Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Anti-SARS-CoV-2 Antibody Tests
2.3. Erythrocyte IMPDH Activity Measurement
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-death in 17 million patients using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; de Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.; Halleck, F.; Weber, U.A.; Szelinski, F.; Jahrsdofrer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schwebe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Herrera, S.; Colmenero, J.; Pascal, M.; Escobedo, M.; Castel, M.A.; Sole-González, E.; Palou, E.; Egri, N.; Ruiz, P.; Mosquera, M.; et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021, 21, 3971–3979. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.L.; Potekhin, A.; Staub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Pross, V.; Hammett, C.; Schrezenmeier, H.; et al. B and T Cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Kroger, H.; Arndt, P.; Sradnick, J.; et al. Cellular and humoral immune responses after three doses of BNT162b2 mRNA SARS-Cov-2 vaccine in kidney transplant. Transplantation 2021, 105, e267–e269. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: A case series. Transplantation 2021, 105, e280–e281. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Yuki, E.F.N.; Borba, E.F.; Pasoto, S.G.; Seguro, L.P.; Lopes, M.; Saad, C.G.S.; Medeiros-Ribeiro, A.C.; Da Silva, C.A.; de Andrade, D.C.O.; de Vinci, L.K.; et al. Impact of distinct therapies on antibody response to SARS-CoV-2 vaccine in systemic lupus erythematosus. Arthritis Care Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bergan, S.; Brunet, M.; Hesselink, D.A.; Johnson-Davis, K.L.; Kunicki, P.K.; Lemaitre, F.; Marquet, P.; Molinaro, M.; Noceti, O.; Pattanaik, S.; et al. Personalized Therapy for Mycophenolate: Consensus Report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2021, 43, 150–200. [Google Scholar] [CrossRef] [PubMed]
- Glander, P.; Sombogaard, F.; Budde, K.; van Gelder, T.; Hambach, P.; Liefeldt, L.; Lorkowski, C.; Mai, M.; Neumayer, H.H.; Vulto, A.G.; et al. Improved assay for the nonradioactive determination of inosine 5′-monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther. Drug Monit. 2009, 31, 351–359. [Google Scholar] [CrossRef]
- Glander, P.; Waiser, J.; Hambach, P.; Bachmann, F.; Budde, K.; Eckardt, K.U.; Friedersdorff, F.; Gaedeke, J.; Kron, S.; Lorkowski, C.; et al. Inosine 5′-monophosphate dehydrogenase activity for the longitudinal monitoring of mycophenolic acid treatment in kidney allograft recipients. Transplantation 2021, 105, 916–927. [Google Scholar] [CrossRef]
- Schmidt, D.; Osmanodja, B.; Pfefferkorn, M.; Graf, V.; Raschke, D.; Duettmann, W.; Naik, M.G.; Gethmann, C.J.; Mayrdorfer, M.; Halleck, F.; et al. TBase—An Integrated Electronic Health Record and Research Database for Kidney Transplant Recipients. J. Vis. Exp. 2021, e61971. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Kling, K.; Vygen-Bonnet, S.; Burchard, G.; Heininger, U.; Kremer, K.; Wiedermann, U.; Bogdan, C. STIKOEmpfehlung zur COVID-19-Impfung bei Personen mit Immundefizienz und die dazugehörige wissenschaftliche Begründung. Epid. Bull. 2021, 39, 11–41. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Marrahi, E.; Cucchiari, E.; Cuadrado-Payán, E.; Cofan, F.; Torregrosa, J.V.; Ventura-Aguiar, P.; Revuelta, I.; Bodro, M.; Pineiro, G.J.; Esforzado, N.; et al. SARS-CoV-2 infection after full vaccination in kidney transplant recipients. Transplantation 2021, 105, e278–e279. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody titers and protection against a SARS-CoV-2 infection. J. Infect. 2021, 82, 248–288. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dols, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Modelli de Andrade, L.G.; de Sandes-Freitas, T.V.; Requião-Moura, L.R.; Viana, L.A.; Cristelli, M.P.; Garcia, V.D.; Cunha Alcântara, A.L.; de Matos Esmeraldo, R.; Filho, M.A.; Pacheco-Silva, A.; et al. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am. J. Transplant. 2022, 22, 610–625. [Google Scholar] [CrossRef]
- Colvin, M.M.; Smith, C.S.; Tullius, S.G.; Goldstein, D.R. Aging and the immune response to organ transplantation. J. Clin. Investig. 2017, 127, 2523–2529. [Google Scholar] [CrossRef] [Green Version]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Chavarot, N.; Morel, A.; Leruez-Ville, M.; Vilain, E.; Divard, G.; Burger, C.; Serris, A.; Sberro-Soussan, R.; Martinez, F.; Amrouche, L.; et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am. J. Transplant. 2021, 21, 4043–4051. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Müller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2021, 21, 16851. [Google Scholar] [CrossRef] [PubMed]
- Connolly, C.M.; Chiang, T.P.; Boyarsky, B.J.; Ruddy, J.A.; Teles, M.; Alejo, J.L.; Massie, A.; Werbel, W.A.; Shah, A.A.; Christopher-Stine, L.; et al. Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: A case series. Ann. Rheum. Dis. 2021, 80, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Rozen-Zvi, B.; Mashraki, T.; Atamna, A.; Ben-Zvi, H.; Bar-Haim, E.; Rahamimov, R. Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: Protocol for a randomised controlled trial (BECAME study). BMJ Open 2021, 11, e055611. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, L.L.; Tedesco-Silva, H.; Medina-Pestana, J.O.; Felipe, C.R. Tolerability of mycophenolate sodium in renal transplant recipients. Int. J. Clin. Pharm. 2018, 40, 1548–1558. [Google Scholar] [CrossRef] [PubMed]

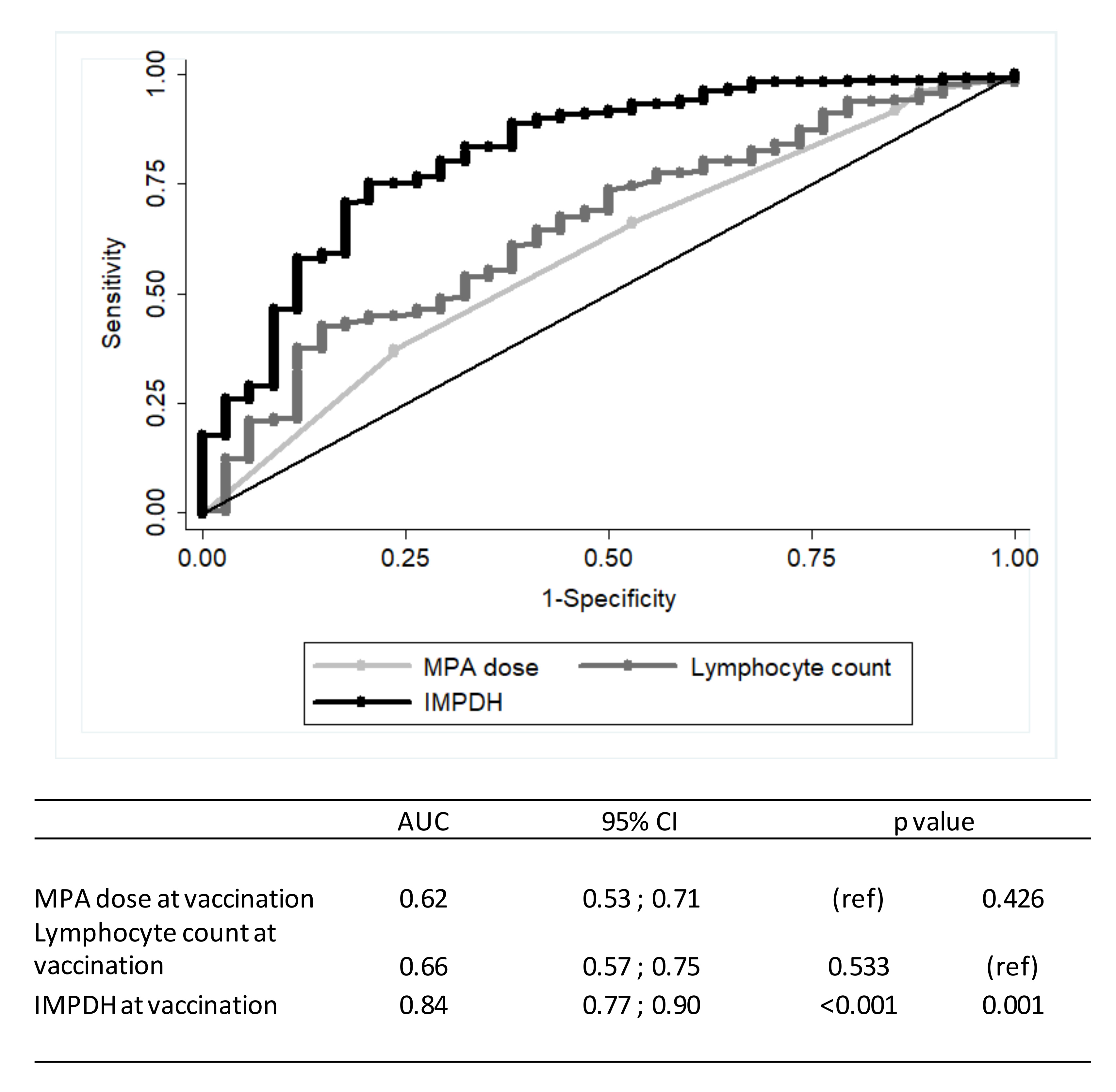
| Characteristic | Total | Responder | Non-Responder |
|---|---|---|---|
| (n = 563) | (n = 148) | (n = 415) | |
| Age at vaccination (years) | |||
| Median (range) | 58.0 (18–88) | 52 (18–81) | 59 (19–88) |
| Mean (standard deviation) | 56.2 (14.2) | 53.0 (13.8) | 57.4 (14.2) |
| Gender | |||
| Female (number; %) | 226; 40.1 | 58; 39.2 | 168; 40.5 |
| Time after transplantation (years) | |||
| Median (range) | 8.3 (0.3–37.7) | 11.5 (0.7–37.7) | 7.8 (0.3–31.3) |
| Mean (standard deviation) | 9.8 (7.5) | 13.5 (9.0) | 8.5 (6.5) |
| eGFR at vaccination (mL/min/1.73 m²) | |||
| Median (range) | 50 (9–98) | 56 (13–95) | 47 (9–98) |
| Mean (standard deviation) | 50.2 (20.2) | 56.9 (18.9) | 47.8 (20.1) |
| Vaccination (number; %) | |||
| BioNTech-Pfizer | 389; 69.1 | 97; 65.5 | 292; 70.4 |
| Moderna | 60; 10.7 | 20; 13.5 | 40; 9.6 |
| AstraZeneca | 72; 12.8 | 12; 8.1 | 60; 14.4 |
| Johnson & Johnson | 1; 0.2 | 0; 0 | 1; 0.2 |
| Mixed | 41; 7.3 | 19; 12.8 | 22; 5.3 |
| Number of immunosuppressants at vaccination | |||
| 1 (number; %) | 5; 0.1 | 4; 2.7 | 1; 0.2 |
| 2 (number; %) | 208; 36.8 | 72; 48.6 | 136; 32.8 |
| ≥3 (number; %) | 350; 62.1 | 72; 48.6 | 278; 67.0 |
| MPA treatment at vaccination (number; %) | 492; 87.4 | 92; 62.2 | 400; 96.4 |
| MPA dose (mg/day) mean (SD) | 1442.6 (495.5) | 1.244.6 (518.8) | 1.488.1 (479.2) |
| Co-immunosuppression at vaccination | |||
| Tacrolimus (number; %) | 395; 70.2 | 94; 63.5 | 301; 72.5 |
| Tacrolimus trough level (ng/mL); mean (SD) | 6.1 (1.5) | 5.7 (1.3) | 6.3 (1.6) |
| Cyclosporine (number; %) | 102; 18.1 | 43; 29.0 | 59; 14.2 |
| Cyclosporine trough level (ng/mL); mean (SD) | 100.8 (32.2) | 93.7 (20.6) | 106 (37.9) |
| Belatacept (number; %) | 45; 8.0 | 0; 0 | 45; 10.8 |
| Other: AZA (number; %) | 22; 3.9 | 17; 11.5 | 5; 1.2 |
| Other: mTORi (number; %) | 17; 3.0 | 12; 8.1 | 5; 1.2 |
| Steroid treatment | |||
| All (number; %) | 398; 70.7 | 106; 71.6 | 292; 70.4 |
| Dose ≥5 mg prednisolone equivalent daily (number; %) | 279; 49.6 | 70; 47.3 | 209; 50.4 |
| Responders | Non-Responders | Responder versus Non-Responder | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 148 | n = 415 | Univariate | Multivariate | ||||||||
| n | %Row | n | %Row | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Female | 58 | 25.7 | 168 | 74.3 | 0.99 | 0.67; 1.46 | 0.965 | 0.75 | 0.46; 1.21 | 0.240 | |
| Age at 2nd vaccination 1 | 53.0 (13.8) | 57.4 (14.2) | 0.98 | 0.97; 0.99 | 0.002 | 0.98 | 0.96; 1.00 | 0.039 | |||
| Time after kidney transplantation (years) 1 | 13.5 (9.0) | 8.5 (6.5) | 1.09 | 1.06; 1.12 | <0.001 | 1.06 | 1.02; 1.10 | 0.001 | |||
| Vaccination | |||||||||||
| AstraZeneca—AstraZeneca | 12 | 16.7 | 60 | 83.3 | 0.64 | 0.33; 1.25 | 0.192 | 0.63 | 0.27; 1.47 | 0.287 | |
| Heterologous scheme 2 | 19 | 47.3 | 22 | 53.7 | 2.53 | 1.31; 4.90 | 0.006 | 2.99 | 1.31; 6.82 | 0.009 | |
| BioNTec-Pfizer—BioNTec-Pfizer | 97 | 24.9 | 292 | 75.1 | 1.00 | 1.00 | |||||
| Moderna—Moderna | 20 | 33.3 | 40 | 66.7 | 1.44 | 0.80; 2.60 | 0.227 | 1.89 | 0.91; 3.90 | 0.086 | |
| Other | 0 | 0.0 | 1 | 100.0 | - | - | - | - | - | - | |
| Tac + MPA + Steroid | 20 | 11.5 | 154 | 88.5 | 0.15 | 0.09; 0.25 | <0.001 | 0.15 | 0.08; 0.28 | <0.001 | |
| Tac + MPA - Steroid | 42 | 23.1 | 140 | 76.9 | 0.49 | 0.32; 0.75 | 0.001 | 0.38 | 0.23; 0.64 | <0.001 | |
| CyA + MPA ± Steroid | 22 | 29.0 | 54 | 71.1 | 0.60 | 0.35; 1.02 | 0.059 | 0.51 | 0.27; 0.96 | 0.038 | |
| Belatacept + MPA ± Steroid | 0 | 0.0 | 42 | 100.0 | - | - | - | - | - | - | |
| Tac/CyA + Azathioprine—Steroid | 16 | 80.0 | 4 | 20.0 | 3.46 | 1.43; 8.32 | 0.006 | 4.22 | 1.51; 11.83 | 0.006 | |
| mTOR + MPA ± Steroid | 6 | 54.6 | 5 | 45.5 | 0.80 | 0.27; 2.35 | 0.679 | 0.79 | 0.24; 2.63 | 0.706 | |
| Tac/CyA + Steroid | 25 | 78.1 | 7 | 21.9 | 4.07 | 1.95; 8.50 | <0.001 | 4.11 | 1.71; 9.90 | 0.002 | |
| eGFR at vaccination (mL/min/1.73 m²) 1 | 56.9 (18.9) | 47.8 (20.1) | 1.02 | 1.01; 1.03 | <0.001 | 1.03 | 1.02; 1.04 | <0.001 | |||
| lymphocyte count at vaccination 1 | 1734 (1,360) | 1325 (582) | 1.07 | 1.03; 1.12 | 0.001 | 1.12 | 1.06; 1.18 | <0.001 | |||
| CNI trough levels at vaccination in % (deviation) 1,3 | 102.2 (24.8) | 109.1 (32.7) | 0.96 | 0.93; 0.99 | 0.034 | 0.94 | 0.90; 1.00 | 0.036 | |||
| Responders | Non-Responders | Responder versus Non-Responder | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 92 | n = 400 | Univariate | Multivariate | ||||||||
| n | %Row | n | %Row | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Female | 28 | 14.7 | 162 | 85.3 | 0.65 | 0.40; 1.06 | 0.085 | 0.41 | 0.20; 0.83 | 0.013 | |
| Age at 2nd vaccination | 51.7 (12.1) | 57.3 (14.2) | 0.97 | 0.96; 0.99 | 0.001 | 0.96 | 0.94; 0.99 | 0.002 | |||
| Time after kidney transplantation (years) | 12.3 (7.6) | 8.3 (6.2) | 1.09 | 1.05; 1.13 | <0.001 | 1.07 | 1.01; 1.13 | 0.031 | |||
| Vaccination | |||||||||||
| AstraZeneca—AstraZeneca | 5 | 8.1 | 57 | 91.9 | 0.41 | 0.16; 1.06 | 0.066 | 0.53 | 0.15; 1.86 | 0.323 | |
| Heterologous scheme 1 | 14 | 40.0 | 21 | 60.0 | 3.08 | 1.48; 6.40 | 0.003 | 2.86 | 0.90; 9.05 | 0.074 | |
| BioNTec-Pfizer—BioNTec-Pfizer | 61 | 17.8 | 282 | 82.2 | 1.00 | 1.00 | |||||
| Moderna—Moderna | 12 | 23.5 | 39 | 76.5 | 1.42 | 0.70; 2.88 | 0.330 | 2.53 | 0.90; 7.08 | 0.077 | |
| Other | 0 | 30.8 | 1 | 100.0 | - | - | - | - | - | - | |
| Immunosuppression | |||||||||||
| MPA + CyA ± Steroid | 22 | 29.0 | 54 | 71.1 | 1.32 | 0.81; 2.14 | 0.262 | 1.30 | 0.57; 2.98 | 0.534 | |
| MPA + Tacrolimus - Steroid | 41 | 22.7 | 140 | 77.4 | 1.05 | 0.70; 1.56 | 0.823 | 1.11 | 0.58; 2.15 | 0.750 | |
| MPA + Tacrolimus + Steroid | 20 | 11.5 | 154 | 88.5 | 0.36 | 0.22; 0.58 | <0.001 | 0.43 | 0.20; 0.95 | 0.036 | |
| MPA + Belatacept ± Steroid | 0 | 0.0 | 42 | 100.0 | - | - | - | - | - | - | |
| eGFR at vaccination (mL/min/1.73 m²) 2 | 59.3 (17.4) | 48.2 (19.6) | 1.03 | 1.02; 1.04 | <0.001 | 1.03 | 1.01; 1.05 | 0.014 | |||
| Lymphocyte count at vaccination 2 | 1781.1 (1597.6) | 1321.4 (582.1) | 1.14 | 1.08; 1.20 | <0.001 | 1.06 | 0.99; 1.14 | 0.077 | |||
| CNI trough levels at vaccination (% deviation) 2,3 | 101.8 (23.8) | 108.9 (32.7) | 0.95 | 0.91; 1.00 | 0.038 | 0.92 | 0.84; 1.00 | 0.040 | |||
| MPA dose at vaccination (MMF equivalent in g/day) 2 | 1244.6 (518.8) | 1488.1 (479.2) | 0.78 | 0.69; 0.88 | <0.001 | 0.72 | 0.59; 0.87 | 0.001 | |||
| IMPDH activity 2 | 595.1 (437.2) | 1209.6 (614.3) | 0.29 | 0.22; 0.37 | <0.001 | 0.34 | 0.25; 0.46 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liefeldt, L.; Glander, P.; Klotsche, J.; Straub-Hohenbleicher, H.; Budde, K.; Eberspächer, B.; Friedersdorff, F.; Halleck, F.; Hambach, P.; Hofmann, J.; et al. Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring. J. Clin. Med. 2022, 11, 1697. https://doi.org/10.3390/jcm11061697
Liefeldt L, Glander P, Klotsche J, Straub-Hohenbleicher H, Budde K, Eberspächer B, Friedersdorff F, Halleck F, Hambach P, Hofmann J, et al. Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring. Journal of Clinical Medicine. 2022; 11(6):1697. https://doi.org/10.3390/jcm11061697
Chicago/Turabian StyleLiefeldt, Lutz, Petra Glander, Jens Klotsche, Henriette Straub-Hohenbleicher, Klemens Budde, Bettina Eberspächer, Frank Friedersdorff, Fabian Halleck, Pia Hambach, Jörg Hofmann, and et al. 2022. "Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring" Journal of Clinical Medicine 11, no. 6: 1697. https://doi.org/10.3390/jcm11061697
APA StyleLiefeldt, L., Glander, P., Klotsche, J., Straub-Hohenbleicher, H., Budde, K., Eberspächer, B., Friedersdorff, F., Halleck, F., Hambach, P., Hofmann, J., Koch, N., Schmidt, D., Schrezenmeier, E., Seelow, E., Weber, U., Zukunft, B., Eckardt, K.-U., Choi, M., Bachmann, F., & Waiser, J. (2022). Predictors of Serological Response to SARS-CoV-2 Vaccination in Kidney Transplant Patients: Baseline Characteristics, Immunosuppression, and the Role of IMPDH Monitoring. Journal of Clinical Medicine, 11(6), 1697. https://doi.org/10.3390/jcm11061697






