- Article
Perceived Transparency from Dynamic Luminance Modulation in Uniform Center–Surround Displays
- Soomin Kim and
- Sung-Ho Kim
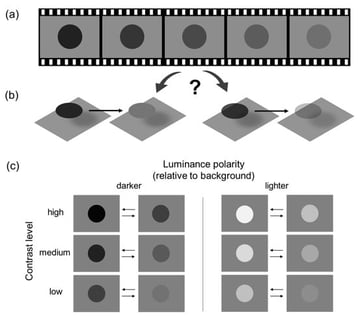
We report a novel phenomenon in which dynamic changes in luminance are perceived as changes in transparency rather than as changes in surface lightness. Participants viewed an achromatic disc on a uniform gray background and indicated whether the observed change was best described in terms of lightness or transparency. In Experiment 1, transparency-change responses were more frequent at low contrast and were strongly biased toward sequences in which contrast decreased over time, revealing a pronounced asymmetry between decreasing and increasing contrast trajectories. Experiment 2 introduced a size manipulation, such that the disc either expanded or contracted during the luminance modulation. Transparency-change responses were highest when contrast decreased and the disc expanded, indicating that spatial expansion further amplifies transparency-related interpretations of the disc’s surface appearance. Overall, the results reveal a systematic asymmetry in how contrast-change direction shapes visual appearance, consistent with a forward bias in the processing of continuously changing visual signals. When contrast dynamically approached the background level, perceptual representations appeared to be weighted toward the upcoming low-contrast state, enhancing impressions of increasing transparency. These findings demonstrate that even minimal displays lacking traditional geometric cues to transparency can evoke strong transparency impressions, driven by predictive weighting of spatiotemporal contrast trajectories rather than by static image properties alone.
6 February 2026