM cine loops. After CNI cessation, estimated glomerular filtration rate increased by 4.8 mL/min/1.73 m
2 (16%). Despite good quality of fit and comparable regions of interest in baseline and follow-up CEUS examinations, quantification of perfusion imaging showed a slightly improved cortical perfusion without reaching statistical significance after CNI cessation. This is the first study that systematically investigates the suitability of CEUS to detect changes of microvascular perfusion in kidney transplant recipients in vivo. No significant differences could be detected in perfusion measurements before and after CNI cessation.
Full article
►▼
Show Figures
Open AccessArticle
Declining Course of Humoral Immune Response in Initially Responding Kidney Transplant Recipients after Repeated SARS-CoV-2 Vaccination
by
Simon Ronicke, Bilgin Osmanodja, Klemens Budde, Annika Jens, Charlotte Hammett, Nadine Koch, Bianca Zukunft, Friederike Bachmann, Mira Choi, Ulrike Weber, Bettina Eberspächer, Jörg Hofmann, Fritz Grunow, Michael Mikhailov, Fabian Halleck and Eva Schrezenmeier
Cited by 2 | Viewed by 2263
Abstract
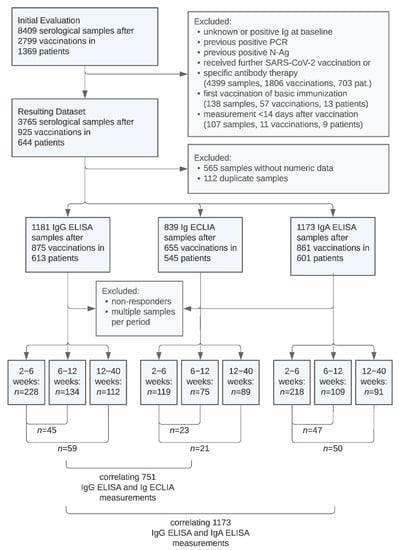
The immunogenicity of SARS-CoV-2 vaccines in kidney transplant recipients is limited, resulting in inadequately low serological response rates and low immunoglobulin (Ig) levels, correlating with reduced protection against death and hospitalization from COVID-19. We retrospectively examined the time course of anti-SARS-CoV-2 Ig antibody
[...] Read more.
The immunogenicity of SARS-CoV-2 vaccines in kidney transplant recipients is limited, resulting in inadequately low serological response rates and low immunoglobulin (Ig) levels, correlating with reduced protection against death and hospitalization from COVID-19. We retrospectively examined the time course of anti-SARS-CoV-2 Ig antibody levels after up to five repeated vaccinations in 644 previously nonresponding kidney transplant recipients. Using anti SARS-CoV-2 IgG/IgA ELISA and the total Ig ECLIA assays, we compared antibody levels at 1 month with levels at 2 and 4 months, respectively. Additionally, we correlated the measurements of the used assays. Between 1 and 2 months, and between 1 and 4 months, mean anti-SARS-CoV-2 Ig levels in responders decreased by 14% and 25%, respectively, depending on the assay. Absolute Ig values and time course of antibody levels showed high interindividual variability. Ig levels decreased by at least 20% in 77 of 148 paired samples with loss of sufficient serological protection over time occurring in 18 out of 148 (12.2%). IgG ELISA and total Ig ECLIA assays showed a strong positive correlation (Kendall’s tau = 0.78), yet the two assays determined divergent results in 99 of 751 (13.2%) measurements. IgG and IgA assays showed overall strong correlation but divergent results in 270 of 1.173 (23.0%) cases and only weak correlation of antibody levels in positive samples. Large interindividual variability and significant loss of serological response after 4 months supports repeated serological sampling and consideration of shorter vaccination intervals in kidney transplant recipients.
Full article
►▼
Show Figures
Open AccessArticle
Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients
by
Bilgin Osmanodja, Simon Ronicke, Klemens Budde, Annika Jens, Charlotte Hammett, Nadine Koch, Evelyn Seelow, Johannes Waiser, Bianca Zukunft, Friederike Bachmann, Mira Choi, Ulrike Weber, Bettina Eberspächer, Jörg Hofmann, Fritz Grunow, Michael Mikhailov, Lutz Liefeldt, Kai-Uwe Eckardt, Fabian Halleck and Eva Schrezenmeier
Cited by 75 | Viewed by 5962
Abstract
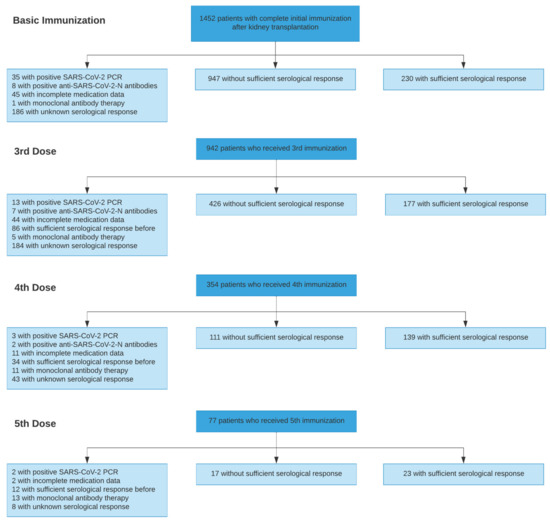
Mortality from COVID-19 among kidney transplant recipients (KTR) is high, and their response to three vaccinations against SARS-CoV-2 is strongly impaired. We retrospectively analyzed the serological response of up to five doses of the SARS-CoV-2 vaccine in KTR from 27 December 2020 until
[...] Read more.
Mortality from COVID-19 among kidney transplant recipients (KTR) is high, and their response to three vaccinations against SARS-CoV-2 is strongly impaired. We retrospectively analyzed the serological response of up to five doses of the SARS-CoV-2 vaccine in KTR from 27 December 2020 until 31 December 2021. Particularly, the influence of the different dose adjustment regimens for mycophenolic acid (MPA) on serological response to fourth vaccination was analyzed. In total, 4277 vaccinations against SARS-CoV-2 in 1478 patients were analyzed. Serological response was 19.5% after 1203 basic immunizations, and increased to 29.4%, 55.6%, and 57.5% in response to 603 third, 250 fourth, and 40 fifth vaccinations, resulting in a cumulative response rate of 88.7%. In patients with calcineurin inhibitor and MPA maintenance immunosuppression, pausing MPA and adding 5 mg prednisolone equivalent before the fourth vaccination increased the serological response rate to 75% in comparison to the no dose adjustment (52%) or dose reduction (46%). Belatacept-treated patients had a response rate of 8.7% (4/46) after three vaccinations and 12.5% (3/25) after four vaccinations. Except for belatacept-treated patients, repeated SARS-CoV-2 vaccination of up to five times effectively induces serological response in kidney transplant recipients. It can be enhanced by pausing MPA at the time of vaccination.
Full article
►▼
Show Figures
Open AccessArticle
Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring
by
Lutz Liefeldt, Petra Glander, Jens Klotsche, Henriette Straub-Hohenbleicher, Klemens Budde, Bettina Eberspächer, Frank Friedersdorff, Fabian Halleck, Pia Hambach, Jörg Hofmann, Nadine Koch, Danilo Schmidt, Eva Schrezenmeier, Evelyn Seelow, Ulrike Weber, Bianca Zukunft, Kai-Uwe Eckardt, Mira Choi, Friederike Bachmann and Johannes Waiser
Cited by 8 | Viewed by 2856
Abstract
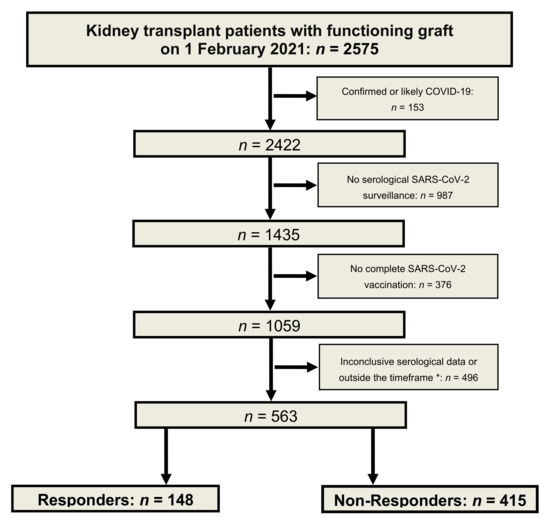
Immunosuppression increases the risk of severe coronavirus disease 2019 (COVID-19). Morbidity and mortality of this disease in kidney transplant patients are higher than in the general population. As the vaccination response of transplant patients is weak, serological monitoring was performed. In this cohort
[...] Read more.
Immunosuppression increases the risk of severe coronavirus disease 2019 (COVID-19). Morbidity and mortality of this disease in kidney transplant patients are higher than in the general population. As the vaccination response of transplant patients is weak, serological monitoring was performed. In this cohort study, we analyzed the determinants of vaccination response. All patients had no history of COVID-19. With anti-spike IgG monitoring, 148 responders and 415 non-responders were identified. We compared both groups using multivariate analyses of the cohort and a sub-cohort of mycophenolic-acid-treated patients. We investigated the influence of patient characteristics, immunosuppression, and erythrocyte inosine monophosphate dehydrogenase (IMPDH) activity. In responders, the time after transplantation was longer (13.5 vs. 8.5 years), the glomerular filtration rate was higher (56.9 vs. 47.8 mL/min/1.73 m
2), and responders were younger (53.0 vs. 57.4 years). Heterologous vaccination was more effective than homologous vaccination. Calcineurin inhibitors plus mycophenolate reduced the seroconversion rate. No seroconversion was observed in belatacept patients. In mycophenolate-treated patients, IMPDH activity was a significantly better predictor of response than mycophenolate dose (AUC 0.84 vs. 0.62,
p < 0.001). Immunosuppression strongly affects vaccine response. Modifications to immunosuppression should be considered in order to facilitate this response. Erythrocyte IMPDH activity can be used to guide mycophenolate treatment.
Full article
►▼
Show Figures
Open AccessArticle
Influence of Belatacept- vs. CNI-Based Immunosuppression on Vascular Stiffness and Body Composition
by
Zbigniew Heleniak, Sarah Illersperger, Marcel G. Naik, Bilgin Osmanodja, Simon Ronicke, Georgios Eleftheriadis, Fabian Halleck and Klemens Budde
Cited by 1 | Viewed by 1919
Abstract
Background: Arterial stiffness and phase angle (PhA) have gained importance as a diagnostic and prognostic parameter in the management of cardiovascular disease. There are few studies regarding the differences in arterial stiffness and body composition between renal transplant recipients (RTRs) receiving belatacept (BELA)
[...] Read more.
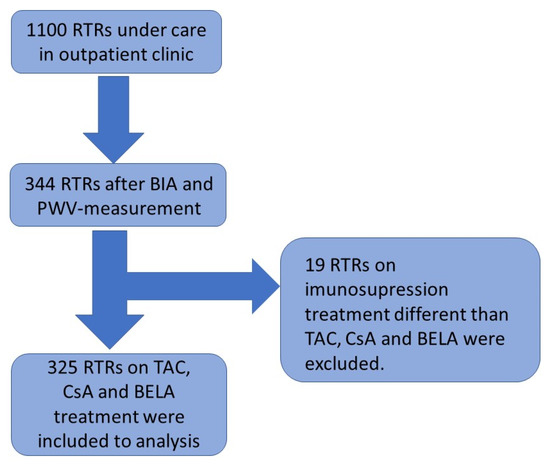
Background: Arterial stiffness and phase angle (PhA) have gained importance as a diagnostic and prognostic parameter in the management of cardiovascular disease. There are few studies regarding the differences in arterial stiffness and body composition between renal transplant recipients (RTRs) receiving belatacept (BELA) vs. calcineurin inhibitors (CNI). Therefore, we investigated the differences in arterial stiffness and body composition between RTRs treated with different immunosuppressants, including BELA. Methods: In total, 325 RTRs were enrolled in the study (mean age 52.2 years, M −62.7%). Arterial stiffness was determined with an automated oscillometric device. All body composition parameters were assessed, based on bioelectrical impedance analysis (BIA), and laboratory parameters were obtained from the medical files of the patients. Results: We did not detect any significant difference in terms of arterial stiffness and PhA in RTRs undergoing different immunosuppressive regimens, based on CsA, Tac, or BELA. Age was an essential risk factor for greater arterial stiffness. The PhA was associated with age, BMI, time of dialysis before transplantation, and kidney graft function. Conclusion: No significant differences in arterial stiffness and PhA were observed in RTRs under different immunosuppressive regimens. While our data provide additional evidence for arterial stiffness and PhA in RTRs, more research is needed to fully explore these cardiovascular risk factors and the impact of different immunosuppressive regimens.
Full article
►▼
Show Figures
Open AccessArticle
Poor Long-Term Renal Allograft Survival in Patients with Chronic Antibody-Mediated Rejection, Irrespective of Treatment—A Single Center Retrospective Study
by
Kaiyin Wu, Danilo Schmidt, Covadonga López del Moral, Bilgin Osmanodja, Nils Lachmann, Qiang Zhang, Fabian Halleck, Mira Choi, Friederike Bachmann, Simon Ronicke, Wiebke Duettmann, Marcel G. Naik, Eva Schrezenmeier, Birgit Rudolph and Klemens Budde
Cited by 6 | Viewed by 2457
Abstract
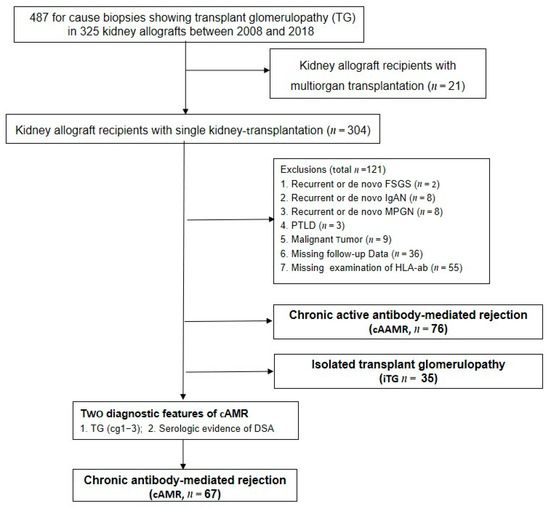
The Banff 2017 report permits the diagnosis of pure chronic antibody-mediated rejection (cAMR) in absence of microcirculation inflammation. We retrospectively investigated renal allograft function and long-term outcomes of 67 patients with cAMR, and compared patients who received antihumoral therapy (cAMR-AHT,
n = 21)
[...] Read more.
The Banff 2017 report permits the diagnosis of pure chronic antibody-mediated rejection (cAMR) in absence of microcirculation inflammation. We retrospectively investigated renal allograft function and long-term outcomes of 67 patients with cAMR, and compared patients who received antihumoral therapy (cAMR-AHT,
n = 21) with patients without treatment (cAMRwo,
n = 46). At baseline, the cAMR-AHT group had more concomitant T-cell-mediated rejection (9/46 (19.2%) vs. 10/21 (47.6%);
p = 0.04), a higher g-lesion score (0.4 ± 0.5 versus 0.1 ± 0.3;
p = 0.01) and a higher median eGFR decline in the six months prior to biopsy (6.6 vs. 3.0 mL/min;
p = 0.04). The median eGFR decline six months after biopsy was comparable (2.6 vs. 4.9 mL/min,
p = 0.61) between both groups, and three-year graft survival after biopsy was statistically lower in the cAMR-AHT group (35.0% vs. 61.0%,
p = 0.03). Patients who received AHT had more infections (0.38 vs. 0.20 infections/patient;
p = 0.04). Currently, antihumoral therapy is more often administered to patients with cAMR and rapidly deteriorating renal function or concomitant TCMR. However, long-term graft outcomes remain poor, despite treatment.
Full article
►▼
Show Figures
Open AccessReview
Kidney Transplantation in COVID Pandemic—A Review of Guidelines
by
Gabriela Gut, Agata Góral, Zofia Dal Canton, Paweł Poznański, Magdalena Krajewska and Mariusz Kusztal
Cited by 9 | Viewed by 3420
Abstract
The paper describes problems with the transplantation process during the COVID-19 pandemic. Transplantation procedures and programs have been impacted by COVID-19. The number of transplants has fallen noticeably. The first part of the paper points out changes in service organization, in particular donor
[...] Read more.
The paper describes problems with the transplantation process during the COVID-19 pandemic. Transplantation procedures and programs have been impacted by COVID-19. The number of transplants has fallen noticeably. The first part of the paper points out changes in service organization, in particular donor and recipient pre-transplant and peri-transplant management. If the patients during pre-transplant evaluation need to attend face-to-face appointments, such as blood testing or other investigations, the risk of contracting or spreading COVID-19 should be minimized. “Clear green areas”, which are COVID-19-free pathways, are highly recommended in hospitals during transplant procedures. Diagnostic procedures concerning donors, including CT scans and coronavirus testing (nasopharyngeal swab), are necessary before transplant surgery. COVID-19 symptoms and risks of the transplant population are described. Detailed guidelines from transplant societies concerning changes in immunosuppression in infected recipients are discussed. Management of infected or suspected medical staff is mentioned. The paper ends with guidelines concerning vaccination against COVID-19 in transplant recipients.
Full article
Open AccessArticle
Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection
by
Stephan R. Marticorena Garcia, Christian E. Althoff, Michael Dürr, Fabian Halleck, Klemens Budde, Ulrike Grittner, Christian Burkhardt, Korinna Jöhrens, Jürgen Braun, Thomas Fischer, Bernd Hamm, Ingolf Sack and Jing Guo
Cited by 10 | Viewed by 3181
Abstract
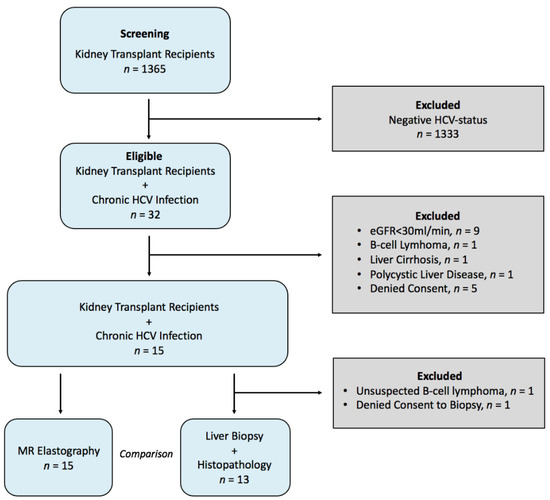
Besides the liver, hepatitis C virus (HCV) infection also affects kidney allografts. The aim of this study was to longitudinally evaluate viscoelasticity changes in the liver and in kidney allografts in kidney transplant recipients (KTRs) with HCV infection after treatment with direct-acting antiviral
[...] Read more.
Besides the liver, hepatitis C virus (HCV) infection also affects kidney allografts. The aim of this study was to longitudinally evaluate viscoelasticity changes in the liver and in kidney allografts in kidney transplant recipients (KTRs) with HCV infection after treatment with direct-acting antiviral agents (DAAs). Fifteen KTRs with HCV infection were treated with DAAs (daclatasvir and sofosbuvir) for 3 months and monitored at baseline, end of treatment (EOT), and 3 (FU1) and 12 (FU2) months after EOT. Shear-wave speed (SWS) and loss angle of the complex shear modulus (φ), reflecting stiffness and fluidity, respectively, were reconstructed from multifrequency magnetic resonance elastography data with tomoelastography post-processing. After virus elimination by DAAs, hepatic stiffness and fluidity decreased, while kidney allograft stiffness and fluidity increased compared with baseline (hepatic stiffness change at FU1: −0.14 m/s,
p < 0.01, and at FU2: −0.11 m/s,
p < 0.05; fluidity at FU1: −0.05 rad,
p = 0.04 and unchanged at FU2:
p = 0.20; kidney allograft stiffness change at FU1: +0.27 m/s,
p = 0.01, and at FU2: +0.30 m/s,
p < 0.01; fluidity at FU1 and FU2: +0.06 rad,
p = 0.02). These results suggest the restoration of mechanically sensitive structures and functions in both organs. Tomoelastography can be used to monitor the therapeutic results of HCV treatment non-invasively on the basis of hepatic and renal viscoelastic parameters.
Full article
►▼
Show Figures
Open AccessReview
Donor-Derived Cell-Free DNA in Kidney Transplantation as a Potential Rejection Biomarker: A Systematic Literature Review
by
Adrian Martuszewski, Patrycja Paluszkiewicz, Magdalena Król, Mirosław Banasik and Marta Kepinska
Cited by 42 | Viewed by 9310
Abstract
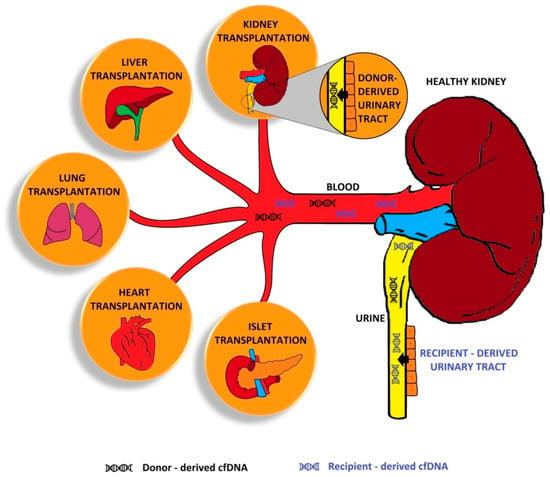
Kidney transplantation (KTx) is the best treatment method for end-stage kidney disease. KTx improves the patient’s quality of life and prolongs their survival time; however, not all patients benefit fully from the transplantation procedure. For some patients, a problem is the premature loss
[...] Read more.
Kidney transplantation (KTx) is the best treatment method for end-stage kidney disease. KTx improves the patient’s quality of life and prolongs their survival time; however, not all patients benefit fully from the transplantation procedure. For some patients, a problem is the premature loss of graft function due to immunological or non-immunological factors. Circulating cell-free DNA (cfDNA) is degraded deoxyribonucleic acid fragments that are released into the blood and other body fluids. Donor-derived cell-free DNA (dd-cfDNA) is cfDNA that is exogenous to the patient and comes from a transplanted organ. As opposed to an invasive biopsy, dd-cfDNA can be detected by a non-invasive analysis of a sample. The increase in dd-cfDNA concentration occurs even before the creatinine level starts rising, which may enable early diagnosis of transplant injury and adequate treatment to avoid premature graft loss. In this paper, we summarise the latest promising results related to cfDNA in transplant patients.
Full article
►▼
Show Figures
Open AccessArticle
Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients
by
Michael Duerr, Lutz Liefeldt, Frank Friedersdorff, Mira Choi, Robert Öllinger, Jörg Hofmann, Klemens Budde, Eva Schrezenmeier and Fabian Halleck
Cited by 6 | Viewed by 2828
Abstract
Transplant candidates are facing incremental mortality risks on the waiting list. Here, we report a novel strategy to expand the donor pool by including hepatitis C seropositive (HCV+) donors. We investigated a pre-exposure prophylactic (PrEP) treatment with direct-acting antivirals (DAA) to allow transplantation
[...] Read more.
Transplant candidates are facing incremental mortality risks on the waiting list. Here, we report a novel strategy to expand the donor pool by including hepatitis C seropositive (HCV+) donors. We investigated a pre-exposure prophylactic (PrEP) treatment with direct-acting antivirals (DAA) to allow transplantation for HCV seronegative (HCV−) kidney transplant recipients (KTR) with the aim to prevent HCV infection post transplantation. In this prospective trial, a pan-genotypic PrEP with daclatasvir and sofosbuvir once daily for 12 week was administered at transplantation. The primary endpoint sustained virological negativity (SVN) 12 weeks after the end of PrEP. Seven patients received a transplantation from four HCV+ donors. Accumulated waiting time was 70 ± 31.3 months already. Of note, study subjects underwent transplantation 24.7 ± 16.1 days after given consent. All KTR developed excellent graft function without any rejection episodes. One patient died with a functioning graft due to sepsis 13 months after transplantation. PrEP demonstrated efficacy with no signs of HCV transmission with excellent tolerability. Two out of four HCV+ donors were viremic at the time of explantation. Interestingly, KTR developed HCV antibodies also from non viramic donors. The acceptance of HCV+ donor was safe and reduced waiting time under the protection of PrEP DAA in kidney transplantation.
Full article
►▼
Show Figures