Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection
Abstract
1. Introduction
2. Materials and Methods
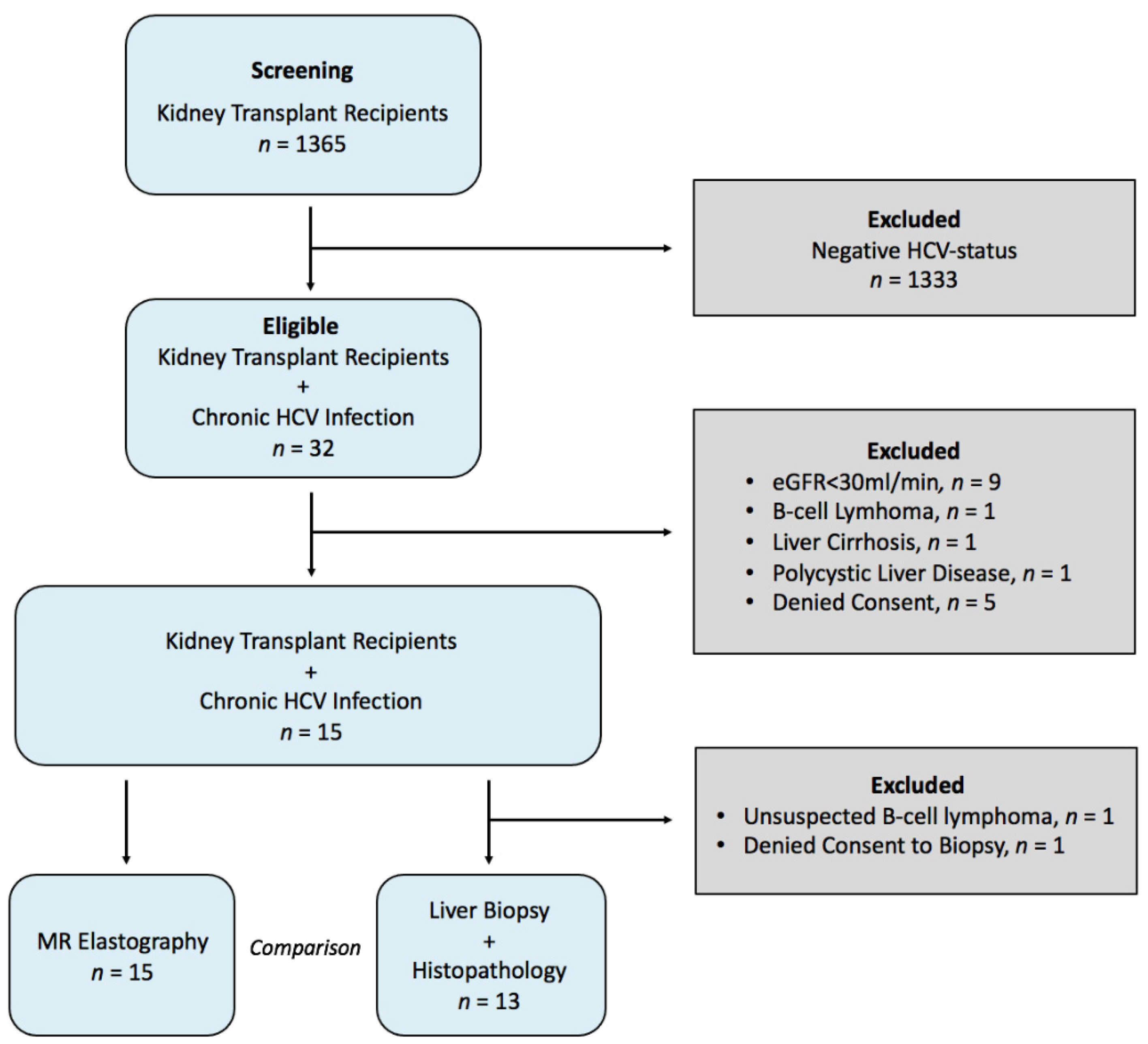
2.1. Study Population
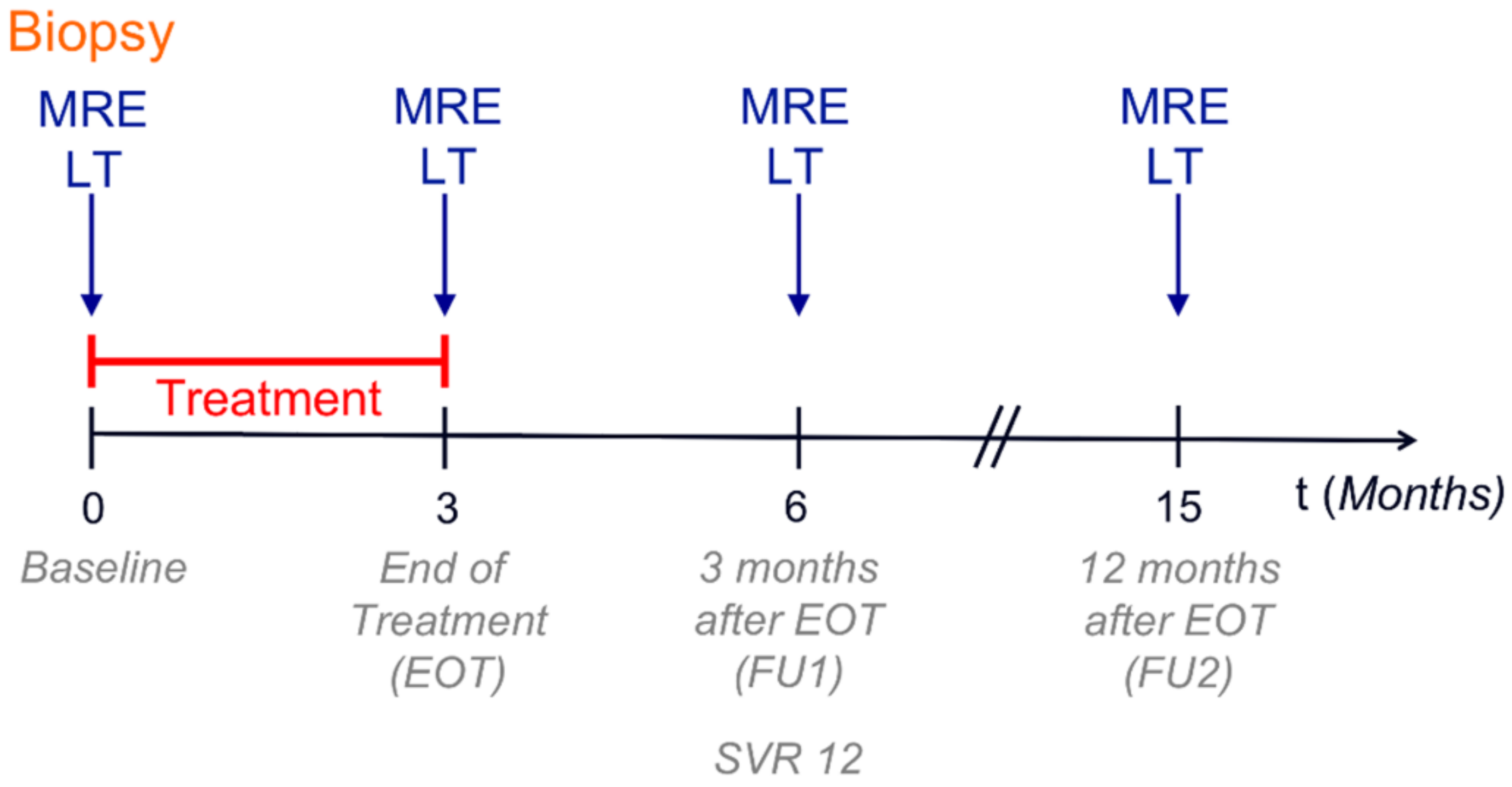
2.2. Study Protocol
2.3. Response to Therapy
2.4. Tomoelastography
2.5. Data Processing
2.6. Biopsy and Histological Staging
2.7. Laboratory Tests
2.8. Statistical Data Analysis
3. Results
3.1. Study Population
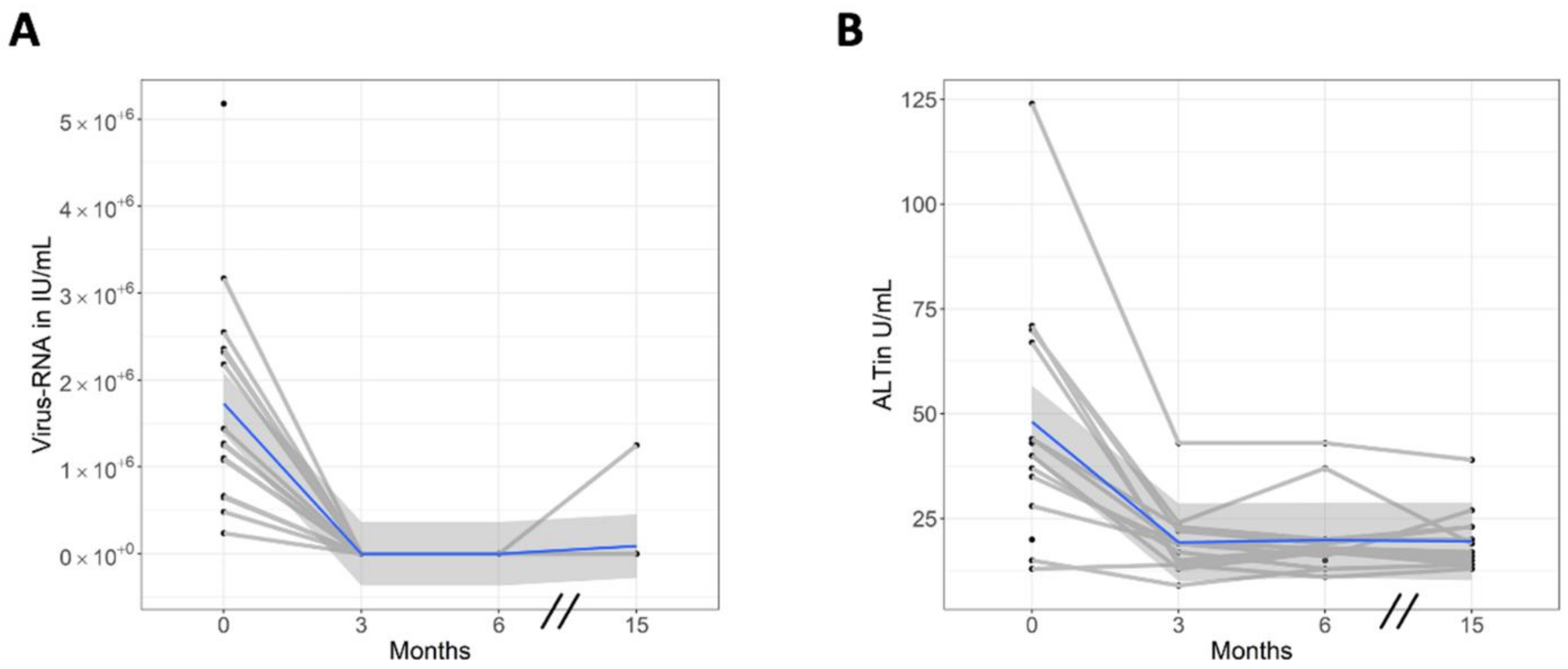
3.2. Viral Response and Laboratory Values
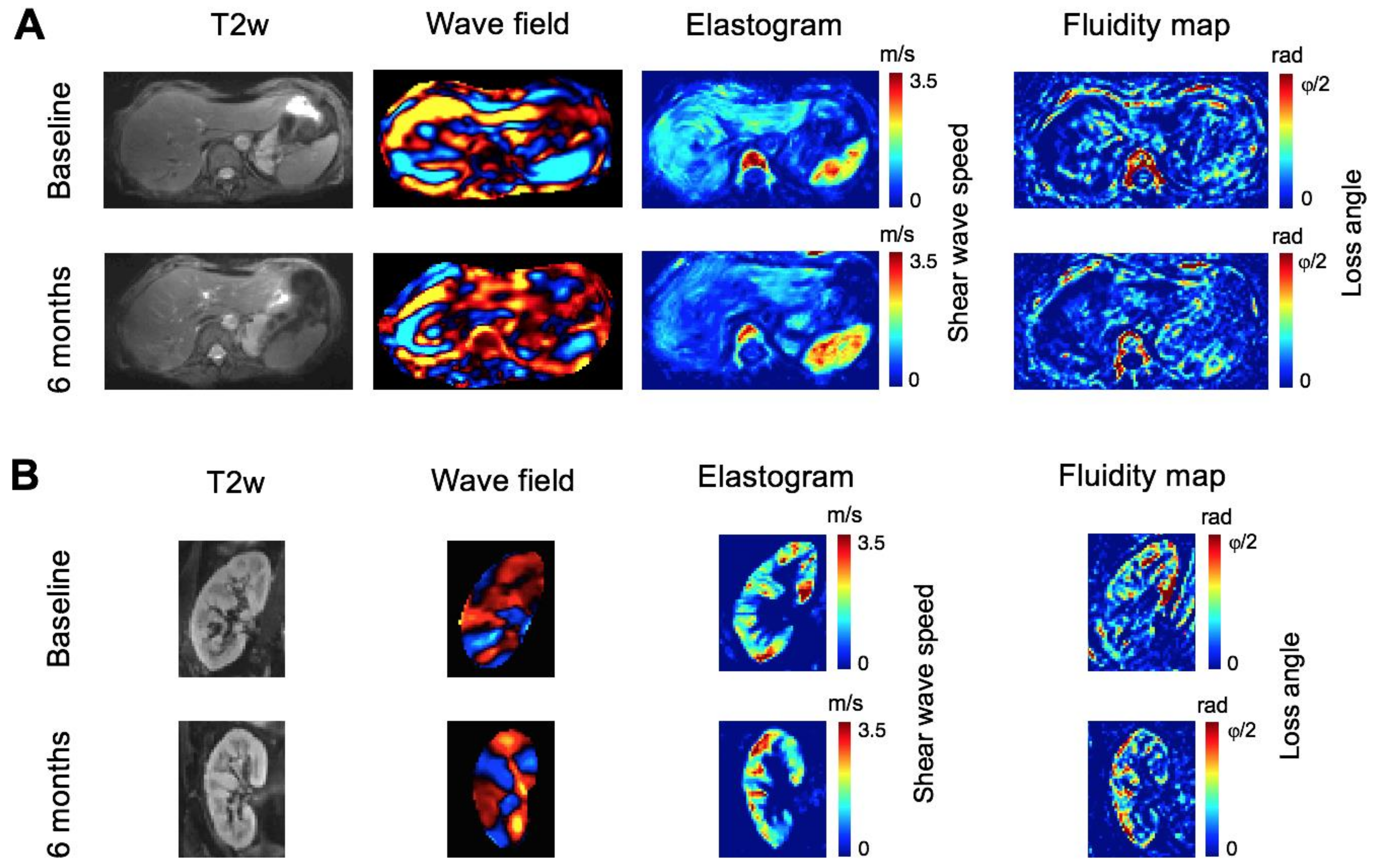
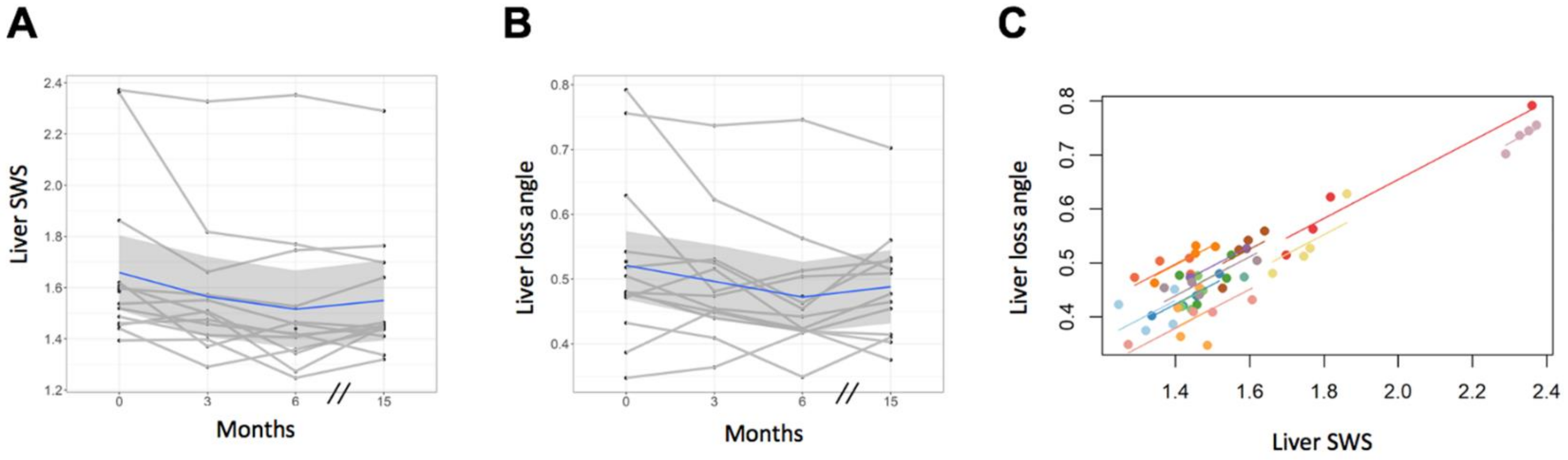
3.3. Tomoelastography—Liver
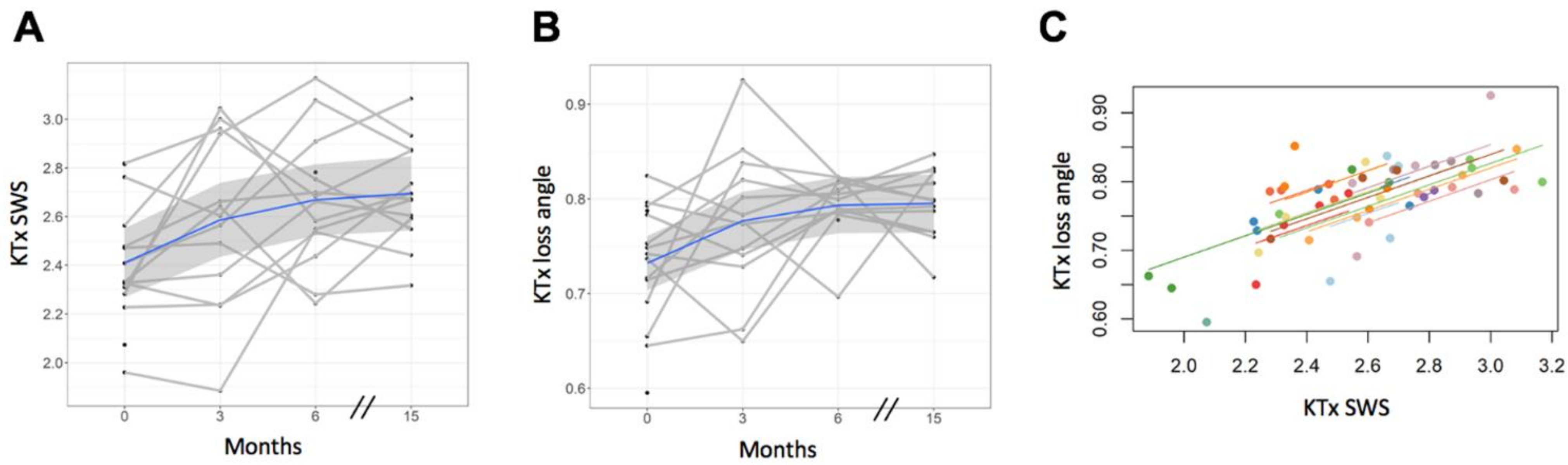
3.4. Tomoelastography—Kidney Allograft
3.5. Histopathology
3.6. Serological Fibrosis Score
4. Discussion
4.1. Viscoelastic DAA Response in the Liver
4.2. Viscoelastic DAA Response in Kidney Allografts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Poynard, T.; Bedossa, P.; Opolon, P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997, 349, 825–832. [Google Scholar] [CrossRef]
- Fabrizi, F.; Verdesca, S.; Messa, P.; Martin, P.L. Hepatitis C Virus Infection Increases the Risk of Developing Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2015, 60, 3801–3813. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Ghazinian, H.; Manch, R.A.; Gish, R.G. Hepatitis C virus as a systemic disease: Reaching beyond the liver. Hepatol. Int. 2016, 10, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Ono, T.; Matsumori, A.; Nogaki, F.; Kusano, H.; Watanabe, H.; Yodoi, J.; Muso, E. Hepatitis C virus-associated tubulointerstitial injury. Am. J. Kidney Dis. 2003, 41, 767–775. [Google Scholar] [CrossRef]
- Ladino, M.; Pedraza, F.; Roth, D. Hepatitis C Virus Infection in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2238–2246. [Google Scholar] [CrossRef]
- Baid-Agrawal, S.; Pascual, M.; Moradpour, D.; Frei, U.; Tolkoff-Rubin, N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev. Med. Virol. 2007, 18, 97–115. [Google Scholar] [CrossRef]
- Kliem, V.; Burg, M.; Haller, H.; Suwelack, B.; Abendroth, D.; Fritsche, L.; Fornara, P.; Pietruck, F.; Frei, U.; Donauer, J.; et al. Relationship of Hepatitis B or C Virus Prevalences, Risk Factors, and Outcomes in Renal Transplant Recipients: Analysis of German Data. Transplant. Proc. 2008, 40, 909–914. [Google Scholar] [CrossRef]
- Izopet, J.; Rostaing, L.; Sandres, K.; Cisterne, J.; Pasquier, C.; Rumeau, J.; Duffaut, M.; Durand, M.; Puel, J. Longitudinal Analysis of Hepatitis C Virus Replication and Liver Fibrosis Progression in Renal Transplant Recipients. J. Infect. Dis. 2000, 181, 852–858. [Google Scholar] [CrossRef]
- Ridruejo, E.; Mandó, O.; Dávalos, M.; Díaz, C.; Vilches, A. Hepatocellular Carcinoma in Renal Transplant Patients. Transplant. Proc. 2005, 37, 2086–2088. [Google Scholar] [CrossRef]
- Lui, F.H.; Moosvi, Z.; Patel, A.; Hussain, S.; Duong, A.; Duong, J.; Nguyen, D.L. Decreased risk of hepatocellular carcinoma recurrence with direct-acting antivirals compared with no treatment for hepatitis C: A meta-analysis. Ann. Gastroenterol. 2020, 33, 293–298. [Google Scholar]
- Virlogeux, V.; Pradat, P.; Hartig-Lavie, K.; Bailly, F.; Maynard, M.; Ouziel, G.; Poinsot, D.; Lebossé, F.; Ecochard, M.; Radenne, S.; et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017, 37, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S., Jr.; Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Gardiner, D.; Rodriguez-Torres, M.; Reddy, K.R.; Hassanein, T.I.; Jacobson, I.; Lawitz, E.J.; Lok, A.S.-F.; Hinestrosa, F.; Thuluvath, P.J.; et al. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N. Engl. J. Med. 2014, 370, 211–221. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Roth, D.; Martin, P.; Nelson, D.R.; Pol, S.; Londoño, M.-C.; Monsour, H.; Silva, M.; Hwang, P.; Arduino, J.-M.; et al. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4–5 chronic kidney disease: Clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 585–594. [Google Scholar] [CrossRef]
- Colombo, M.; Aghemo, A.; Liu, H.; Zhang, J.; Dvory-Sobol, H.; Hyland, R.; Yun, C.; Massetto, B.; Brainard, D.M.; McHutchiso, J.G.; et al. Treatment with Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients with Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Ann. Intern. Med. 2017, 166, 109–117. [Google Scholar] [CrossRef]
- Gane, E.E.; Lawitz, E.; Pugatch, D.D.; Papatheodoridis, G.G.; Bräu, N.N.; Brown, A.A.; Pol, S.; Leroy, V.V.; Persico, M.M.; Moreno, C.; et al. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef]
- Roth, D.; Nelson, D.R.; Bruchfeld, A.; Liapakis, A.; Silva, M.O.; Monsour, H.P.; Martin, P.J.; Pol, S.; Londoño, M.-C.; Hassanein, T.; et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet 2015, 386, 1537–1545. [Google Scholar] [CrossRef]
- Improving Global Outcomes KDIGO Hepatitis C Work Group. KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int. Suppl. 2018, 8, 91–165. [Google Scholar]
- Singh, A.; Kumari, S.; Kumar, P.; De, A.; Singh, V. Sofosbuvir with NS5A inhibitors in hepatitis C virus infection with severe renal insufficiency. J. Viral Hepat. 2018, 25, 1501–1506. [Google Scholar] [CrossRef]
- Eisenberger, U.; Guberina, H.; Willuweit, K.; Bienholz, A.; Kribben, A.; Gerken, G.; Witzke, O.; Herzer, K. Successful Treatment of Chronic Hepatitis C Virus Infection with Sofosbuvir and Ledipasvir in Renal Transplant Recipients. Transplantation 2017, 101, 980–986. [Google Scholar] [CrossRef]
- Goel, A.; Bhadauria, D.S.; Kaul, A.; Prasad, N.; Gupta, A.; Sharma, R.K.; Rai, P.; Aggarwal, R. Experience with direct acting anti-viral agents for treating hepatitis C virus infection in renal transplant recipients. Indian J. Gastroenterol. 2017, 36, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Marion, O.; Rostaing, L.; Cointault, O.; Ribes, D.; Lavayssière, L.; Esposito, L.; Del Bello, A.; Metivier, S.; Barange, K.; et al. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection after Kidney Transplantation. Arab. Archaeol. Epigr. 2016, 16, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Kaur, N.; Ajeti, A.; Trofe-Clark, J.; Lim, M.; Bleicher, M.B.; Goral, S.; Forde, K.A.; Bloom, R.D. Successful Treatment of Hepatitis C in Renal Transplant Recipients with Direct-Acting Antiviral Agents. Arab. Archaeol. Epigr. 2016, 16, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.T.; Guo, J.; Bruns, A.; Dürr, M.; Braun, J.; Hamm, B.; Sack, I.; Garcia, S.R.M. Multiparametric Quantitative MRI for the Detection of IgA Nephropathy Using Tomoelastography, DWI, and BOLD Imaging. Investig. Radiol. 2019, 54, 669–674. [Google Scholar] [CrossRef]
- Garcia, S.R.M.; Grossmann, M.; Bruns, A.; Dürr, M.; Tzschätzsch, H.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. Tomoelastography Paired with T2* Magnetic Resonance Imaging Detects Lupus Nephritis with Normal Renal Function. Investig. Radiol. 2019, 54, 89–97. [Google Scholar] [CrossRef]
- Garcia, S.R.M.; Fischer, T.; Dürr, M.; Gültekin, E.; Braun, J.; Sack, I.; Guo, J. Multifrequency Magnetic Resonance Elastography for the Assessment of Renal Allograft Function. Investig. Radiol. 2016, 51, 591–595. [Google Scholar]
- Duerr, M.; Schrezenmeier, E.; Lehner, L.J.; Bergfeld, L.; Glander, P.; Garcia, S.R.M.; Althoff, C.E.; Sack, I.; Brakemeier, S.; Eckardt, K.-U.; et al. A prospective study of daclatasvir and sofosbuvir in chronic HCV-infected kidney transplant recipients. BMC Nephrol. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Ipek-Ugay, S.; Tzschätzsch, H.; Hudert, C.; Garcia, S.R.M.; Fischer, T.; Braun, J.; Althoff, C.; Sack, I. Time Harmonic Elastography Reveals Sensitivity of Liver Stiffness to Water Ingestion. Ultrasound Med. Biol. 2016, 42, 1289–1294. [Google Scholar] [CrossRef]
- Dittmann, F.; Tzschätzsch, H.; Hirsch, S.; Barnhill, E.; Braun, J.; Sack, I.; Guo, J. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magn. Reson. Med. 2017, 78, 976–983. [Google Scholar] [CrossRef]
- Müller, S. Liver Elastography, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; p. 737. [Google Scholar]
- Dittmann, F.; Hirsch, S.; Tzschätzsch, H.; Guo, J.; Braun, J.; Sack, I. In vivo wideband multifrequency MR elastography of the human brain and liver. Magn. Reson. Med. 2015, 76, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Graduiertenkolleg BIOQIC, GRK 2260. Available online: https://bioqic-apps.charite.de (accessed on 26 January 2021).
- Shahryari, M.; Tzschätzsch, H.; Guo, J.; Garcia, S.R.M.; Böning, G.; Fehrenbach, U.; Stencel, L.; Asbach, P.; Hamm, B.; Käs, J.A.; et al. Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res. 2019, 79, 5704–5710. [Google Scholar] [CrossRef] [PubMed]
- Tzschätzsch, H.; Guo, J.; Dittmann, F.; Hirsch, S.; Barnhill, E.; Jöhrens, K.; Braun, J.; Sack, I. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med. Image Anal. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Gupta, S.; Wallace, M.J.; Cardella, J.F.; Kundu, S.; Miller, D.L.; Rose, S.C. Quality Improvement Guidelines for Percutaneous Needle Biopsy. J. Vasc. Interv. Radiol. 2010, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Kalambokis, G.; Manousou, P.; Vibhakorn, S.; Marelli, L.; Cholongitas, E.; Senzolo, M.; Patch, D.; Burroughs, A. Transjugular liver biopsy—Indications, adequacy, quality of specimens, and complications—A systematic review. J. Hepatol. 2007, 47, 284–294. [Google Scholar] [CrossRef]
- Batts, K.P.; Ludwig, J. An Update on Terminology and Reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J. Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef]
- Wai, C.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 67. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Sone, Y.; Takeshima, K.; Ogawa, S.; Goto, T.; Wakahata, A.; Nakashima, M.; Nakamuta, M.; et al. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment. Pharmacol. Ther. 2018, 47, 1012–1022. [Google Scholar] [CrossRef]
- Bachofner, J.A.; Valli, P.V.; Kröger, A.; Bergamin, I.; Künzler, P.; Baserga, A.; Braun, D.; Seifert, B.; Moncsek, A.; Fehr, J.; et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017, 37, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Iijima, H.; Tada, T.; Kumada, T.; Yoshida, M.; Aoki, T.; Nishimura, T.; Nakano, C.; Takata, R.; Yoh, K.; et al. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur. J. Gastroenterol. Hepatol. 2018, 30, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kohla, M.A.S.; El Fayoumi, A.; Akl, M.; Abdelkareem, M.; Elsakhawy, M.; Waheed, S.; Abozeid, M. Early fibrosis regression by shear wave elastography after successful direct-acting anti-HCV therapy. Clin. Exp. Med. 2020, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, V.; Deterding, K.; Attia, D.; Ringe, K.I.; Heidrich, B.; Cornberg, M.; Gebel, M.; Manns, M.P.; Wedemeyer, H.; Potthoff, A. Long-term changes in liver elasticity in hepatitis C virus-infected patients with sustained virologic response after treatment with direct-acting antivirals. United Eur. Gastroenterol. J. 2018, 6, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.; Nayak, B.; Patel, A.H.; Gunjan, D.; Singh, V.; Kedia, S. Shalimar Therapy with Oral Directly Acting Agents in Hepatitis C Infection Is Associated with Reduction in Fibrosis and Increase in Hepatic Steatosis on Transient Elastography. J. Clin. Exp. Hepatol. 2019, 9, 207–214. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Mizuno, K.; Sone, Y.; Kataoka, S.; Hashinokuchi, S. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J. Gastroenterol. Hepatol. 2017, 32, 1982–1988. [Google Scholar] [CrossRef]
- Peltenburg, H.G.; Hermens, W.T.; Willems, G.M.; Flendrig, J.G.; Schmidt, E. Estimation of the fractional catabolic rate constants for the elimination of cytosolic liver enzymes from plasma. Hepatology 1989, 10, 833–839. [Google Scholar] [CrossRef]
- Leitão, H.S.; Doblas, S.; Garteiser, P.; D’Assignies, G.; Paradis, V.; Mouri, F.; Geraldes, C.F.G.C.; Ronot, M.; Van Beers, B.E. Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology 2017, 283, 98–107. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Wells, M.L.; Miller, F.H.; Jhaveri, K.S.; Silva, A.C.; Taouli, B.; Ehman, R.L. Magnetic resonance elastography: Beyond liver fibrosis—A case-based pictorial review. Abdom. Radiol. 2017, 43, 1590–1611. [Google Scholar] [CrossRef]
- Laursen, T.L.; Siggaard, C.B.; Kazankov, K.; Sandahl, T.D.; Møller, H.J.; Tarp, B.; Kristensen, L.H.; Laursen, A.L.; Leutscher, P.; Grønbaek, H. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J. Viral Hepat. 2019, 27, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S. Daclatasvir and Sofosbuvir Mitigate Hepatic Fibrosis through Downregulation of TNF-α/NF-κB Signaling Pathway. Curr. Mol. Pharmacol. 2020, 13, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Irving, W.; Cheung, M.C.; Walker, A.J.; Hudson, B.E.; Verma, S.; Mclauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.; et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 64, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Knop, V.; Hoppe, D.; Welzel, T.; Vermehren, J.; Herrmann, E.; Friedrich-Rust, M.; Sarrazin, C.; Zeuzem, S.; Welker, M.-W. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J. Viral Hepat. 2016, 23, 994–1002. [Google Scholar] [CrossRef]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and Kinetics of Decrease in Liver Stiffness after Antiviral Therapy in Patients with Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 27–38.e4. [Google Scholar] [CrossRef]
- Yin, M.; Glaser, K.J.; Manduca, A.; Mounajjed, T.; Malhi, H.; Simonetto, D.A.; Wang, R.; Yang, L.; Mao, S.A.; Glorioso, J.M.; et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017, 284, 694–705. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwakiri, Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 733–749. [Google Scholar] [CrossRef]
- Gentil, M.; González-Corvillo, C.; Perelló, M.; Zarraga, S.; Jiménez-Martín, C.; Lauzurica, L.; Alonso, A.; Franco, A.; Hernández-Marrero, D.; Sánchez-Fructuoso, A. Hepatitis C Treatment with Direct-Acting Antivirals in Kidney Transplant: Preliminary Results from a Multicenter Study. Transplant. Proc. 2016, 48, 2944–2946. [Google Scholar] [CrossRef]
- Goetsch, M.R.; Tamhane, A.; Varshney, M.; Kapil, A.; Overton, E.T.; Towns, G.C.; Franco, R.A. Direct-acting Antivirals in Kidney Transplant Patients: Successful Hepatitis C Treatment and Short Term Reduction in Urinary Protein/Creatinine Ratios. Pathog. Immun. 2017, 2, 366–375. [Google Scholar] [CrossRef]
- Lubetzky, M.; Chun, S.; Joelson, A.; Coco, M.; Kamal, L.; Ajaimy, M.; Gaglio, P.; Akalin, E.; De Boccardo, G. Safety and Efficacy of Treatment of Hepatitis C in Kidney Transplant Recipients with Directly Acting Antiviral Agents. Transplantation 2017, 101, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Kidney Allograft Recipients |
|---|---|
| Number of participants | 15 |
| Number of men | 8 |
| Number of women | 7 |
| Age in years | |
| Mean (SD) | 48 (13) |
| Body mass index in kg/m2 | |
| Mean (SD) | 23.3 (4.5) |
| Time since kidney transplantation in years | |
| Mean (SD) | 13.1 (6.9) |
| Variable | Baseline | End of Treatment | FU1 | FU2 |
|---|---|---|---|---|
| (n = 15) | (n = 13) | (n = 14) | (n = 13) | |
| Viral parameters | ||||
| HCV-RNA (106 × IU/mL) | ||||
| Mean (SD) | 1.73 (1.28) | 0 (0) | 0 (0) | 0.09 (0.33) |
| Mean difference vs baseline (95% CI) | −1.72 (−2.35–−1.08) | −1.72 (−2.36–−1.08) | −1.64 (−2.28–−1.01) | |
| p-value, comparison with baseline | <0.001 | <0.001 | <0.001 | |
| Liver parameters | ||||
| ALT (U/L) | ||||
| Mean (SD) | 48 (29) | 19 (8) | 20 (9) | 20 (7) |
| p-value, comparison with baseline | <0.001 | <0.001 | <0.001 | |
| Billirubin (mg/dL) | ||||
| Mean (SD) | 0.52 (0.21) | 0.42 (0.16) | 0.46 (0.24) | 0.47 (0.35) |
| p-value, comparison with baseline | 0.348 | 0.805 | 0.751 | |
| Renal parameters | ||||
| eGFR (mL/min/1.73m2) | ||||
| Mean (SD) | 56 (17) | 57 (19) | 56 (18) | 53 (20) |
| p-value, comparison with baseline | 0.996 | 1.0 | 0.886 | |
| Proteinuria (mg/L) | ||||
| Mean (SD) | 238 (275) | 215 (287) | 239 (235) | 364 (500) |
| p-value, comparison with baseline | 0.887 | 0.614 | 0.642 |
| Variable | Baseline | End of Treatment | FU1 | FU2 |
|---|---|---|---|---|
| (n = 15) | (n = 13) | (n = 14) | (n = 13) | |
| Liver | ||||
| Shear wave speed (ms) | ||||
| Mean (SD) | 1.66 (0.31) | 1.56 (0.27) | 1.52 (0.28) | 1.55 (0.26) |
| p-value, comparison with baseline | 0.088 | 0.005 | 0.047 | |
| Phase angle of the loss modulus (rad) | ||||
| Mean (SD) | 0.52 (0.12) | 0.50 (0.10) | 0.47 (0.09) | 0.49 (0.09) |
| p-value, comparison with baseline | 0.382 | 0.038 | 0.195 | |
| Kidney allograft | ||||
| Shear wave speed (ms) | ||||
| Mean (SD) | 2.41 (0.25) | 2.59 (0.35) | 2.67 (0.27) | 2.70 (0.21) |
| p-value, comparison with baseline | 0.136 | 0.012 | 0.004 | |
| Phase angle of the loss modulus (rad) | ||||
| Mean (SD) | 0.73 (0.06) | 0.78 (0.08) | 0.79 (0.03) | 0.80 (0.04) |
| p-value, comparison with baseline | 0.180 | 0.021 | 0.018 |
| Variable | Fibrosis Score | Portal/Periportal Activity | Lobular Activity |
|---|---|---|---|
| (n = 13) | (n = 13) | (n = 13) | |
| Shear wave speed | 0.358 a | −0.227 a | 0.040 b |
| Phase angle of the loss modulus | 0.521 a | 0.147 a | 0.277 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marticorena Garcia, S.R.; Althoff, C.E.; Dürr, M.; Halleck, F.; Budde, K.; Grittner, U.; Burkhardt, C.; Jöhrens, K.; Braun, J.; Fischer, T.; et al. Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection. J. Clin. Med. 2021, 10, 510. https://doi.org/10.3390/jcm10030510
Marticorena Garcia SR, Althoff CE, Dürr M, Halleck F, Budde K, Grittner U, Burkhardt C, Jöhrens K, Braun J, Fischer T, et al. Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection. Journal of Clinical Medicine. 2021; 10(3):510. https://doi.org/10.3390/jcm10030510
Chicago/Turabian StyleMarticorena Garcia, Stephan R., Christian E. Althoff, Michael Dürr, Fabian Halleck, Klemens Budde, Ulrike Grittner, Christian Burkhardt, Korinna Jöhrens, Jürgen Braun, Thomas Fischer, and et al. 2021. "Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection" Journal of Clinical Medicine 10, no. 3: 510. https://doi.org/10.3390/jcm10030510
APA StyleMarticorena Garcia, S. R., Althoff, C. E., Dürr, M., Halleck, F., Budde, K., Grittner, U., Burkhardt, C., Jöhrens, K., Braun, J., Fischer, T., Hamm, B., Sack, I., & Guo, J. (2021). Tomoelastography for Longitudinal Monitoring of Viscoelasticity Changes in the Liver and in Renal Allografts after Direct-Acting Antiviral Treatment in 15 Kidney Transplant Recipients with Chronic HCV Infection. Journal of Clinical Medicine, 10(3), 510. https://doi.org/10.3390/jcm10030510







