Abstract
Coffee silverskin is generated in large quantities as a co-product during the roasting process of coffee beans. This co-product is rich in bioactive compounds that offer potential health benefits, justifying its consideration as a functional ingredient in food. In this study, silverskin from the species Coffea arabica and Coffea canephora from six different countries was characterized to highlight its potential and applicability as a safe ingredient in new food formulations. The results revealed a dietary fiber content ranging from 71.81 to 76.86 g/100 g, with a high portion of insoluble fiber ranging from 54.02 to 60.58 g/100 g. The mineral content showed that, in all samples, potassium and calcium were the main elements with values ranging from 6.66 to 17.57 mg/g and from 9.25 to 16.44 mg/g, respectively. The caffeine content was quantified with levels ranging from 0.81 to 7.32 mg/g. In addition, high levels of phenolic compounds were identified in free and bound forms, with 5-caffeoylquinic, 3-caffeoylquinic, 4,5-dicaffeoylquinic, and ferulic acids being the main components in both fractions. All samples analyzed showed a good antioxidant capacity in the four different methods used, with values ranging from 8.12 to 10.85 mg Trolox Equivalents (mgTE/g) in the DPPH assay; from 9.69 to 19.68 mgTE/g in the FRAP assay; from 5.96 to 11.05 mgTE/g in the FRAP assay; and from 0.21 to 1.11 and 4.69 mg EDTA/g sample in the FIC assay. In conclusion, coffee silverskin has the potential to play a beneficial role as an ingredient in new food formulations, thus contributing to the development of a circular economy in the food industry.
1. Introduction
Coffee plants are perennial shrubs belonging to the Rubiaceae family. Although more than 120 wild coffee species have been identified, only two are commercially cultivated for global trade and consumption: Coffea arabica (Arabica) and Coffea canephora (Robusta). C. arabica is the most widely cultivated, representing around 55–60% of the world’s coffee supply [1]. The coffee fruits, named cherries, which are approximately 10 mm in size and oval-shaped, contain green coffee beans. These beans are enveloped by several layers: a thin skin referred to as the coffee silverskin, an endocarp known as the parchment, a peptic adhesive layer, the pulp, and the outer skin known as the epicarp [2].
Coffee is one of the most consumed beverages worldwide, mainly due to its stimulating properties [3]. It is worth noting that several studies have demonstrated that coffee has health benefits due to its high levels of antioxidants, particularly phenolic compounds, which are associated with a reduced risk of developing several chronic diseases like cancer and cardiovascular disease [4]. In addition, coffee consumption has been linked to improvements in gut microbiota and a decreased risk of diabetes, obesity, and cardiovascular mortality [5].
According to the International Coffee Organization (ICO), the estimated total world production of coffee in the year 2021–2022 was 10 million tons, while consumption of the beverage increased by 3.3%, showing steady growth over the last few years [6]. The highest production, in 2024–2025, was located in Brazil, followed by Vietnam, Colombia, and Indonesia [7]. Coffee is prepared for consumption by roasting the coffee beans at high temperatures before grinding them, to obtain a final product with a characteristic aroma, flavor, and color [4]. During the roasting process, the coffee bean increases in size, and the silverskin, a thin tegument that covers the green coffee beans, is detached. This thin layer removed from the bean is the only co-product of the roasting stage and represents approximately 4–5% of the bean weight [8]. Due to the high volume of coffee roasted worldwide, large quantities of this co-product are generated. It is thus important to properly manage the accumulation of this co-product to reduce its environmental impact [9]. The main uses given to this co-product have been as biofuel, compost production, and soil fertilization. However, in recent years, several studies have been carried out to find new applications for coffee silverskin, due to the high content of bioactive compounds found in its composition, including dietary fiber, proteins, sugars, methylxanthines like caffeine, phenolic acids (mainly chlorogenic and neochlorogenic acids), and flavonoids, among others [1,10,11]. Due to this composition and the low fat and carbohydrate content, it would be interesting and useful to use the coffee silverskin as an innovative and functional food ingredient. In this sense, several studies explored the possibility of using coffee silverskin as an ingredient in various matrices. Cantele et al. [12] proposed the production of biscuits enriched with coffee silverskin obtained from C. arabica and C. canephora, improving the polyphenolic compound and dietary fiber content. Similarly, Dauber et al. [1] developed cookies enriched with coffee silverskin powder, obtaining a product with higher antioxidant activity and phenolic profile than the control. Bertolino et al. [13] developed a whole cow milk yogurt with added coffee silverskin and stated that silverskin can be used in yogurt production to increase the nutraceutical value of the products. Therefore, the objective of this study was to evaluate the chemical, physico-chemical, techno-functional, antioxidant, and bioactive composition, including the determination of free and bound polyphenolic compounds as well as caffeine content. Coffee silverskin derived from Coffea arabica and Coffea canephora from six different geographical origins and roasted under the same conditions was analyzed in order to assess its suitability as an ingredient for the development of novel functional food products. Unlike earlier studies that focused on single origins, mixed by-products, extract-based analyses, or specific compositional aspects, this work systematically compares coffee silverskin derived from C. arabica and C. canephora from six different geographical origins, all roasted under identical conditions. A major novelty lies in the simultaneous determination of free and bound polyphenolic compounds, offering a more complete evaluation of the antioxidant potential of coffee silverskin within the whole matrix. Additionally, caffeine content and techno-functional properties relevant to food formulation are jointly assessed, providing new insights into the influence of species and origin on the suitability of coffee silverskin for the development of novel functional food products.
2. Materials and Methods
2.1. Plant Material
The silverskin samples were obtained from two different species, Coffea arabica (Arabica) and Coffea canephora (Robusta), and supplied by the CoffeeShop company (Elche, Spain). Within these species, a distinction was made between the silverskins obtained from roasting coffee beans from six different countries: Kenya (SSFK), Guatemala (SSFG), Ethiopia (SSFE), Rwanda (SSFR), and Burundi (SSFB), belonging to the species C. arabica, and India (SSFI), belonging to the species C. canephora. The different coffee beans were subjected to a light roasting process at a temperature of 195 °C for 12 min.
To obtain the flour, the silverskins were ground in a Black + Decker coffee grinder ( Black + Decker, Towson, MD, USA) for 40 s. After that, the samples obtained were passed through a mesh (0.417 mm) and stored in vacuum bags until use.
2.2. Chemical Composition
The chemical composition (fat, protein, total dietary fiber, soluble dietary fiber, and insoluble dietary fiber) was determined following the official methods described by the Association of Official Agricultural Chemists [14]. The mineral content was assessed using inductively coupled plasma-mass spectrometry (ICP-MS, Shimadzu MS-2030, Shimadzu, Kioto, Japan). The ICP-MS was operated under the following conditions: carrier gas 42 L/h; plasma gas 540 L/h; auxiliary gas 66 L/h; radio frequency 1200 W; and energy filter 7.0 V. The results were expressed as mg/100 g of flour.
2.3. Physico-Chemical Properties
The pH of the samples was measured with a pH-meter Crison-507 (Crison, Barcelona, Spain) from the solution formed by mixing the flour with distilled water in a ratio of 1:10. The pH meter was calibrated daily using standard buffer solutions (pH 4.00, 7.00, and 10.00) at room temperature before each measurement. Automatic temperature compensation was applied during all readings. The water activity (Aw) of dry flours was directly determined at 25 °C with an electric hygrometer Novasina TH-500 (Novasina, Pfaeffikon, Switzerland). As regards color coordinates, L* (lightness), a* (redness), and b* (yellowness) of silverskin flours were evaluated. For this purpose, the different silverskin flours were placed in the granular materials attachment, compacted, and measured using a Minolta CM-700 spectrocolorimeter (Minolta Camera Co., Osaka, Japan) with the following settings: illuminant D65, SCI mode, and 10° standard observer angle.
2.4. Techno-Functional Properties
Water holding capacity (WHC) and oil holding capacity (OHC) were determined following the methodology described by Chau et al. [15]. The WHC of each sample was expressed as the weight of water held by 1 g of the corresponding sample, whilst OHC was expressed as the weight of oil held by 1 g of the corresponding sample. The swelling capacity (SWC) was determined following the methodology described by Robertson et al. [16], and the results were expressed as mL water per g of sample (mL/g).
2.5. Polyphenolic Profile
2.5.1. Extraction Method
The extraction of polyphenolic compounds was separated into two fractions: (i) extracts containing free polyphenolic compounds and (ii) extracts containing bound polyphenolic compounds. To obtain the extracts with free polyphenolic compounds, the procedure described by Genskowsky et al. [17] was used. To obtain the extract with bound polyphenolic compounds, the pellet produced after the free polyphenolic compounds extraction was used, following the methodology described by Mpofu et al. [18]. The extracts obtained (free and bound) were maintained at −40 °C until high-performance liquid chromatography analysis.
2.5.2. High Performance Liquid Chromatography Analysis
Polyphenolic profiles of free and bound extracts obtained from all samples were determined by high-performance liquid chromatography following the methodology described by Genskowsky et al. [17]. The identified compounds were quantified according to the peak area measurements, which were reported in calibration curves of the corresponding authentic standards. The values were expressed as mg/100 g of flour.
2.6. Caffeine Content
The caffeine content of the extracts obtained in Section 2.5.1 was assessed. The HPLC assay was performed with the method proposed by Grillo et al. [19]. The caffeine content was determined based on peak area measurements, which were compared to calibration curves from the authentic standard.
2.7. Antioxidant Analysis
2.7.1. Extraction Method
Silverskin samples (1 g) were mixed with 10 mL of methanol: water (80:20, v/v) and then sonicated for 15 min. After centrifugation for 18 min, 4800× g at 4 °C, the supernatants were collected, and the pellets were mixed with 10 mL of acetone: water (70:30, v/v), and the same procedure was performed. Then, both supernatants were combined and evaporated to dryness in a rotary-evaporator Labtech EV311 Plus (Labtech, Sorisole BG, Italy). Eight milliliters of methanol were added to the residue, and the mixture was thoroughly shaken in a Vortex for 2 min. The methanolic extract was filtered through a 0.45 μm filter and stored at −40 °C until use.
2.7.2. Methodology
The antioxidant capacity was assessed by means of the following four in vitro spectrophotometric assays. The DPPH assay was performed using the stable radical 2,2-diphenyl-1-picrylhydrazyl, following the method proposed by Brand-Williams et al. [20]. The ferric reducing antioxidant power (FRAP) was assessed by means of the potassium ferricyanide-ferric chloride method described by Oyaizu [21]. The TEAC-ABTS assay was applied following the method proposed by Gullón et al. [22]. In all three methods, Trolox was used as a reference standard, and results were expressed as mg Trolox equivalents/g of sample. Finally, the ferrous ions chelating activity (FIC) was determined by means of the method described by Mahdavi [23]. EDTA was used as a reference standard, and the results were expressed as mg EDTA/g of sample.
2.8. Statistical Analysis
Results obtained from chemical composition, physico-chemical and techno-functional properties, as well as antioxidant capacity of the different silverskin flours were reported as average ± standard deviation. These data, from the three independent trials, were subjected to one-way analysis of variance (ANOVA). To evaluate the statistical significance (p < 0.05) between the different samples. The means comparisons were made using the Tukey HDS test. The Statgraphics Centurion XVI program (Statgraphics Technologies, Inc., The Plains, VA, USA) was used for these statistical analyses.
3. Results and Discussions
3.1. Chemical Composition
Table 1 shows the chemical composition values of the different coffee silverskin flours. Regarding moisture content, the values obtained ranged between 4.50 and 6.41 g/100 g of sample, with statistical differences (p < 0.05) between samples. In previous studies conducted by Costa Vimercati et al. [24], the moisture content of coffee silverskin obtained from C. arabica was 5.03 g/100 g; more recently, Gottstein et al. [10] reported that the moisture content of silverskin obtained from C. arabica and C. canephora was 6.15 and 6.57 g/100 g, respectively. These values were consistent with those obtained in this study. Moisture is a very important factor to consider since higher moisture can be indicative of lower stability and greater susceptibility to microbiological degradation, which could affect the storage and potential use of silverskin as an ingredient in the food industry. For the protein content, the silverskin flour obtained from C. canephora had the highest value (p < 0.05). On the other hand, the flours obtained from C. arabica showed values contained between 12.37 and 14.21 g/100 g, with statistical differences (p < 0.05) among them. The values obtained in this study were lower than those reported in the scientific literature, which ranged between 17.0 and 20.0 g/100 g of sample [10,25]. The fat content of the silverskin assessed in this work ranged from 1.20 to 2.10 g/100 g. These values were slightly lower than those described by Bobková et al. [26] or Bertolino et al. [13], who reported values ranging from 2.4 to 3.5 g/100 g in coffee silverskins obtained from C. arabica and C. canephora. It is important to highlight that the samples obtained from C. arabica showed a higher fat content (p < 0.05) than those obtained for C. canephora. In reference to ash content, the values obtained for all samples analyzed were comprised between 4.23 and 5.34 g/100 g with statistical differences between samples (p < 0.05). The values obtained for this parameter, in all samples, were lower than those reported in the literature, which were around 7–8 g/100 g [27,28].

Table 1.
Chemical composition of silverskin flours obtained from roasting coffee beans from six different origins.
The chemical composition is strongly influenced by several factors, such as soil characteristics and agricultural practices, as well as the roasting conditions. Additionally, genetic background, cultivation environment, and processing steps collectively shape silverskin chemical composition [10].
The total dietary fiber (TDF), insoluble dietary fiber (IDF), and soluble dietary fiber (SDF) contents of the silverskin flours are summarized in Table 1. In reference to TDF content, the silverskin flours analyzed in this study showed values ranging from 71.81 to 76.86 g/100 g, with the samples cultivated in Ethiopia and Guatemala those that showed the highest value (p < 0.05). The total dietary fiber content of silverskin shows great variability, although it is usually between 60 and 85%. Thus, Thangavelu et al. [29] and Franca et al. [30] reported that coffee silverskin from C. arabica species showed a TDF content of 69.89 and 68.30 g 100 g, respectively, while Gottstein et al. [10] informed that the TDF content of C. arabica and C. canephora silverskins were 67.0 and 62.0 g/100 g values lower than those obtained in this work. However, several authors [31,32] reported similar values (around 70–75 g/100 g) to those obtained in this study. In all cases, the IDF fraction was higher than the SDF, with the sample obtained from Rwanda showing the highest (p < 0.05) values, without statistical differences with the flours obtained from India and Guatemala (p > 0.05). A high content of IDF in powders or flours obtained from co-products may be considered a benefit because IDF can be used by the food industry to increase the water-holding and oil-holding properties, as well as the swelling capacity [33]. Additionally, a high IDF content might have valuable health effects related to increasing satiety, the fecal bulk, and accelerating intestinal transit, thus promoting improved functioning of the digestive system [34].
In reference to mineral content, Table 2 displays the mineral profile of silverskin flours obtained from roasting coffee beans from six different origins. In SSFI (C. canephora), the predominant element (p < 0.05) was potassium, followed by calcium and phosphorus. On the other hand, in samples obtained from C. arabica, the main compound (p < 0.05) was calcium (except in SSFR), followed by potassium, and depending on the type of sample, in some cases phosphorus (SSFG, SSFE, and SSFR) and in others magnesium (SSFK and SSFB). Iron and sodium were also detected in considerable amounts (Table 2). The elements zinc, manganese, and copper were detected, in trace amounts, in all coffee silverskin flour analyzed.

Table 2.
Mineral profile of silverskin flours obtained from roasting coffee beans from six different origins.
In the scientific literature, there is a great diversity of results relating to the composition and concentration of minerals in coffee silverskin. Thus, Iriondo-DeHond et al. [35] reported that the coffee silverskin from C. arabica species had a micronutrient profile composed of potassium (56.0 mg/g), magnesium (5.30 mg/g), sodium (4.60 mg/g), and calcium (3.50 mg/g). Similarly, Costa et al. [25] stated that the mineral composition of coffee silverskin consisted mainly of potassium (50 mg/g), magnesium (20 mg/g), and calcium (5 mg/g). In a more recent study, Martuscelli et al. [36] reported that the predominant elements in coffee silverskin obtained from a blend of C. arabica and C. canephora were calcium (5.46 mg/g), followed by magnesium (2.22 mg/g) and phosphorus (1.46 mg/g). These differences may be attributed to the conditions in which the coffee beans were cultivated. Although all the flours correspond to the same variety, C. arabica (except for SSFI, which corresponds to the species C. canephora), the coffee beans are grown in different parts of the world, meaning that climate, soil, water, and altitude, among other factors, may influence the mineral profile of the coffee bean and, consequently, the silverskin [37,38].
3.2. Physico-Chemical Properties
Table 3 shows the results of the physico-chemical properties of the flours obtained from coffee silverskin of different origins. In reference to pH, the values ranged between 4.30 (SSFE) and 5.79 (SSFI), with statistical differences (p < 0.05) between all samples analyzed. It is important to highlight that the flour obtained from variety C. canephora had the highest pH value. The values obtained in this work were lower than those reported in scientific literature for coffee silverskin from different varieties and origins. Thus, Vargas-Sanchez et al. [39] reported that pH values of coffee silverskin flakes and powder from dark C. arabica were 6.09 and 6.30, respectively. The water activity values (Aw) varied from 0.400 to 0.586. Thus, SSFG and SSFE showed the highest values (p < 0.05) without statistical differences between them (p > 0.05). These results agree with those reported by Martuscelli et al. [36] in silverskin flour obtained from a blend of C. arabica and C. canephora varieties. The low value obtained on coffee silverskin flours examined indicates that the risk of deterioration provoked by bacterial or mold strains, enzymes, and non-enzymatic reactions is minimal [40]. With the obtained values of water activity (less than 0.586) and pH (less than 4.5 in many cases), these flours would be stable against degradation caused by the growth of microorganisms.

Table 3.
Physico-chemical Properties of silverskin flours obtained from roasting coffee beans from six different origins.
Color is one of the most important parameters to characterize when using flours or powders from the valorization of co-products as ingredients in the development of new foods, since these flours or powders could change the appearance of the product to which they are added. The color properties of coffee silverskin flours obtained from different origins are given in Table 3. For lightness (L*), no statistical (p > 0.05) differences were found among samples SSFB, SSFK, SSFE, and SSFI, with values ranging between 53.92 and 55.45, whilst SSFR and SSFG showed lower (p < 0.05) values without differences between them (p > 0.05). The L* of the flour will depend on several factors, such as the moisture content, the type of drying, and particle size. For all samples, the values obtained were higher than those reported by Quagliata et al. [41] in coffee silverskins obtained from Arabica–Robusta blends subjected to sugar-glazing treatment (L* = 38.30) and without treatment (L* = 46.82). As regards redness (a*), SSFB, SSFK, and SSFR had the highest (p < 0.05) values, followed by SSFB and SSFE without differences between them (p > 0.05). It is important to highlight that the flour obtained from C. arabica showed the lowest (p < 0.05) values for this coordinate. The values obtained were similar to those reported by Vargas-Sánchez et al. [39] on coffee silverskin powder from dark C. arabica. In reference to yellowness (b*), the values for this coordinate range between 21.34 and 27.20, with the SSFK sample showing the highest (p < 0.05) value. Again, the flour obtained from C. arabica showed the lowest (p < 0.05) values. The results of this study regarding the b* color coordinate suggest a trend towards yellow that could be related to the quality and drying process of the samples.
It is worth noting that color primarily depends on the interaction of the constitutive polyphenols and anthocyanins involved in oxidation and polymerization reactions during the coffee roasting stage. Furthermore, roasting processes lead to the degradation of proteins, and Maillard reactions occur, so melanoidins also contribute to the characteristic color of coffee silverskin [42].
3.3. Techno-Functional Properties
When assessing the feasibility of flours or powders derived from fruit or vegetable co-products as potential functional ingredients, one of the most critical parameters to consider is their hydration properties, including water-holding capacity, oil-holding capacity, and swelling capacity [43]. These characteristics play a key role in determining food stability and directly influence texture-related attributes such as hardness and juiciness [44]. Figure 1 shows the results of the techno-functional properties of the different flours obtained from coffee silverskin. Regarding WHC, SSFB and SSFR had the highest (p < 0.05) WHC of all coffee silverskin flours analyzed, with values of 5.46 and 5.10 g water/g flour, respectively. On the other hand, SSFG showed the lowest (p < 0.05) value (3.76 g water/g flour). In this case, the species C. arabica or C. canephora did not influence this property.
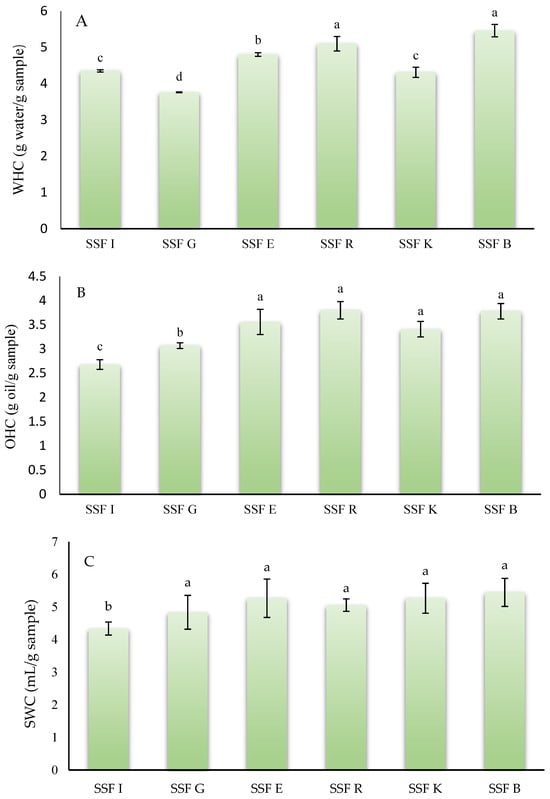
Figure 1.
Techno-functional properties of silverskin flours obtained from roasting coffee beans from six different origins. (A): water holding capacity; (B): oil holding capacity; (C): swelling capacity. SSFI: coffee silverskin flour from India; SSFG: Coffee silverskin flour from Guatemala; SSFE: coffee silverskin flour from Ethiopia; SSFR: coffee silverskin flour from Rwanda; SSFK: coffee silverskin flour from Kenia; SSFB: Coffee silverskin flour from Burundi. For the same analysis (WHC, OHC, and SWC), histograms with different letters indicate significant differences (p < 0.05) according to Tukey’s multiple range test.
The results obtained were similar to those reported by Ballesteros et al. [45], who reported that the powder obtained from mixtures of C. arabica or C. canephora coffee species had a WHC of 5.11 g water/g sample. On the other hand, Thangavelu et al. [29] reported that the WHC of coffee silverskin from arabica species treated with ultrasound ranged between 3.03 and 3.62 g water/g sample. Vargas-Sanchez et al. [39] reported that the WHC value of coffee silverskin powder from dark C. arabica was 2.9 g water/g sample. The variability in WHC values among the different samples and with the studies found in the literature may be due to several factors, such as granulometry of flours, porosity, insoluble dietary fiber content, and hydrophilic surface group availability that generally influence the WHC [46].
In reference to the oil holding capacity, SSFE, SSFR, SSFK, and SSFB had an OHC that ranged between 3.41 and 3.80 g oil/g sample, with no statistically significant differences (p > 0.05) between samples. In this case, the sample SSFI, which belongs to the species canephora, showed the lowest value (p < 0.05) for this property. The values obtained in this work were similar to those reported by Martuscelli et al. [36] in silverskin flour obtained from a blend of C. arabica and C. canephora species, who stated that this flour had an OHC of 3.02 g oil/g sample. However, Ateş and Elmacı [47] reported that coffee silverskins from the arabica species showed an OHC of 4.80 g oil/g sample, higher than reported in this study. OHC depends on several factors, including the chemical structure of the matrix, viscosity, total charge density, as well as the thickness and hydrophobic nature of the particle, as mentioned by Wang et al. [48] and Song et al. [49].
Finally, for swelling capacity, no statistical differences (p > 0.05) were found between the samples belonging to C. arabica (SSFG, SSFE, SSFR, SSFK, and SSFB) with values ranging between 4.48 and 5.45 mL/g sample. For this parameter (SWC), the flour obtained from the silverskin of C. canephora beans (SSFI) had the lowest value (4.34 mL/g sample). To the best of our knowledge, no studies have been reported in the scientific literature that have analyzed the swelling capacity of flours or powders derived from coffee silverskin. Swelling capacity is influenced by factors such as chemical structure (with higher soluble fiber content resulting in increased swelling capacity), porosity, and particle size [50]. A high swelling capacity is particularly advantageous in food applications, as it enhances product texture and overall palatability [51]. For instance, flours or powders that exhibit substantial swelling capacity can improve the juiciness of meat formulations, the structural consistency of baked goods, and the functional properties of functional food products [52].
3.4. Polyphenolic Profile
The polyphenolic profile of silverskin flours obtained from roasted coffee beans from six distinct geographical origins reveals substantial variability in both the qualitative and quantitative distribution of free and bound phenolic compounds, as shown in Table 4. It is important to highlight that, for all silverskin flour samples analyzed, the concentration of free polyphenolic compounds was lower than that of bound polyphenolic compounds. In the free fraction, seven compounds were identified in all samples, while in the bound fraction 5 compounds were detected. Statistically significant differences (p < 0.05) demonstrate that origin exerts a decisive influence on both free and bound phenolic composition, suggesting that environmental factors, post-harvest processing, and roasting behavior collectively shape the bioactive content of coffee silverskin. Overall, the results highlight the complexity of the coffee co-product matrix and underscore its potential as a functional ingredient with origin-dependent bioactive properties.

Table 4.
Polyphenolic profile of silverskin flours obtained from roasting coffee beans from six different origins.
In the free polyphenolic compounds fraction, marked disparities (p < 0.05) emerge among the samples. For instance, free vanillin concentrations vary significantly, ranging from 0.41 µg/g in SSFG to 1.63 µg/g in SSFE, differing significantly from all other origins (p < 0.05). A similar pattern is observed for free ferulic acid, which is most abundant (p < 0.05) in SSFE (2.60 µg/g) and SSFB (2.55 µg/g). The quinic acid derivatives display the most striking variation: SSFR and SSFK exhibit high levels of 3-caffeoylquinic acid (47.44 and 29.79 µg/g, respectively), whereas SSFG shows no detectable levels. These large inter-origin discrepancies, supported by significant statistical differences (p < 0.05) between samples, indicate that the stability of 3-caffeoylquinic acid during roasting may vary by bean type or roasting associated with the original raw material. Similarly, 4-caffeoylquinic, 5-caffeoylquinic, and 4,5-dicaffeoylquinic acids display heterogeneous distributions. The total free polyphenols show a distinct pattern: SSFR exhibits the highest (p < 0. 05) content (75.65 µg/g), followed by SSFK (64.95 µg/g), whereas SSFG had the lowest (p < 0. 05) concentration. On the other hand, the bound polyphenolic fraction provides even more remarkable contrasts, especially for compounds such as caffeic acid, ferulic acid, and vanillic acid derivatives. The bound caffeic acid content ranges from only 13.84 µg/g in SSFG to an outstanding 65.11 µg/g in SSFE, with significant differences (p < 0. 05) between all origins. Bound ferulic acid shows the highest concentration in SSFI, with 109.10 µg/g, almost double or more compared with the other samples. One fact worth highlighting is that the silverskin flours obtained from C. canephora have a higher content of these bioactive compounds than the silverskin flours obtained from C. arabica. These results agree with Mannino et al. [53] and Panusa et al. [54], who reported that the polyphenolic content of C. arabica beans and their by-products is lower than found in C. canephora.
The observation that bound polyphenols predominate over free polyphenols in all samples is consistent with reports for numerous plant matrices and agro-food by-products. Several studies have shown that a substantial fraction of phenolic compounds is esterified or non-covalently associated with cell wall components, such as structural polysaccharides and lignin, which limits their extractability using conventional solvents [55,56]. This matrix–phenol association explains why free polyphenols usually represent only a minor fraction of the total phenolic content. In this context, the predominance of bound polyphenols suggests a strong interaction with the plant matrix, characteristic of fiber-rich tissues, and underscores the need to consider this fraction for a more realistic assessment of the functional potential of the ingredient [57].
Roasting can markedly affect the balance between free and bound polyphenols. Thermal treatment may partially cleave bonds between phenols and the cell wall, promoting the release of previously bound compounds, while higher roasting intensities can induce thermal degradation or chemical transformation of certain polyphenols [58]. Consequently, the net effect of roasting depends on the degree and duration of the treatment: light to medium roasting may enhance the release of bound phenols, whereas more intense roasting can reduce total phenolic content. These dynamics provide a mechanistic explanation for observed shifts in the proportion of free and bound polyphenols after roasting and should be taken into account when interpreting compositional data [59]. From a nutritional and functional perspective, the high proportion of bound polyphenols carries important implications for bioaccessibility and bioactivity. While free polyphenols have traditionally been considered the primary bioactive fraction, increasing evidence shows that bound polyphenols can be released during gastrointestinal digestion, particularly in the colon via microbial action, contributing to antioxidant and metabolic effects at the intestinal level [60,61]. This delayed release can extend the functional impact of the ingredient beyond the immediately bioaccessible fraction. Therefore, the predominance of bound polyphenols should not be viewed as a limitation, but rather as a feature with potential physiological relevance, especially in fiber-rich foods and plant co-products.
The content and concentration of polyphenolic compounds in silverskin depend on several factors, such as coffee species and variety, environmental conditions of cultivation, origin, and fundamentally on the conditions of time and temperature in which roasting takes place [27,62]. In this context, Nzekoue et al. [8] reported that in C. arabica silverskin obtained from beans of Ethiopian origin, eighteen phenolic compounds with 3-caffeoylquinic acid, 5-caffeoylquinic acid, and 3,5-dicaffeoylquinic acid were detected as the most abundant polyphenols, with values compressed between 3.11 mg/g and 5.44 mg/g. More recently, Giordano et al. [63] reported that the principal polyphenolic compounds found in silverskin obtained from C. arabica and C. canephora dark roasted beans were 5-caffeoylquinic acid with values of 0.61 and 0.48 mg/g, respectively, and dicaffeoylquinic acid with values of 0.14 and 0.30 mg/g for C. arabica and C. canephora silverskin. Similarly, Peixoto et al. [64] stated that the main polyphenolic compounds found in coffee silverskin obtained from a blend of both Arabica and Robusta were 4-feruloylquinic acid, 5-caffeoylquinic acid, and 5-feruloylquinic acid with values of 0.39, 1.04, and 1.05 mg/g, respectively. Several recent studies have investigated how phenolic compounds such as caffeoylquinic acids and derivatives such as ferulic acid are related to the sensory characteristics of coffee and other food products. In this sense, Linne et al. [65] reported that 3-caffeoylquinic acid and 4-caffeoylquinic acid isomers can influence mouthfeel attributes such as “body” and oral coating in coffee, with perceptible sensory effects even at ecologically relevant concentrations, although not in a linear manner with respect to compound concentration. In a similar study, Tieghi et al. [66] reported that in specialty coffees, the presence of phenolic acids, including ferulic acid, was statistically correlated with sensory scores such as sweetness and acidity, which suggests a clear relationship between chemical profile and perceived quality. In addition, research evaluating coffee blends with differing contents of caffeoylquinic acids has reported variations in attributes such as astringency and overall sensory appeal, further supporting the relevance of these compounds to coffee sensory perception [67].
3.5. Caffeine Content
Caffeine, a methylxanthine, is another main type of phytochemical present in coffee silverskin. This phytocompound has been related to several positive effects on human health, acting as anticarcinogenic, anti-obesity, or diuretic agents, among other effects [68]. Figure 2 shows the caffeine content of coffee silverskin flours of different origins. SSFB showed the highest (p < 0.05) value (7.32 mg/g), followed by SSFK, SSFE, SSFI, and SSFR with values of 5.83, 5.35, 4.37, and 4.23 mg/g, respectively, without statistical differences (p > 0.05) between them. Finally, SSFG showed the lowest (p < 0.05) value (0.85 mg/g). In the scientific literature, there is significant variation in the concentration of caffeine present in silverskin co-products, since this concentration depends on many factors such as the coffee variety, growing conditions, the origin, the roasting process, the extraction methods, and silverskin storage and collection methods, among others [8]. Thus, the values obtained in this work were similar to those reported by Machado et al. [62], who reported a caffeine content of silverskin obtained from a blend of C. arabica and C. canephora, approximately 40%:60%, respectively, of 7.1 mg/g. Gottstein et al. [10] reported that the caffeine content of C. arabica and C. canephora silverskins was 8.0 and 8.6 mg/g. Similarly, Bessada et al. [27] reported that the caffeine content in coffee silverskin from five different geographical origins ranged between 7.1 and 12.15 mg/g. On the other hand, Quagliata et al. [41] reported that silverskin obtained from Arabica–Robusta blends subjected to sugar-glazing had a caffeine content of 13.2 mg/g, while the silverskin obtained from 100% Arabica without the torrefacto treatment showed a value of 8.70 mg/g. The recovery of caffeine from coffee silverskin deeply depends on the extraction methodology. Thus, the use of novel technologies, such as ultrasonic-assisted extraction (UAE) or Pressurized Liquid Extraction (PLE) process, substantially increased the concentration of this compound. In this sense, Peixoto et al. [64] reported that the caffeine content of coffee silverskin obtained from a blend of both C. arabica and C. canephora beans and extracted using UAE was 27.70 mg/g. Similarly, Koskinakis et al. [69] reported a value of 56.7 mg/g dry extract from coffee silverskin extracted by means of the PLE process.
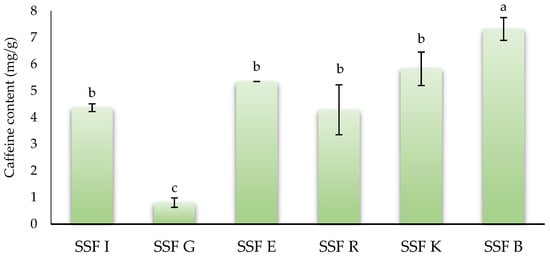
Figure 2.
Caffeine content of silverskin flours obtained from roasting coffee beans from six different origins. SSFI: coffee silverskin flour from India; SSFG: coffee silverskin flour from Guatemala; SSFE: coffee silverskin flour from Ethiopia; SSFR: coffee silverskin flour from Rwanda; SSFK: coffee silverskin flour from Kenia; SSFB: coffee silverskin flour from Burundi. Histograms with different letters indicate significant differences (p < 0.05) according to Tukey’s multiple range test.
3.6. Antioxidant Capacity
To thoroughly evaluate the antioxidant activity of a flour or powder derived from the valorization of co-products, it is necessary to employ more than one analytical methodology that encompasses different mechanisms of action. In this study, four distinct assays were utilized: ABTS, FRAP, FIC, and DPPH. Figure 3A–D illustrates the results obtained for the antioxidant capacity measurement of silverskin flours obtained from roasting coffee beans from six different origins. For the DPPH assay (Figure 3A), no significant differences (p > 0.05) were observed among SSFI, SSFG, SSFK, and SSFB, while SSFR and SSFE showed lower (p < 0.05) values without statistical differences (p > 0.05) between them. In the FRAP assay (Figure 3B), which measures the ability to reduce ferric ions, the analyzed silverskin flours showed a wide range of antioxidant capacities, with values ranging between 9.69 (SSFE) and 19.68 mg Trolox equivalents (TE)/g of sample (SSFK) with statistically significant differences (p < 0.05) between samples. For the ABTS assay (Figure 3C), the SSFE sample showed the highest (p < 0.05) value (11.05 mg TE/g), followed by SSFK (10.02 mg TE/g), whilst SSFR and SSFI showed the lowest (p < 0.05) values without statistical differences (p > 0.05) between them. Finally, for the ferrous ion chelating activity (Figure 3D), the results indicated that SSFI had the highest (p < 0.05) chelating capacity without statistical differences (p > 0.05) with SSFE.
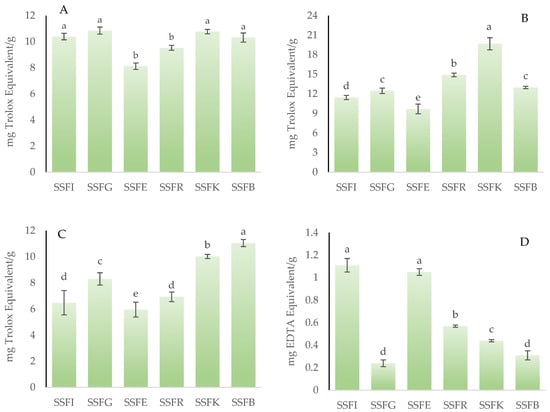
Figure 3.
Antioxidant capacity of silverskin flours obtained from roasting coffee beans from six different origins. (A): measured with DPPH assay; (B): measured with FRAP assay; (C): measured with ABTS assay; (D): measured with FIC assay. SSFI: coffee silverskin flour from India; SSFG: coffee silverskin flour from Guatemala; SSFE: coffee silverskin flour from Ethiopia; SSFR: coffee silverskin flour from Rwanda; SSFK: coffee silverskin flour from Kenia; SSFB: coffee silverskin flour from Burundi. For each antioxidant assay, histograms with different letters indicate significant differences (p < 0.05) according to Tukey’s multiple range test.
In the scientific literature, there is a lot of variability regarding the results obtained for the antioxidant activity of extracts or flours derived from coffee silverskin. This variability is attributable to multiple factors, including the species of coffee used, the method employed, and the type of extraction, among others. Furthermore, the different ways in which the results obtained can be expressed make comparing data extremely difficult. In this context, Del Castillo et al. [70] indicates that the silverskin obtained from C. canephora beans exhibits higher antioxidant activity, as measured by DPPH and ABTS assays, with values of 23.1 and 22.5 mmol TE/100 g, respectively, compared to samples obtained silverskin from C. arabica beans, which yielded values of 22.5 and 8.5 mmol TE/100 g. More recently, Nzekoue et al. [8] reported that with the DPPH assay, the methanolic extract obtained from coffee (C. arabica beans of Ethiopian origin) silverskin had an IC50 (which is the concentration of the extract necessary to cause 50% of DPPH inhibition) of 101.7 µg/mL. In a study carried out by Vargas-Sanchez et al. [39], the antioxidant capacity, measured with ABTS and FRAP assay, of coffee silverskin powder from dark C. arabica was analyzed. These authors found that this extract had % ABTS·+ inhibition of 87.89%, whereas the FRAP value was 2.65 mg Fe2+g sample. Franca et al. [30] stated that the antioxidant capacity of methanolic and ethanolic coffee silverskin extracts measured with the FRAP assay was 170.64 and 98.37 µmol Fe2SO4/g, respectively, while the DPPH-IC50 for methanolic and ethanolic coffee silverskin extracts was 1727.6 and 251.15 (g/g DPPH). Dauber et al. [1] reported that the antioxidant capacity of coffee silverskin obtained from a blend of C. arabica and C. canephora, and measured with ABTS assay, was 49.8 μmol TE/g sample. The antioxidant activity of coffee silverskin might be due to its rich profile of polyphenolic compounds, particularly chlorogenic acids and related caffeoylquinic derivatives. In this way, Dong et al. [71] showed that fractions obtained from coffee silverskin, which contain 3-caffeoylquinic acid, feruloylquinic acids, and various dicaffeoylquinic acids, exhibit the highest antioxidant capacity. These compounds, well known for their strong radical-scavenging ability, were especially concentrated in the free phenolic fraction, which correlated directly with superior in vitro antioxidant performance. Complementing these findings, Jirarat et al. [72] demonstrated that extraction techniques optimized for phenolic release significantly enhance the antioxidant potential of silverskin extracts. Their results confirmed that chlorogenic acids, other phenolic acids, and additional polyphenols are the dominant contributors to antioxidant activity, while caffeine and interactions with dietary fiber provide secondary roles.
4. Conclusions
The results obtained highlight the considerable potential of coffee silverskin, generated during the roasting process, as a promising ingredient for the development of new food products. This potential is primarily attributed to (i) its high dietary fiber content, and (ii) its notable concentration of bioactive compounds of interest, such as caffeine and polyphenolic compounds. From a circular economy perspective, the valorization of coffee silverskin as a food ingredient represents a high-value upcycling strategy that closes material loops within the coffee production chain. Given that silverskin is the only solid co-product generated during roasting and accounts for approximately 4–5% of bean weight, its conversion into a functional ingredient can significantly reduce waste streams while generating added economic value. Moreover, the demonstrated techno-functional properties allow its incorporation without extensive chemical modification, aligning with clean-label trends and sustainable food design.
Thus, silverskin flour from India (C. canephora) stands out for having the highest protein content, highest pH, and remarkably high bound polyphenol content, particularly ferulic and caffeic acids, together with strong metal chelating activity (FIC). This profile suggests its suitability for nutritionally enriched formulations such as fiber-rich supplements or functional snacks. Silverskin flour from Guatemala (C. arabica) exhibits the highest total dietary fiber content, with a particularly high soluble dietary fiber fraction, but the lowest polyphenol and caffeine contents. This makes it especially suitable for fiber enrichment purposes in food products where low stimulant content and mild bioactivity are desirable, such as bakery products or fiber-enhanced foods. Silverskin flour from Ethiopia (C. arabica) shows a balanced composition, combining high total dietary fiber, elevated bound polyphenol content, high oil-holding capacity, and strong antioxidant activity (ABTS). This sample appears well-suited for functional foods requiring lipid retention and antioxidant protection, such as meat analogues or emulsified products. Silverskin flour from Rwanda (C. arabica) is characterized by the highest insoluble dietary fiber content, very high free polyphenol concentration, particularly caffeoylquinic acids, and excellent water-holding capacity. These properties make it particularly attractive for applications aimed at texture enhancement, satiety promotion, and antioxidant delivery, such as baked goods or high-fiber formulations. Silverskin flour from Kenya (C. arabica) combines high antioxidant capacity (FRAP and ABTS), high caffeine content, good swelling capacity, and low water activity, indicating strong stability. This profile supports its potential use in energy-oriented or stimulant functional foods and formulations requiring high oxidative stability. Finally, Silverskin flour from Burundi (C. arabica) exhibits high water- and oil-holding capacities, high soluble dietary fiber, high caffeine content, and a rich bound polyphenolic profile. These characteristics suggest its suitability for food formulations demanding moisture retention and enhanced mouthfeel, such as bakery, dairy, or plant-based products.
It is also important to consider that, prior to its application as a food ingredient, the possible formation of roasting-derived compounds, such as 5-hydroxymethylfurfural or acrylamide, must be assessed, as these substances may exert detrimental effects both on the food matrix to which the ingredient is added and on the health of the final consumer.
Author Contributions
Conceptualization, M.V.-M. and R.L.-G.; methodology, L.C.-S.; validation, J.F.-L.; formal analysis, J.F.-L. and M.V.-M.; investigation, L.C.-S. and R.L.-G.; resources, R.L.-G.; data curation, J.F.-L.; writing—original draft preparation, L.C.-S.; writing—review and editing, M.V.-M.; visualization, L.C.-S.; supervision, M.V.-M. and J.A.P.-Á.; project administration, J.A.P.-Á.; funding acquisition, J.A.P.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Estatal de Investigacion, grant number CPP2021-008937” through the Research Project “Obtención de productos de alto valor añadido para el sector alimentario y cosmético del dátil del Palmeral de Elche”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the CoffeeShop company (Elche, Spain) for the donations in kind of the different coffee silverskin samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A.; et al. Cookies enriched with coffee silverskin powder and coffee silverskin ultrasound extract to enhance fiber content and antioxidant properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Yang, W.; Su, P.; Ge, Y.; Jiang, K.; Huang, J. Optimized carbonization of coffee shell via response surface methodology: A circular economy approach for environmental remediation. Environm. Pollution 2023, 346, 123018. [Google Scholar] [CrossRef] [PubMed]
- Al-Fawaeir, S.; Alawneh, J.M.; Al-Odat, I. Influence of coffee consumption on serum lipid profile parameters: Can coffee consumption lead to health consequences in humans? J. Agric. Food Res. 2023, 14, 100904. [Google Scholar] [CrossRef]
- Therdtatha, P.; Jareontanahun, N.; Chaisuwan, W.; Yakul, K.; Paemanee, A.; Manassa, A.; Moukamnerd, C.; Phimolsiripol, Y.; Sommano, S.R.; Seesuriyachan, P. Production of functional Arabica and Robusta green coffee beans: Optimization of fermentation with microbial cocktails to improve antioxidant activity and metabolomic profiles. Biocatal. Agric. Biotechnol. 2023, 53, 102869. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Market Report: March 2023. Available online: https://www.ico.org/documents/cy2022-23/cmr-0323-e.pdf (accessed on 15 July 2025).
- Foreign Agricultural Service. U.S. Department of Agriculture. Available online: https://www.fas.usda.gov/data/production/commodity/0711100 (accessed on 22 November 2025).
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of coffee industry wastes: Comprehensive physico-chemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Cantele, C.; Tedesco, M.; Ghirardello, D.; Zeppa, G.; Bertolino, M. Coffee silverskin as a functional ingredient in vegan biscuits: Physicochemical and sensory properties and in vitro bioaccessibility of bioactive compounds. Foods 2022, 11, 717. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- Chau, C.F.; Huang, Y.L. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus Sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2023, 51, 2615–2618. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.F. Hydration properties of dietary fibre and resistant starch: A european collaborative study. J. Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. J. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Jap. J. Nut. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Gullón, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Mahdavi, B.; Yaacob, W.A.; Din, L.B. Chemical composition, antioxidant, and antibacterial activity of essential oils from Etlingera sayapensis A.D. Poulsen & Ibrahim. Asian Pac. J. Trop. Med. 2017, 10, 819–826. [Google Scholar] [CrossRef]
- Costa Vimercati, W.; da Silva Araújo, C.; Levate Macedo, L.; Gomes Correa, J.L.; Pimenta, C.J. Encapsulation of coffee silverskin extracts by foam mat drying and comparison with powders obtained by spray drying and freeze-drying. J. Food Sci. 2022, 87, 1767–1779. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bobková, A.; Poláková, K.; Demianová, A.; Belej, L.; Bobko, M.; Jurčaga, L.; Gálik, B.; Novotná, I.; Iriondo-DeHond, A.; Del Castillo, M.D. Comparative analysis of selected chemical parameters of Coffea arabica, from cascara to silverskin. Foods 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P. Coffea canephora silverskin from different geographical origins: A comparative study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. J. Food Sci Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Tiwari, B.K.; Kerry, J.P.; Álvarez, C.A. Comprehensive study on the characterisation properties of power ultrasound-treated apple pomace powder and coffee silverskin powder. Eur. Food Res. Technol. 2022, 248, 1939–1949. [Google Scholar] [CrossRef]
- Franca, A.S.; Basílio, E.P.; Resende, L.M.; Fante, C.A.; Oliveira, L.S. Coffee silverskin as a potential ingredient for functional foods: Recent advances and a case study with chocolate cake. Foods 2024, 13, 3935. [Google Scholar] [CrossRef]
- Alghooneh, A.; Mohammad Amini, A.; Behrouzian, F.; Razavi, S.M.A. Characterisation of cellulose from coffee silverskin. Int. J. Food Prp. 2017, 20, 2830–2843. [Google Scholar] [CrossRef]
- Behrouzian, F.; Amini, A.S.; Alghooneh, A.; Razavi, S.M.A. Characterization of dietary fiber from coffee silverskin: An optimization study using response surface methodology. Bioact. Carbohydr. Diet. Fibre 2016, 8, 58–64. [Google Scholar] [CrossRef]
- Mammolenti, D.; Lupi, F.R.; Baldino, N.; Gabriele, D. Technological advancements of insoluble dietary fiber from food by-product processing: A review. Foods 2025, 14, 1822. [Google Scholar] [CrossRef]
- Alahmari, L.A. Dietary fiber influence on overall health, with an emphasis on CVD, diabetes, obesity, colon cancer, and inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, F.; Fontanella, M.C.; Lambri, M.; Beone, G.M. Specialty and high-quality coffee: Discrimination through elemental characterization via ICP-OES, ICP-MS, and ICP-MS/MS of origin, species, and variety. J. Sci. Food Agric. 2023, 103, 4303–4316. [Google Scholar] [CrossRef] [PubMed]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Torres-Martínez, B.M.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Physicochemical, techno-functional and antioxidant characterization of coffee silverskin. Biotecnia 2023, 25, 43–50. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Quagliata, E.; Gazzara, S.; Dauber, C.; Rodríguez, A.; Panizzolo, L.; Irigaray, B.; Gámbaro, A.; Mendiola, J.A.; Vieitez, I.; del Castillo, M.D. Novel insights into torrefacto and natural coffee silverskin: Composition, bioactivity, safety, and environmental impact for sustainable food applications. Foods 2025, 14, 3388. [Google Scholar] [CrossRef]
- Lu, T.; Sun, Y.; Huang, Y.; Chen, X. Effects of roasting on the chemical compositions, color, aroma, microstructure, and the kinetics of changes in coffee pulp. J. Food Sci. 2023, 88, 1430–1444. [Google Scholar] [CrossRef]
- López-Marcos, M.C.; Bailina, C.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Properties of dietary fibers from agroindustrial coproducts as source for fiber-enriched foods. Food Biopr. Technol. 2015, 8, 2400–2408. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Laguna, L.; Haros, C.M.; Tárrega, A. Techno-functional and rheological properties of alternative plant-based flours. Foods 2023, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Wagner, C.E.; Richter, J.K.; Ikuse, M.; Ganjyal, G.M. Classification of select functional dietary fiber ingredients based on quantitative properties and latent qualitative criteria. J. Food Sci. 2024, 89, 6098–6112. [Google Scholar] [CrossRef] [PubMed]
- Ateş, G.; Elmacı, Y. Coffee silverskin as fat replacer in cake formulations and its effect on physical, chemical and sensory attributes of cakes. J. Food Sci. Technol. 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Xu, Y.; Zhu, B.; Piao, J.; Zhu, L.; Yao, L.; Liu, K.; Wang, S.; Zhang, Q.; et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu. Fruits. J. Funct. Food 2022, 93, 105081. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Liu, Y.; Ma, Y.; Lin, C.; Nie, N.; Song, X.; Yang, J. Physicochemical properties of soluble dietary fiber from passion fruit peel based on various extraction methods. Agric. Nat. Resour. 2025, 15, 44. [Google Scholar] [CrossRef]
- Kalla-Bertholdt, A.-M.; Baier, A.K.; Rauh, C. Potential of modification of techno-functional properties and structural characteristics of citrus, apple, oat, and pea dietary fiber by high-intensity ultrasound. Foods 2023, 12, 3663. [Google Scholar] [CrossRef]
- Manoliu, D.-R.; Ciobotaru, M.C.; Ciobanu, M.M.; Boișteanu, P.C. Development and characterization of meat-based pasta enriched with apple and sugar beet fibers. Foods 2025, 14, 3837. [Google Scholar] [CrossRef]
- Feng, J.; Kong, B.; Sun, F.; Xia, X. Effect of potato dietary fiber on the quality, microstructure, and thermal stability of chicken patty. Foods 2022, 11, 3978. [Google Scholar] [CrossRef]
- Mannino, G.; Kunz, R.; Maffei, M.E. Discrimination of green coffee (Coffea arabica and Coffea canephora) of different geographical origin based on antioxidant activity, high-throughput metabolomics, and DNA RFLP fingerprinting. Antioxidants 2023, 12, 1135. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of Arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Intern. 2017, 99, 155–165. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Yang, Y.; Luo, J.; Yang, R.; Li, W. Profiles of Free and Bound Phenolics and Their Antioxidant Capacity in Rice Bean (Vigna umbellata). Foods 2023, 12, 2718. [Google Scholar] [CrossRef] [PubMed]
- Pinaffi, A.C.D.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S. Insoluble-bound polyphenols released from guaraná powder: Inhibition of alpha-glucosidase and proanthocyanidin profile. Molecules 2020, 25, 679. [Google Scholar] [CrossRef]
- Suwannachot, J.; Collado Reginio, F.; Hamauzu, Y.Y.; Kiharu Ogawa, Y. Assessment of free, esterified, and insoluble-bound phenolics of green and red perilla leaves and changes during simulated gastrointestinal digestion. Food Chem. Adv. 2022, 1, 100018. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A. R: Bioaccessibility and bioactivities of phenolic compounds from roasted coffee beans during in vitro digestion and colonic fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gómez, C.I.; Morales-Castro, J.; Barragan-Zuñiga, J.; Denise Herrera, M.; Zamilpa-Álvarez, A.; Gónzalez, J.L.; Martínez-Aguilar, G.; Morales-Castro, E.P.; Anese, M.; Alongi, M. Influence of coffee roasting degree on antioxidant and metabolic parameters: Comprehensive in vitro and in vivo analysis. Curr. Res. Food Sci. 2024, 9, 100861. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound polyphenols from red quinoa prevailed over free polyphenols in reducing postprandial blood glucose rises by inhibiting α-glucosidase activity and starch digestion. Nutrients 2022, 14, 728. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and bioavailability of diet polyphenols and their modulation of gut microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive potential and chemical composition of coffee by-products: From pulp to silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef]
- Giordano, M.; Bertolino, M.; Belviso, S.; Ghirardello, D.; Zeppa, G. Effects of species, post-harvest treatment, and roasting on fibre, volatile compounds, and polyphenol contents in coffee silverskin. Foods 2022, 11, 3132. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.G.; Puga, H.; Oliveira, M.B.P.P.; Martel, F.; Alves, R.C. Valorizing coffee silverskin based on its phytochemicals and antidiabetic potential: From lab to a pilot scale. Foods 2022, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Linne, B.M.; Tello, E.; Simons, C.T.; Peterson, D.G. Characterization of the impact of chlorogenic acids on tactile perception in coffee through an inverse effect on mouthcoating sensation. Food Res. Int. 2023, 172, 113167. [Google Scholar] [CrossRef] [PubMed]
- Tieghi, H.; de Almeida Pereira, L.; Silva Viana, G.; Katchborian-Neto, A.; Brandão Santana, D.; Mincato, R.L.; Ferreira Dias, D.; Chagas-Paula, D.A.; Gomes Soares, M.; de Araújo, W.G.; et al. Effects of geographical origin and post-harvesting processing on the bioactive compounds and sensory quality of Brazilian specialty coffee beans. Food Res. Int. 2024, 186, 114346. [Google Scholar] [CrossRef] [PubMed]
- Šeremet, D.; Fabečić, P.; Vojvodić Cebin, A.; Mandura Jarić, A.; Pudić, R.; Komes, D. Antioxidant and sensory assessment of innovative coffee blends of reduced caffeine content. Molecules 2022, 27, 448. [Google Scholar] [CrossRef]
- Saavedra Velásquez, N.; Cuadrado Peñafiel, V.; de la Vega Marcos, R. Can caffeine improve your performance? Psychophysiological effects. A systematic review. Nutr. Hosp. 2024, 41, 677–685. [Google Scholar] [CrossRef]
- Koskinakis, S.E.; Stergiopoulos, C.; Vasileiou, C.; Krokida, M. Sustainable valorization of coffee silverskin waste: Pressurized liquid extraction of bioactive compounds. Foods 2025, 14, 615. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-Dehond, A.; Martirosyan, D.M.; Mesa, M.D.; Dolores, M.; Castillo, D. Coffee silverskin extract for aging and chronic diseases. In Functional Foods for Chronic Diseases; Martirosyan, D.M., Ed.; Food Science Publisher: Dallas, TX, USA, 2016; pp. 386–409. [Google Scholar]
- Dong, S.; Ding, L.; Zheng, X.; Wang, O.; Cai, S. Phenolic compositions of different fractions from coffee silver skin and their antioxidant activities and inhibition towards carbohydrate-digesting enzymes. Foods 2024, 13, 3083. [Google Scholar] [CrossRef]
- Jirarat, W.; Kaewsalud, T.; Yakul, K.; Rachtanapun, P.; Chaiyaso, T. Sustainable valorization of coffee silverskin: Extraction of phenolic compounds and proteins for enzymatic production of bioactive peptides. Foods 2024, 13, 1230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.