Exploring Variations in Physical and Chemical Characteristics of Barringtonia Nuts: A Novel Forest Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Oil Extraction and Chemical Analyses
2.3. Statistical Analysis
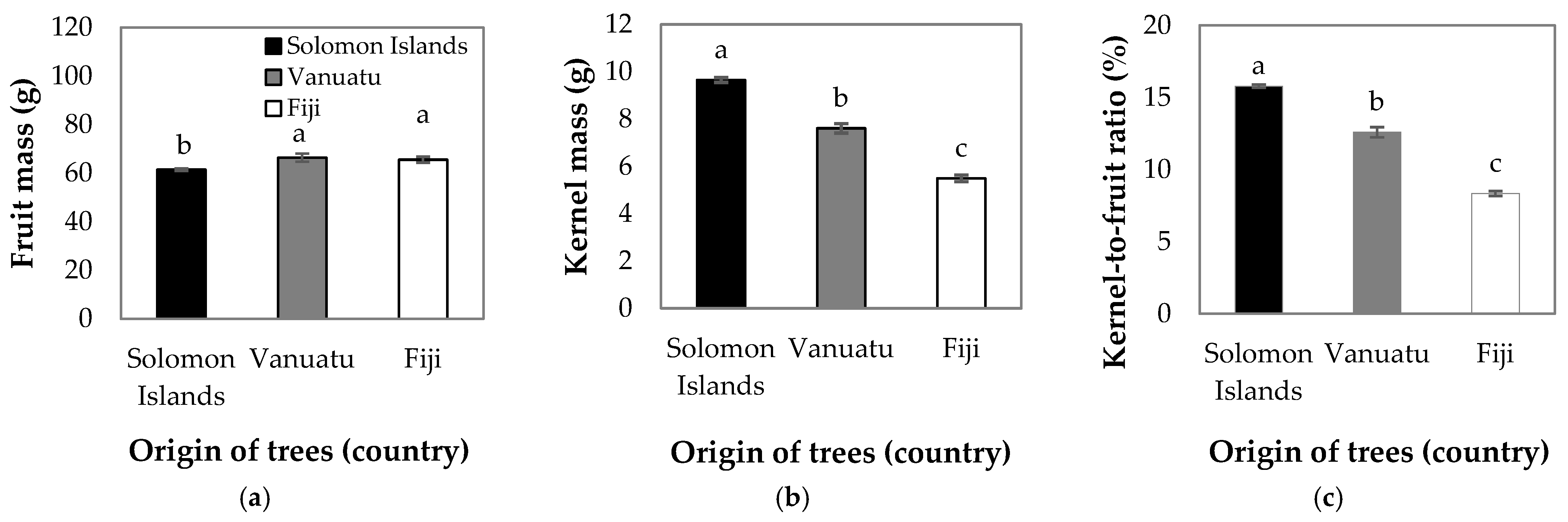
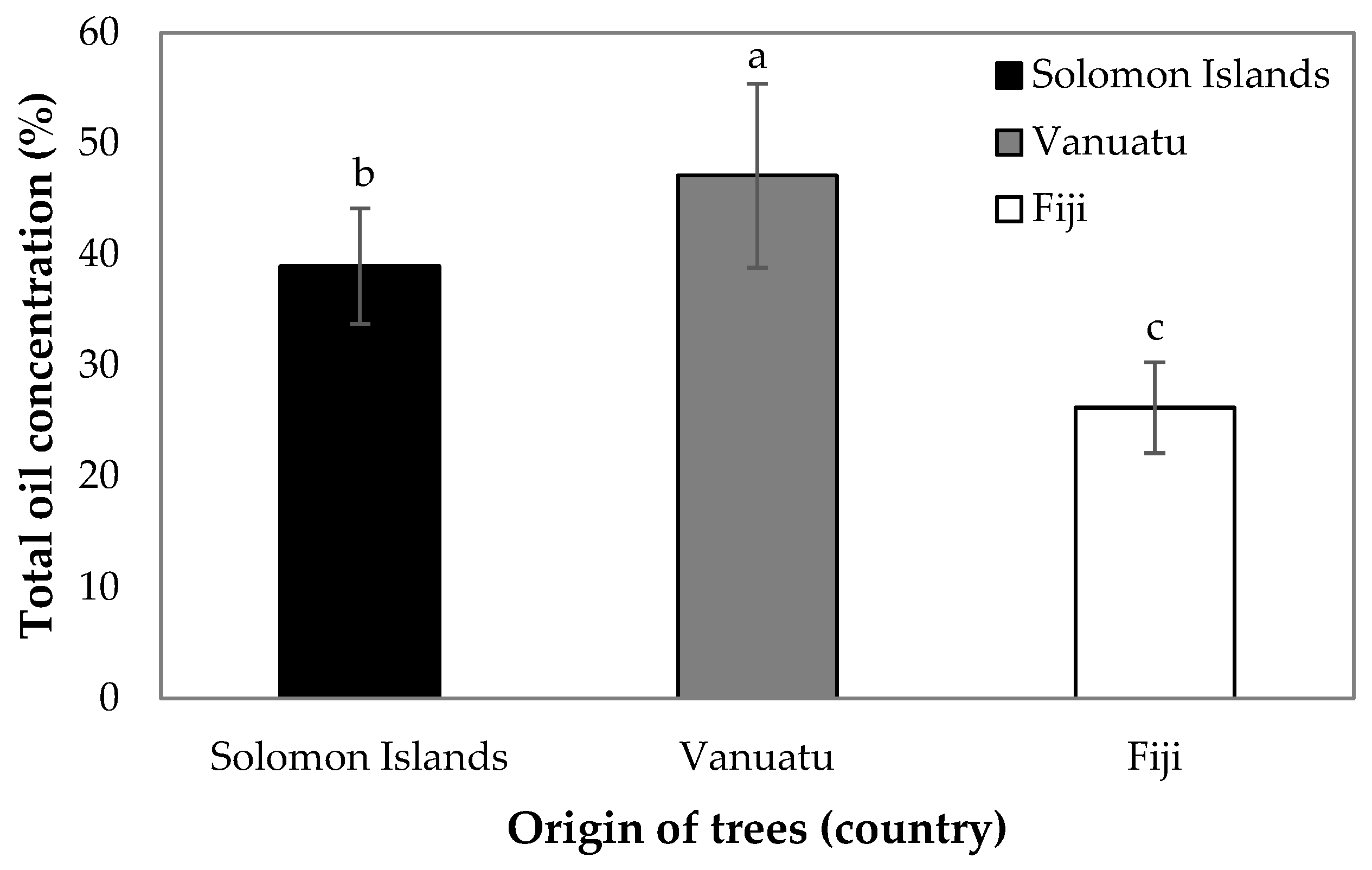
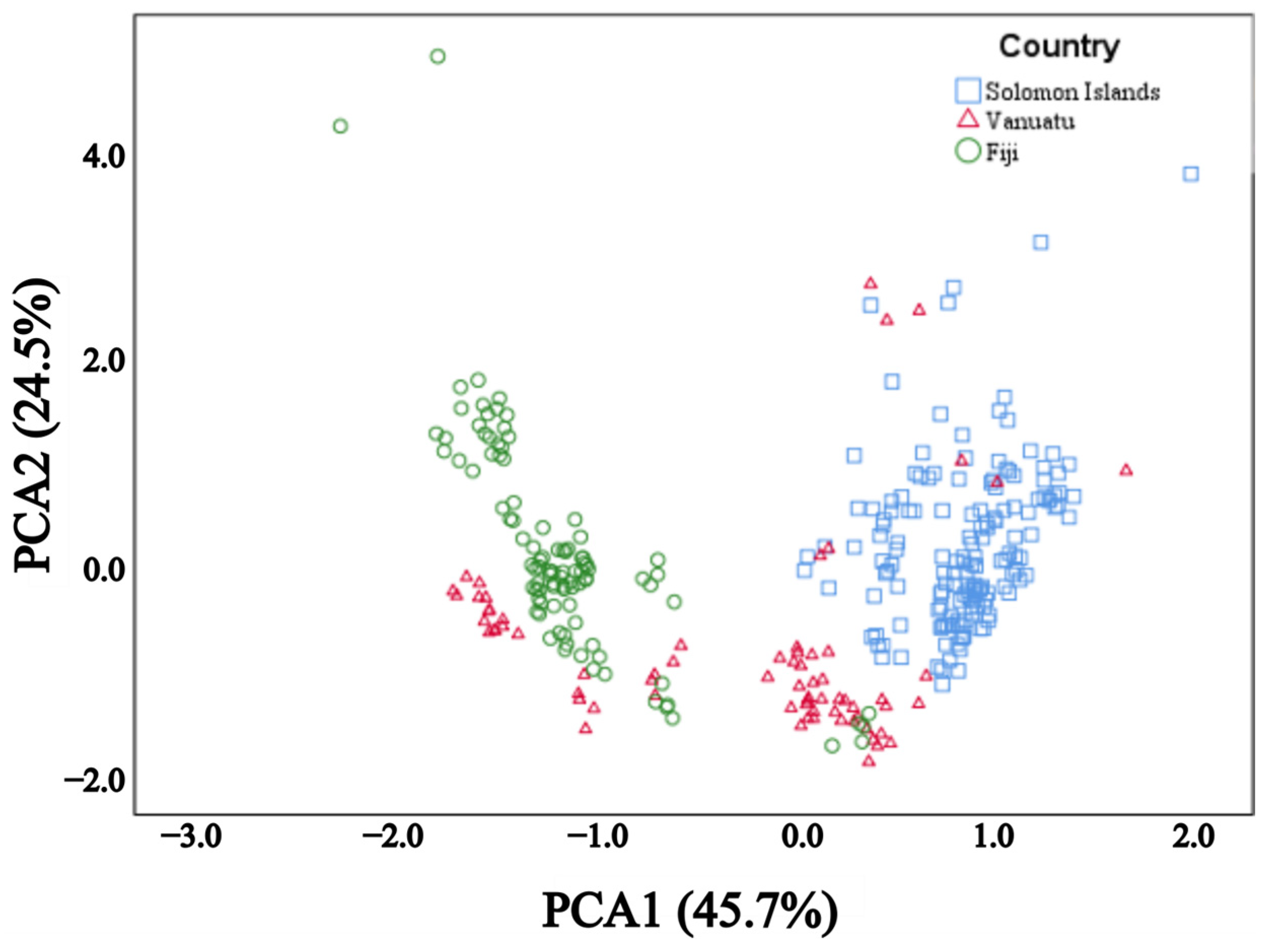
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organisation of the United Nations. The State of Food Security and Nutrition in the World 2017; FAO: Rome, Italy, 2017; Volume Building resilience for peace and food security. [Google Scholar]
- Leakey, R.R. Converting ‘trade-offs’ to ‘trade-ons’ for greatly enhanced food security in Africa: Multiple environmental, economic and social benefits from ‘socially modified crops’. Food Secur. 2018, 10, 505–524. [Google Scholar] [CrossRef]
- Ngadze, R.T.; Verkerk, R.; Nyanga, L.K.; Fogliano, V.; Linnemann, A.R. Improvement of traditional processing of local monkey orange (Strychnos spp.) fruits to enhance nutrition security in Zimbabwe. Food Secur. 2017, 9, 621–633. [Google Scholar] [CrossRef]
- Wallace, H.; Komolong, B.; Nevenimo, T.; Waaii, C.; Hannett, D.; Hannett, G.; Kapi Ling, S.; Grant, E.; Hodges, B.; Kill, E.; et al. Enhancing Private Sector-Led Development of the Canarium Industry in PNG: ACIAR Project FST/2014/099 Final Report; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2021; Available online: https://aciar.gov.au/project/fst–2014-2099 (accessed on 20 March 2023).
- Wallace, H.; Hosseini Bai, S.; Grant, E.; Hodges, B.; Kill, E.; Randall, B.; Komolong, B.; Waaii, C.; Hannett, D.; Hannett, G.; et al. Enhancing private-sector-led development of the Canarium industry in Papua New Guinea. In Raising Trees and Livelihoods: Experiences of Integrating Trees into Smallholder Farming Systems; Race, D., Wettenhall, G., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2024; pp. 255–277. [Google Scholar]
- Wallace, H.M.; Hannet, D.; Hannet, G.; Hosseini-Bai, S.; Jones, K.; Komolong, B. Commercialising an indigenous agroforestry tree: overview of commercial processing methods for Canarium indicum (galip) nuts in Papua New Guinea. Acta Hortic. 2022, 1355, 345–350. [Google Scholar] [CrossRef]
- Bai, S.H.; Brooks, P.; Gama, R.; Nevenimo, T.; Hannet, G.; Hannet, D.; Randall, B.; Walton, D.; Grant, E.; Wallace, H.M. Nutritional quality of almond, canarium, cashew and pistachio and their oil photooxidative stability. J. Food Sci. Technol. 2019, 56, 792–798. [Google Scholar] [CrossRef]
- Borges, O.; de Carvalho, J.L.S.; Gonçalves, B.; Silva, A.P.; Correia, P. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- da Silva, A.; de Freitas, B.V.M.; Silveira, B.K.S.; Hermsdorff, H.H.M.; Bressan, J. Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods 2022, 11, 2925. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- US Department of Health & Human Services. Nutrient Recommendations: Dietary Reference Intakes (DRI). Available online: https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx (accessed on 15 September 2016).
- Hosseini Bai, S.; Nevenimo, T.; Hannet, G.; Hannet, D.; Jones, K.; Trueman, S.; Grant, E.; Walton, D.A.; Randall, B.; Wallace, H.M. Freezing, roasting and salt dipping impacts on peroxide value, free fatty acid and fatty acid concentrations of nut kernels. Acta Hortic. 2019, 1256, 71–76. [Google Scholar] [CrossRef]
- Walton, D.A.; Randall, B.W.; Poienou, M.; Moxon, J.; Wallace, H.M. A roasting study for the tropical nut Canarium indicum (Burseraceae). Acta Hortic. 2016, 1109, 43–48. [Google Scholar] [CrossRef]
- Randall, B.W.; Walton, D.A.; Grant, E.L.; Zekele, P.; Gua, B.; Pauku, R.; Wallace, H.M. Selection of the tropical nut Canarium indicum for early fruiting, nut-in-shell size and kernel size. Acta Hortic. 2016, 1109, 169–174. [Google Scholar] [CrossRef]
- Wallace, H.; Randall, B.; Grant, E.; Jones, K.; Walton, D.; Poienou, M.; Nevenimo, T.; Moxon, J.; Pauku, R.L. Processing methods for Canarium nuts in the Pacific. Acta Hortic. 2016, 1128, 145–149. [Google Scholar] [CrossRef]
- Bai, S.H.; Randall, B.; Grant, E.; Gama, R.; Gua, B.; Keli, D.; Negalevu, P.; Oakeshott, J.; Wallace, H.M. Tree-to-tree variation of kernel size in two underutilized tree nuts in Pacific. Acta Hortic. 2022, 1340, 141–144. [Google Scholar] [CrossRef]
- Grant, E.; Macdonell, P.; Tungon, J.; Tabi, M.; David, M.; Kaku, S.; Page, T. Geographical variation in Canarium indicum (Burseraceae) nut characteristics across Vanuatu. Genet. Resour. Crop Evol. 2024, 71, 1325–1340. [Google Scholar] [CrossRef]
- Leakey, R.; Fuller, S.; Treloar, T.; Stevenson, L.; Hunter, D.; Nevenimo, T.; Binifa, J.; Moxon, J. Characterization of tree-to-tree variation in morphological, nutritional and medicinal properties of Canarium indicum nuts. Agrofor. Syst. 2008, 73, 77–87. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.; Trueman, S.; Bai, S. Variability in crude protein and mineral nutrient concentrations of almonds. Acta Hortic. 2018, 1219, 213–218. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Hosseini Bai, S. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef]
- Akter, S.; Netzel, M.E.; Fletcher, M.T.; Tinggi, U.; Sultanbawa, Y. Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods 2018, 7, 60. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Chen, C.-Y.O.; McKay, D.L.; Bolling, B.W. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L. Tree nut consumption is associated with better nutrient adequacy and diet quality in adults: National Health and Nutrition Examination Survey 2005–2010. Nutrients 2015, 7, 595–607. [Google Scholar] [CrossRef]
- Ng, S.; Lasekan, O.; Sulaiman, R.; Muhammad, K.S.; Hussain, N. Physicochemical properties of Malaysian-grown tropical almond nuts (Terminalia catappa). J. Food Sci. Technol. 2015, 52, 6623–6630. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, W.; Wang, Y.; Yang, W.; Cai, M.; Xu, X.; Matsumoto, T.; Zhang, S.; Ma, X.; Zhu, M.; et al. Signatures of selection in recently domesticated macadamia. Nat. Commun. 2022, 13, 242. [Google Scholar] [CrossRef]
- Dicenta, F.; Cremades, T.; Rubio, M.; Martínez-García, P. Early selection for flowering time in almond breeding programs. Sci. Hortic. 2017, 220, 1–3. [Google Scholar] [CrossRef]
- Kodad, O.; R. Socias i Company. Variability of oil content and of major fatty acid composition in almond (Prunus amygdalus Batsch) and its relationship with kernel quality. J. Agric. Food Chem. 2008, 56, 4096–4101. [Google Scholar] [CrossRef]
- Alasalvar, C.; Ros, E.; Salvadó, J.-S. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Bodoira, R.; Martínez, M.; Maestri, D.; Cittadini, M.C. Tree Nut Oils: Chemical Profiles, Extraction, Stability, and Quality Concerns. Eur. J. Lipid Sci. Technol. 2020, 122, 1900450. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Jones, K.; Hosseini-Bai, S. Late-dropping macadamia nuts have reduced shelf life. Sci. Hortic. 2020, 268, 109378. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Gama, R.; Jones, K.; Hannet, D.; Hannet, G.; Komolong, B.; Brooks, P.; Grant, E.; Elliott, B.; Wallace, H.M. Presence of Testa and Shell Maintains Oil Stability in Almond and Canarium Nuts. Horticulturae 2023, 9, 1003. [Google Scholar] [CrossRef]
- Thomson, L.; Doran, J.; Clarke, B. Conservation and utilisation of genetic diversity. In Trees for Life in Oceania; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2018; Volume Monograph No. 201. [Google Scholar]
- Jebb, M. Edible Barringtonias. Curtis’s Bot. Mag. 1992, 9, 164–172. [Google Scholar] [CrossRef]
- Payens, J.P.D.W. A monograph of the genus Barringtonia (Lecythidaceae). Blumea Biodivers. Evol. Biogeogr. Plants 1967, 15, 157–263. [Google Scholar]
- Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:469127-1 (accessed on 30 July 2021).
- Evans, B. A Variety Collection of Edible Nut Tree Crops in Solomon Islands; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1999; p. 96. [Google Scholar]
- Stevens, M.; Bourke, R.; Evans, B. South Pacific Indigenous Nuts. In Proceedings of the a Workshop, Port Vila, Vanuatu, 31 October–4 November 1994; Australian Centre for International Agricultural Research: Port Vila, Vanuatu, 1996; p. 176. [Google Scholar]
- Evans, B.R. Edible Nut Trees in Solomon Islands: A Variety Collection of Canarium, Terminalia and Barringtonia; Australian Centre for International Agricultural Research: Canberra, Australia, 1999; pp. 12–66. [Google Scholar]
- Holmes, M.; Cunningham, J.; Hamilton, K.; Brooks, P.; Russell, F.D. Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr. Diet. 2010, 67, 182–189. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Von Felten, S. Analysis of variance with unbalanced data: An update for ecology & evolution. J. Anim. Ecol. 2010, 79, 308–316. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Moya-Laraño, J. Unexpected failures of recommended tests in basic statistical analyses of ecological data. Web Ecol. 2008, 8, 67–73. [Google Scholar] [CrossRef]
- Bamgboye, A.I.; Ogunsina, B.S. Pre-shelling parameters and conditions that influence the whole kernel out-turn of steam-boiled cashew nuts. J. Saudi Soc. Agric. Sci. 2014, 13, 29–34. [Google Scholar] [CrossRef]
- Trueman, S.J.; Richards, S.; McConchie, C.A.; Turnbull, C.G.N. Relationships between kernel oil content, fruit removal force and abscission in macadamia. Aust. J. Exp. Agric. 2000, 40, 859–866. [Google Scholar] [CrossRef]
- Radunić, M.; Vidaković, A.; Poljak, I. Variation in chemical composition and fruit morphometric traits of almond-leaved pear (Pyrus spinosa Forssk.) natural populations. Genet. Resour. Crop Evol. 2025, 72, 1495–1510. [Google Scholar] [CrossRef]
- Kucukyumuk, Z.; Erdal, I. Rootstock and cultivar effect on mineral nutrition, seasonal nutrient variation and correlations among leaf, flower and fruit nutrient concentrations in apple trees. Bulg. J. Agric. Sci. 2011, 17, 633–641. [Google Scholar]
- Sathe, S.; Seeram, N.; Heber, D.; Kshirsagar, H.; Lapsley, K. Fatty Acid Composition of California Grown Almonds. J. Food Sci. 2008, 73, C607–C614. [Google Scholar] [CrossRef] [PubMed]
- Nevenimo, T.; Moxon, J.; Bunt, C.; Wemin, J.; Leakey, R.R.B.; Johnston, M. Domestication potential and marketing of Canarium indicum nuts in the Pacific: 1. A literature review. Agrofor. Syst. 2007, 69, 117–134. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Randall, B.; Gama, R.; Gua, B.; Keli, D.; Jones, K.; Elliott, B.; Wallace, H.M. Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts. Horticulturae 2025, 11, 540. [Google Scholar] [CrossRef]
- Bai, S.H.; Tahmasbian, I.; Zhou, J.; Nevenimo, T.; Hannet, G.; Walton, D.; Randall, B.; Gama, T.; Wallace, H.M. A non-destructive determination of peroxide values, total nitrogen and mineral nutrients in an edible tree nut using hyperspectral imaging. Comput. Electron. Agric. 2018, 151, 492–500. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Darby, I.; Nevenimo, T.; Hannet, G.; Hannet, D.; Poienou, M.; Grant, E.; Brooks, P.; Walton, D.; Randall, B.; et al. Effects of roasting on kernel peroxide value, free fatty acid, fatty acid composition and crude protein content. PLoS ONE 2017, 12, e0184279. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.J.; Aune, D.; Freisling, H.; Mohammadifard, N.; Kendall, C.W.C.; Salas-Salvadó, J.; Jenkins, D.J.A.; Hu, F.B.; Sievenpiper, J.L. Nuts and Cardiovascular Disease Outcomes: A Review of the Evidence and Future Directions. Nutrients 2023, 15, 911. [Google Scholar] [CrossRef] [PubMed]
- Leakey, R.R.; Page, T. The ‘ideotype concept’ and its application to the selection of cultivars of trees providing agroforestry tree products. For. Trees Livelihoods 2006, 16, 5–16. [Google Scholar] [CrossRef]

| Solomon Islands | Vanuatu | Fiji | |
|---|---|---|---|
| C14_0 | 0.11 ± 0.0034 a | 0.043 ± 0.0042 b | 0.03 ± 0.0011 c |
| C16_0 | 39.46 ± 0.2349 a | 29.40 ± 0.8748 b | 21.80 ± 0.4054 c |
| C18_0 | 6.06 ± 0.0573 c | 8.84 ± 0.3819 b | 9.47 ± 0.1693 a |
| C20_0 | 0.41 ± 0.0082 a | 0.26 ± 0.0180 b | 0.30 ± 0.0081 b |
| C22_0 | 0.05 ± 0.0019 a | 0.04 ± 0.0024 a | 0.04 ± 0.0040 a |
| C16_1 | 0.12 ± 0.0046 a | 0.03 ± 0.0036 b | 0.02 ± 0.0011 c |
| C18_1C | 32.58 ± 0.2274 b | 37.77 ± 0.4744 a | 32.13 ± 0.7948 b |
| C18_1T | 0.86 ± 0.0231 a | 0.29 ± 0.0326 b | nd |
| C20_1 | 0.05 ± 0.0019 a | 0.03 ± 0.0025 b | 0.05 ± 0.0037 a |
| C18_2 | 20.33 ± 0.2484 c | 23.32 ± 0.6568 b | 36.16 ± 0.6598 a |
| TSFA | 46.08 ± 0.2339 a | 38.57 ± 0.5467 b | 31.64 ± 0.4199 c |
| TUSFA | 53.94 ± 0.2358 c | 61.44 ± 0.5478 b | 68.36 ± 0.4199 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.H.; Randall, B.; Gama, R.; Gua, B.; Keli, D.; Brooks, P.; Elliott, B.; Wallace, H.M. Exploring Variations in Physical and Chemical Characteristics of Barringtonia Nuts: A Novel Forest Food. Foods 2025, 14, 2147. https://doi.org/10.3390/foods14122147
Bai SH, Randall B, Gama R, Gua B, Keli D, Brooks P, Elliott B, Wallace HM. Exploring Variations in Physical and Chemical Characteristics of Barringtonia Nuts: A Novel Forest Food. Foods. 2025; 14(12):2147. https://doi.org/10.3390/foods14122147
Chicago/Turabian StyleBai, Shahla Hosseini, Bruce Randall, Repson Gama, Basil Gua, Doni Keli, Peter Brooks, Brittany Elliott, and Helen M. Wallace. 2025. "Exploring Variations in Physical and Chemical Characteristics of Barringtonia Nuts: A Novel Forest Food" Foods 14, no. 12: 2147. https://doi.org/10.3390/foods14122147
APA StyleBai, S. H., Randall, B., Gama, R., Gua, B., Keli, D., Brooks, P., Elliott, B., & Wallace, H. M. (2025). Exploring Variations in Physical and Chemical Characteristics of Barringtonia Nuts: A Novel Forest Food. Foods, 14(12), 2147. https://doi.org/10.3390/foods14122147






