Kinases and Cancer
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Tumor Microenvironment".
Submission Status: Closed | Viewed by 169480Editor
2. MAP Kinase Resource, Bioinformatics, Melchiorstrasse 9, CH-3027 Bern, Switzerland
Interests: breast cancer; prostate cancer; acute myeloid leukemia (AML); kinases
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
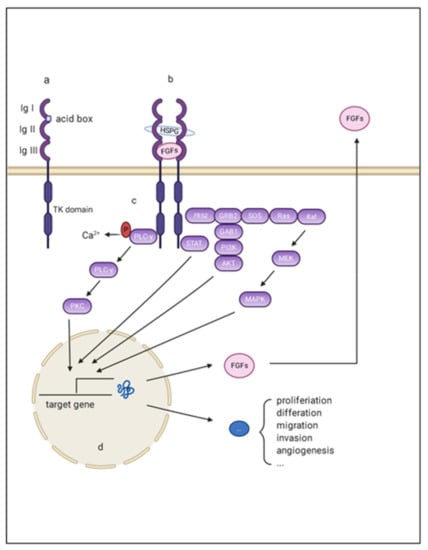
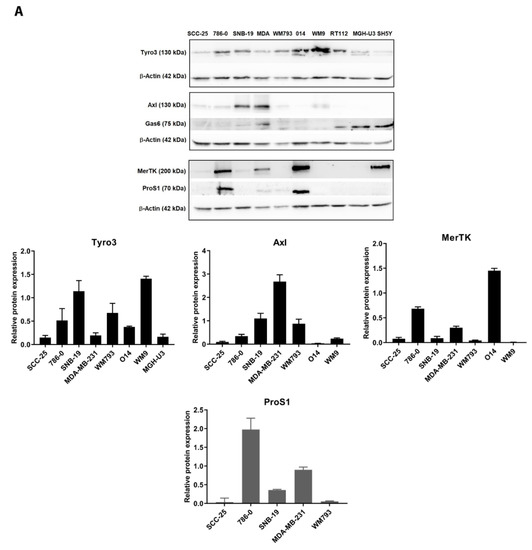
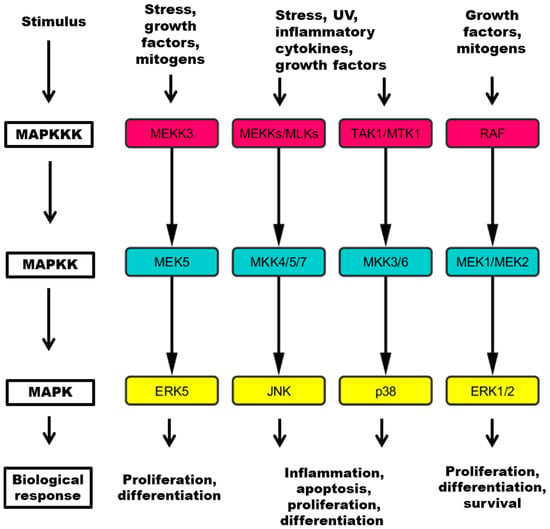
Protein phosphorylation is one of the most critical mechanisms for regulating various cellular functions. Consequently, protein kinase dysfunctions have been associated with the development of various types and subtypes of human cancers. Some examples include amplification and overexpression of the epidermal growth factor receptor (EGFR) and the ERBB2 receptor in lung and breast tumors, mutation of the BRAF oncogene in a wide range of human tumors, and the chromosomal translocation known as the Philadelphia chromosome, which results in ABL1 tyrosine kinase activation.
In the drug discovery field, despite early skepticism on the potential and selectivity of protein kinase inhibitors, enormous progress has been made. New classes of drugs, such as antibodies to receptors, and small-molecule inhibitors, were developed and tested in the clinics. The success of such drugs (e.g., imatinib and trastuzumab) encouraged development of new, second-generation drugs, which target protein kinases in cancer. Moreover, kinases have been shown to be invaluable diagnostic, prognostic, and predictive biomarkers for many types of cancers. In addition to protein kinases, lipid kinases, such as phosphatidylinositol-3-kinase (PI3K) and phosphatidylinositol-4-kinase (PI4K), have also been involved in the pathology of many tumors. In this issue of Cancers, “Kinases and Cancers”, experts are invited to contribute original research papers or review articles that will provide further insights on the various functions of kinases in cancers, and on their role as drug targets and biomarkers.
Dr. Jonas Cicenas
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- kinase inhibitors in cancer therapy
- monoclonal antibodies against kinases in cancer therapy
- kinases as oncogenes
- kinases as tumor supressors
- kinases as biomarkers
- protein phosphorylation and cancer
Full article