Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients
Abstract
1. Introduction
2. Methods
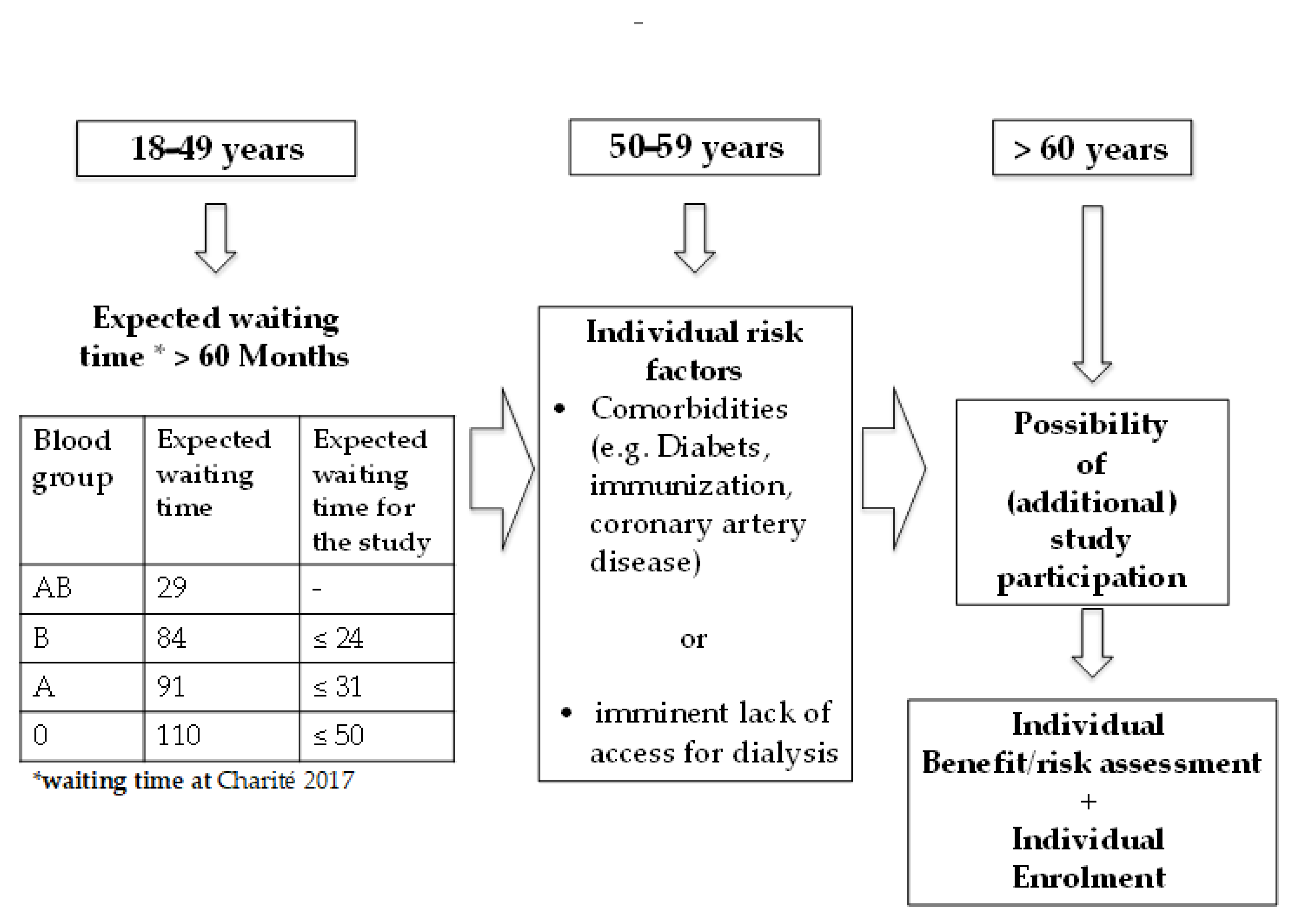
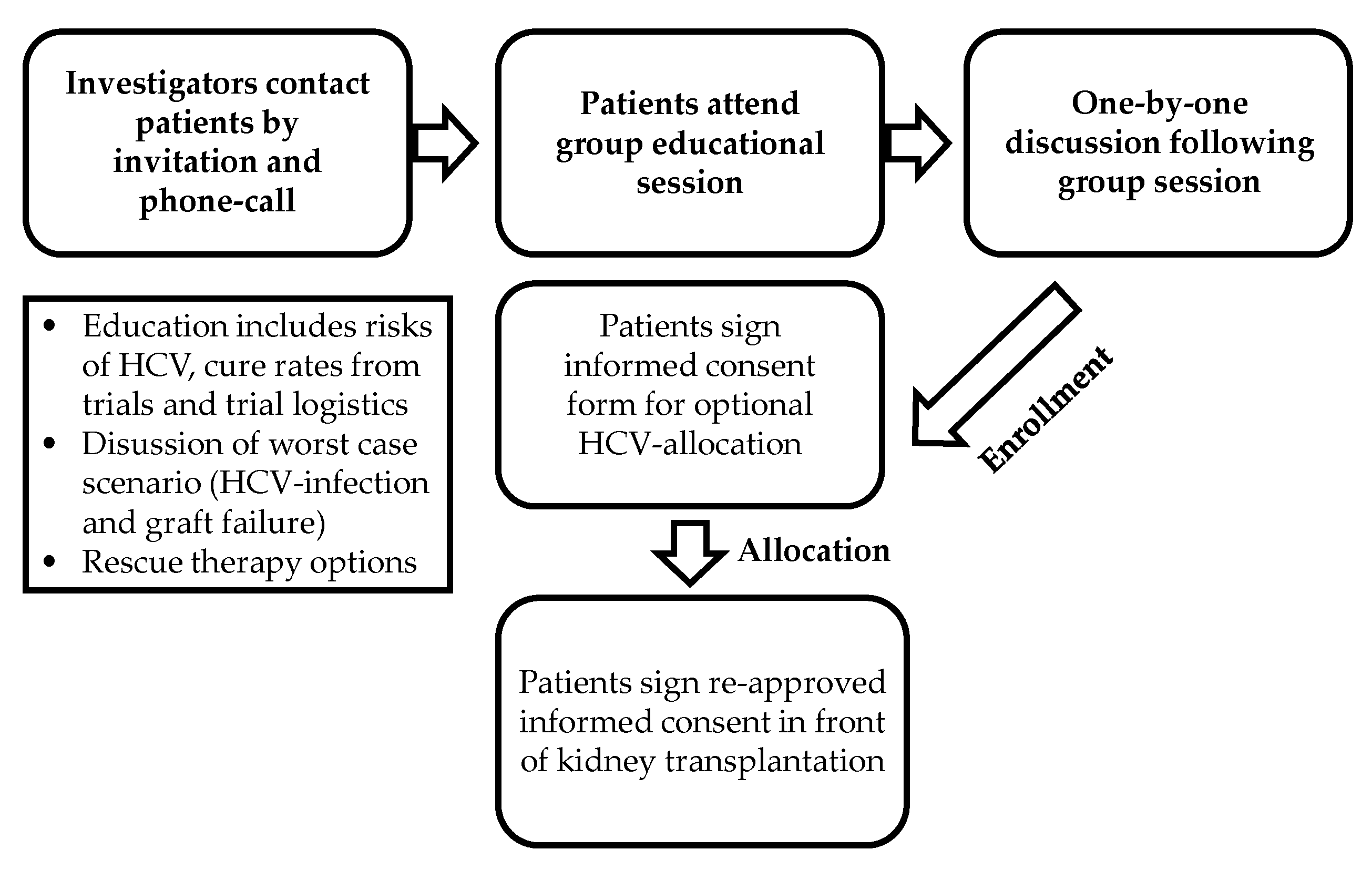
2.1. Study Design
2.2. Key Inclusion Criteria
2.3. Key Exclusion Criteria
2.4. Treatment Regimen
2.5. Efficacy Endpoints
2.6. Safety
2.7. Virological Testing
2.8. Statistical Analysis
3. Results
3.1. Efficacy of PrEP
3.2. Safety Data
3.3. SAEs
3.4. Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glander, P.; Budde, K.; Schmidt, D.; Fuller, T.; Giessing, M.; Neumayer, N.; Liefeldt, L. The ‘blood group O problem’ in kidney transplantation--time to change? Nephrol. Dial. Transplant. 2010, 25, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Scalea, J.R.; Barth, R.N.; Munivenkatappa, R.; Philosophe, B.; Cooper, M.; Whitlow, V.; LaMattina, J.C. Shorter Waitlist Times and Improved Graft Survivals Are Observed in Patients Who Accept Hepatitis C Virus+ Renal Allografts. Transplantation 2015, 99, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Carpio, R.; Pamugas, G.; Danguilan, R.; Que, E. Outcomes of Renal Allograft Recipients with Hepatitis C. Transplant. Proc. 2016, 48, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Bowring, L. Utilization and Post-Transplant Outcomes Associated with Hepatitis C+ Donor Kidneys in Advent of Direct-Acting Antivirals. Am. J. Transplant. 2016, 16, 203–404. [Google Scholar]
- Kamar, N.; Marion, O.; Rostaing, L.; Cointault, O.; Ribes, D.; Lavayssière, L.; Esposito, L.; Del Bello, A.; Metivier, S.; Barange, K.; et al. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Arab. Archaeol. Epigr. 2016, 16, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Kaur, N.; Ajeti, A.; Trofe-Clark, J.; Lim, M.; Bleicher, M.B.; Goral, S.; Forde, K.A.; Bloom, R.D. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Arab. Archaeol. Epigr. 2016, 16, 1588–1595. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Abt, P.L.; Blumberg, E.A.; Van Deerlin, V.M.; Levine, M.; Reddy, K.R.; Bloom, R.D.; Nazarian, S.M.; Sawinski, D.; Porrett, P.; et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N. Engl. J. Med. 2017, 376, 2394–2395. [Google Scholar] [CrossRef]
- Reese, P.P.; Abt, P.L.; Blumberg, E.A.; van Deerlin, V.M.; Bloom, R.D.; Potluri, V.S.; Levine, M.; Porrett, P.; Sawinski, D.; Nazarian, S.M.; et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann. Intern. Med. 2018, 169, 273–281. [Google Scholar] [CrossRef]
- Martins, P.N.; Movahedi, B.; Bozorgzadeh, A.; Halleck, F.; Budde, K.; Duerr, M.; Staeck, O.; Hofmann, J.; Eisenberger, U.; Herzer, K.; et al. Transplanting HCV-Infected Kidneys into Uninfected Recipients. N. Engl. J. Med. 2017, 377, 1103–1105. [Google Scholar] [CrossRef]
- Eisenberger, U.; Friebus-Kardash, J.; Guberina, H.; Kribben, A.; Witzke, O.; Willuweit, K.; Gerken, G.; Herzer, K. Treatment With Grazoprevir/Elbasvir for Renal Transplant Recipients With Chronic Hepatitis C Virus Infection and Impaired Allograft Function. Transplant. Direct 2019, 5, e419. [Google Scholar] [CrossRef]
- Friebus-Kardash, J.; Gäckler, A.; Kribben, A.; Witzke, O.; Wedemeyer, H.; Treckmann, J.; Herzer, K.; Eisenberger, U. Successful early sofosbuvir-based antiviral treatment after transplantation of kidneys from HCV-viremic donors into HCV-negative recipients. Transpl. Infect. Dis. 2019, 21, e13146. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Hatzenbuehler, M.L.; Keyes, K.; Ogburn, E. Substance use disorders:Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) andInternational Classification of Diseases, tenth edition (ICD-10). Addiction 2006, 101, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Van Deerlin, V.M.; Goldberg, D.S.; Reese, P.P.; Hasz, R.D.; Abt, P.; Blumberg, E.; Farooqi, M.S. Hepatitis C virus genotyping of organ donor samples to aid in transplantation of HCV-positive organs. Clin. Transplant. 2018, 32, e13172. [Google Scholar] [CrossRef]
- Sise, M.E.; Goldberg, D.S.; Kort, J.J.; Schaubel, D.E.; Alloway, R.R.; Durand, C.M.; Fontana, R.J.; Brown, R.S.; Friedewald, J.J.; Prenner, S.; et al. Multicenter Study to Transplant Hepatitis C–Infected Kidneys (MYTHIC): An Open-Label Study of Combined Glecaprevir and Pibrentasvir to Treat Recipients of Transplanted Kidneys from Deceased Donors with Hepatitis C Virus Infection. J. Am. Soc. Nephrol. 2020, 31, 2678–2687. [Google Scholar] [CrossRef]
- Durand, C.M. Four-Week Direct-Acting Antiviral Prophylaxis for Kidney Transplantation from Hepatitis C-Viremic Donors to Hepatitis C-Negative Recipients: An Open-Label Nonrandomized Study. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Feld, J.J.; Cypel, M.; Kumar, D.; Dahari, H.; Ribeiro, R.V.P.; Marks, N.; Kamkar, N.; Bahinskaya, I.; Onofrio, F.Q.; Zahoor, M.A.; et al. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: A phase 3, single-centre, open-label study. Lancet Gastroenterol. Hepatol. 2020, 5, 649–657. [Google Scholar] [CrossRef]
- Gupta, G.; Yakubu, I.; Bhati, C.S.; Zhang, Y.; Kang, L.; Patterson, J.A.; Andrews-Joseph, A.; Alam, A.; Ferreira-Gonzalez, A.; Kumar, D.; et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Arab. Archaeol. Epigr. 2019, 20, 739–751. [Google Scholar] [CrossRef]
- Dharancy, S.; Coilly, A.; Fougerou-Leurent, C.; Duvoux, C.; Kamar, N.; Leroy, V.; Tran, A.; Houssel-Debry, P.; Canva, V.; Moreno, C.; et al. Direct-acting antiviral agent-based regimen for HCV recurrence after combined liver-kidney transplantation: Results from the ANRS CO23 CUPILT study. Arab. Archaeol. Epigr. 2017, 17, 2869–2878. [Google Scholar] [CrossRef][Green Version]
- Schrezenmeier, E.; Hoffmann, F.; Jaeger, C.; Schrezenmeier, J.; Lisec, J.; Glander, P.; AlGharably, E.; Kreutz, R.; Budde, K.; Duerr, M.; et al. Pharmacokinetics of Daclatasvir, Sofosbuvir, and GS-331007 in a Prospective Cohort of Hepatitis C Virus–Positive Kidney Transplant Recipients. Ther. Drug Monit. 2019, 41, 53–58. [Google Scholar] [CrossRef]
- Borgia, S.M.; Dearden, J.; Yoshida, E.M.; Shafran, S.D.; Brown, A.; Ben-Ari, Z.; Cramp, M.E.; Cooper, C.; Foxton, M.; Rodriguez, C.F.; et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J. Hepatol. 2019, 71, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Malhotra, V.; Agrawal, D.; Singh, S.K.; Beniwal, P.; Sharma, S.; Jhorawat, R.; Rathore, V.; Joshi, H. Sofosbuvir–Velpatasvir Fixed Drug Combination for the Treatment of Chronic Hepatitis C Infection in Patients With End-Stage Renal Disease and Kidney Transplantation. J. Clin. Exp. Hepatol. 2020, 10, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Nair, S.; Cseprekal, O.; Yazawa, M.; Talwar, M.; Balaraman, V.; Podila, P.S.B.; Mas, V.; Maluf, D.; Helmick, R.A.; et al. Transplantation of kidneys from hepatitis C–infected donors to hepatitis C–negative recipients: Single center experience. Arab. Archaeol. Epigr. 2019, 19, 3046–3057. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Ramirez-Sanchez, C.; Ramirez, S.I.; Logan, C.; Law, N.; Mekeel, K.; Pretorius, V.; Aslam, S. Post-transplant survey to assess patient experiences with donor-derived HCV infection. Transpl. Infect. Dis. 2020, 24. [Google Scholar] [CrossRef]
- Agbim, U.; Cseprekal, O.; Yazawa, M.; Talwar, M.; Balaraman, V.; Bhalla, A.; Podila, P.S.B.; Maliakkal, B.; Nair, S.; Eason, J.D.; et al. Factors associated with hepatitis C antibody seroconversion after transplantation of kidneys from hepatitis C infected donors to hepatitis C naïve recipients. Ren. Fail. 2020, 42, 767–775. [Google Scholar] [CrossRef]
- Porrett, P.M.; Reese, P.P.; Holzmayer, V.; Coller, K.E.; Kuhns, M.; Van Deerlin, V.M.; Gentile, C.; Smith, J.R.; Sicilia, A.; Woodards, A.; et al. Early emergence of anti-HCV antibody implicates donor origin in recipients of an HCV-infected organ. Arab. Archaeol. Epigr. 2019, 19, 2525–2532. [Google Scholar] [CrossRef]
- Kaballo, M.A.; Canney, M.; O’Kelly, P.; Williams, Y.; O’Seaghdha, C.M.; Conlon, P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018, 11, 389–393. [Google Scholar] [CrossRef]

| HCV Positive Transplant Donors (n = 4) | |
|---|---|
| Mean age, y (SD) | 46.4 (±7.8) |
| Male, n | 4 |
| History of IV drug abuse | 3 |
| Cause of death | |
| ICB (traumatic) | 2 |
| Drug overdose | 1 |
| Bolus aspiration | 1 |
| Creatinine (mg/dl) (SD) | 0.6 (±0.02) |
| HCV negative transplant recipients (n = 7) | |
| Mean age at transplantation, y (SD) | 59.4 (±8.4) |
| Female, n | 3 |
| Blood group, n | |
| AB | 2 |
| B | 3 |
| A | 0 |
| 0 | 2 |
| Median time on ET waiting list (SD) | 24.7 (±16.1) days |
| At study entry (SD) | 70 (±31.3) months |
| After study entry until transplantation (SD) | 24.7 (±16.1) days |
| Median broad HLA mismatches (IQR) | 4 (3/5) |
| Cause of kidney failure with replacement therapy (patient No.) | 1 diabetic nephropathy 2 glomerulonephritis 3 IgA nephropathy 4 interstitial nephritis 5 unknown 6 thrombotic thrombocytopenic purpura 7 unknown |
| HCV Positive Kidney Donors | HCV Negative Kidney Transplant Recipients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | GT | HCV Ab | HCV RNA Level, IU/mL | No. | HCV RNA Level, IU/mL | HCV Ab Status | ||||||
| Day 0 | TW1 | TW4 | TW12 | EOT | SVN12 | Day 0 | SVN12 | |||||
| 1 | nd | positive | neg | 1 | neg | neg | neg | nd | neg | neg | neg | neg |
| 2 | neg | neg | neg | nd | neg | neg | neg | neg | ||||
| 2 | nd | positive | neg | 3 | neg | neg | neg | nd | neg | neg | neg | reactive |
| 4 | neg | neg | neg | nd | neg | neg | neg | reactive | ||||
| 3 | 2a | positive | >200.000 | 5 | <15 | neg | neg | neg | neg | neg | neg | reactive |
| 6 | <15 | neg | neg | neg | neg | neg | neg | reactive | ||||
| 4 | 2a | positive | >200.000 | 7 | <15 | neg | neg | neg | neg | neg | neg | reactive |
| EOT | SVN12 | |
|---|---|---|
| Creatinine, mg/dl | 1.3 (±0.4) | 1.1 (±0.3) |
| Biopsy proven acute rejection | none | none |
| SAE | n | |
| Delayed graft function | 3 | |
| Renal biopsy | 1 | |
| Major bleeding | 1 | |
| Urosepsis | 1 |
| Patient No. | Creatinine mg/dL 2 Years after Transplantation | Albuminuria mg/g Creatinine in Spot Urine | Rejection/Biopsies | Unscheduled Hospitalizations |
|---|---|---|---|---|
| 1 | 1.11 | 3 | no/no | none |
| 2 | 1.32 | 9 | no/yes (showing ATN) | none |
| 3 | 1.52 | 3 | no/no | none |
| 4 | 1.09 | 18 | no/no | none |
| 5 | 0.90 | 7 | no/no | none |
| 6 | 1.04 | 100 | no/no | none |
| 7 | 3.83 | 39 | no/no | 2 hospitalizations due to pseudomonas urosepsis Exitus letalis due to MSSA-Sepsis; 12 months post kidney Tx |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duerr, M.; Liefeldt, L.; Friedersdorff, F.; Choi, M.; Öllinger, R.; Hofmann, J.; Budde, K.; Schrezenmeier, E.; Halleck, F. Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients. J. Clin. Med. 2021, 10, 89. https://doi.org/10.3390/jcm10010089
Duerr M, Liefeldt L, Friedersdorff F, Choi M, Öllinger R, Hofmann J, Budde K, Schrezenmeier E, Halleck F. Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients. Journal of Clinical Medicine. 2021; 10(1):89. https://doi.org/10.3390/jcm10010089
Chicago/Turabian StyleDuerr, Michael, Lutz Liefeldt, Frank Friedersdorff, Mira Choi, Robert Öllinger, Jörg Hofmann, Klemens Budde, Eva Schrezenmeier, and Fabian Halleck. 2021. "Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients" Journal of Clinical Medicine 10, no. 1: 89. https://doi.org/10.3390/jcm10010089
APA StyleDuerr, M., Liefeldt, L., Friedersdorff, F., Choi, M., Öllinger, R., Hofmann, J., Budde, K., Schrezenmeier, E., & Halleck, F. (2021). Pan-Genotype Pre-Exposure Prophylaxis (PrEP) Allows Transplantation of HCV-Positive Donor Kidneys to Negative Transplant Recipients. Journal of Clinical Medicine, 10(1), 89. https://doi.org/10.3390/jcm10010089






