Development of a Natural Coating Based on Fermented Milk Whey for Biopreservation of Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microbiological Culture
2.3. Isolation of Bacterial Culture
2.4. Preparation of LAB-Fermented MRS Broth and LAB-Fermented Whey
2.5. Qualitative Antifungal Activity of Biocontrol Strains
2.6. Quantitative Antifungal Activity of Biocontrol Strains
2.7. Identification of Phenolic Compounds in the Fermented Whey
2.8. Antifungal Activity of 5% HPMC Coating with LAB-Fermented Whey Lyophilizate
2.9. Reduction in Fungal Contamination in Cheese Slices Treated with 5% HPMC Coating LAB-Fermented Whey Lyophilizate
2.10. Reduction in Fungal Contamination in Cheese Treated with 5% HPMC Coating LAB-Fermented Whey Lyophilizate
2.11. Statistical Analysis
3. Results and Discussion
3.1. Isolation, Selection, and Identification of Bacterial Cultures
3.2. Qualitative Antifungal Activity of the LAB
3.3. Quantitative Antifungal Activity of LAB-Fermented Whey
| Fungal Strain | LAB Strain | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F10 | F13 | KK13 | KB2 | KB3 | KB4 | Q1 | Q3 | Q4 | ||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| P. camemberti | 125.0 | >250.0 | 62.5 | 125.0 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 |
| P. expansum | 62.5 | 125.0 | 62.5 | 125.0 | 31.3 | 62.5 | 62.5 | 125.0 | 62.5 | 125.0 | 31.3 | 62.5 | 125.0 | 250.0 | 62.5 | 125.0 | 62.5 | 125.0 |
| P. roqueforti | 125.0 | 250.0 | 62.5 | 125.0 | 2.0 | 3.9 | 3.9 | 7.8 | 15.6 | 31.3 | 3.9 | 7.8 | 125.0 | 250.0 | 62.5 | 125.0 | 31.3 | 62.5 |
| P. digitatum | 250.0 | >250.0 | 125.0 | 250.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 250.0 | >250.0 | 125.0 | 250.0 |
| P. brevicopactum | 125.0 | 250.0 | 62.5 | 125.0 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 |
| P. nordicum | 125.0 | 250.0 | 62.5 | 125.0 | 31.3 | 62.5 | 31.3 | 62.5 | 15.6 | 31.3 | 31.3 | 62.5 | 125.0 | 250.0 | 62.5 | 125.0 | 31.3 | 62.5 |
| P. commune | 125.0 | 250.0 | 62.5 | 125.0 | 15.6 | 31.3 | 31.3 | 62.5 | 15.6 | 31.3 | 31.3 | 62.5 | 125.0 | 250.0 | 62.5 | 125.0 | 125.0 | 250.0 |
| P. solitum | 125.0 | 250.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 31.3 | 62.5 | 125.0 | 250.0 | 125.0 | 250.0 | 31.3 | 62.5 | 62.5 | 125.0 |
| P. verrucosum | 125.0 | 250.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 125.0 | 250.0 | 62.5 | 125.0 | 31.3 | 62.5 |
| Mean | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| 131.9 | 236.1 | 69.4 | 138.9 | 47.1 | 94.2 | 52.5 | 105.0 | 46.9 | 93.7 | 56.0 | 112.0 | 111.1 | 222.2 | 86.8 | 145.8 | 78.1 | 145.8 | |
3.4. Identification of Phenolic Compounds in the Fermented Whey
3.5. Antifungal Activity of 5% HPMC Coating with LAB-Fermented Whey
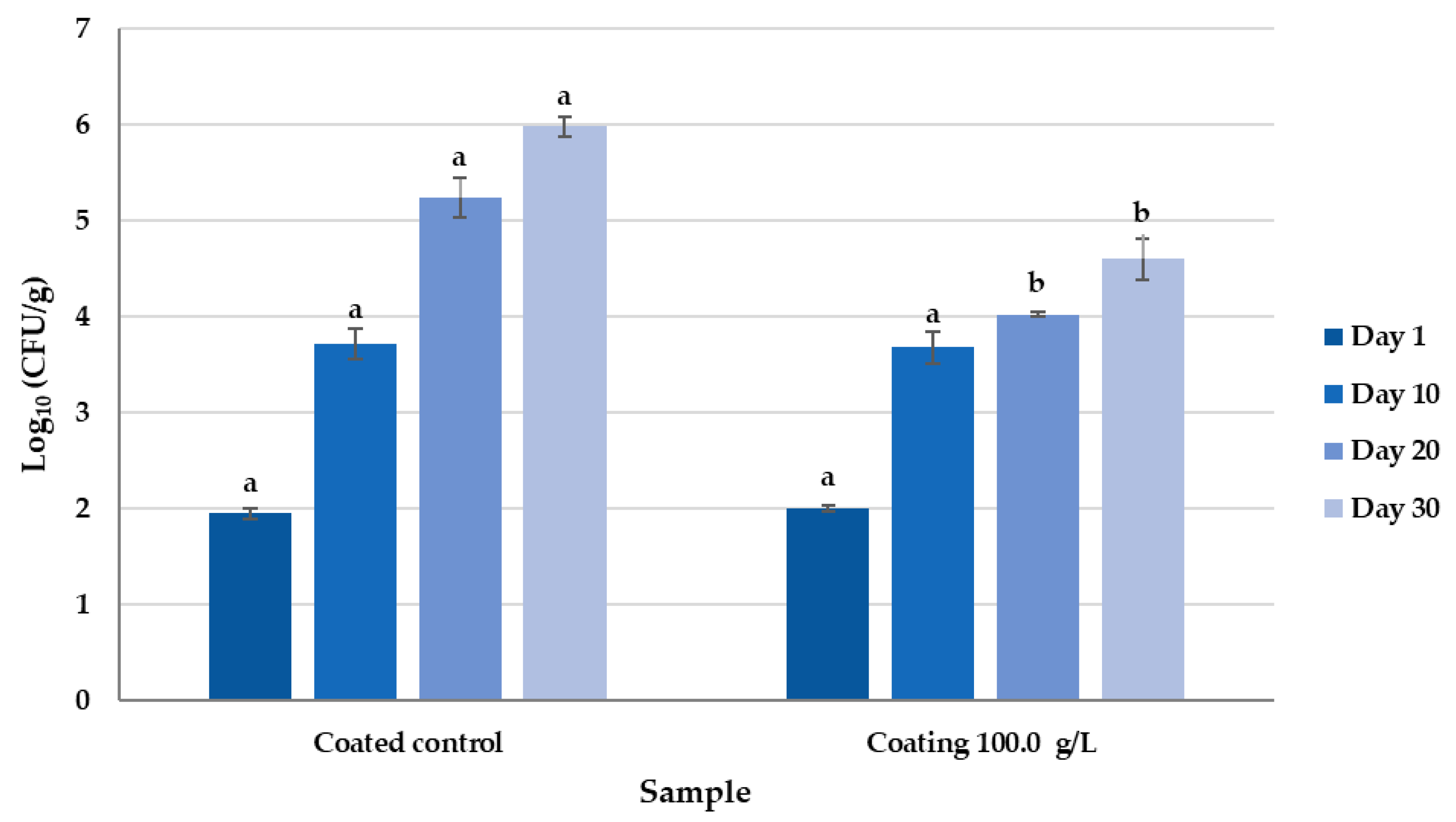
3.6. Reduction in Fungal Contamination in Cheeses Treated with 5% HPMC Coating LAB-Fermented Whey
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CECT | Spanish Type Culture Collection |
| F10 | Lactiplantibacillus plantarum F10 |
| F13 | Lactiplantibacillus plantarum F13 |
| HPMC | Hydroxypropylmethylcellulose |
| KB2 | Lactiplantibacillus plantarum KB2 |
| KB3 | Lactiplantibacillus plantarum KB3 |
| KB4 | Lactiplantibacillus plantarum KB4 |
| KK13 | Lactiplantibacillus plantarum KK13 |
| LAB | Lactic acid bacteria |
| MIC | Minimum inhibitory concentration |
| MFC | Minimum fungicide concentration |
| MRS | Man–Rogosa–Sharpe |
| PDA | Potato dextrose agar |
| PDB | Potato dextrose broth |
| Q1 | Lactiplantibacillus plantarum Q1 |
| Q3 | Lactiplantibacillus plantarum Q3 |
| Q4 | Lactiplantibacillus plantarum Q4 |
References
- Khuntia, A.; Ghosh, S.; Mitra, J. Food preservatives: Food application, legislation and preservative techniques. In Trends & Prospects in Food Technology, Processing and Preservation, 1st ed.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2020. [Google Scholar]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Nájera, A.I.; Nieto, S.; Barron, L.J.R.; Albisu, M. A review of the preservation of hard and semi-hard cheeses: Quality and safety. Int. J. Environ. Res. Public Health 2021, 18, 9789. [Google Scholar] [CrossRef] [PubMed]
- Bearth, A.; Cousin, M.E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Bischoff, N.S.; de Kok, T.M.; Sijm, D.T.H.M.; van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.M.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D.; et al. Review possible adverse effects of food additive E171 (titanium dioxide) related to particle specific human toxicity, including the immune system. Int. J. Mol. Sci. 2021, 22, 207. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Morovic, W.; Roper, J.M.; Smith, A.B.; Mukerji, P.; Stahl, B.; Rae, J.C.; Ouwehand, A.C. Safety evaluation of HOWARU® restore (Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Bifidobacterium animalis subsp. Lactis Bl-04 and B. lactis Bi-07) for antibiotic resistance, genomic risk factors, and acute toxicity. Food Chem. Toxicol. 2017, 110, 316–324. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal Fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria: Biodiversity and Taxonomy, 1st ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Escrivá, L.; Manyes, L.; Vila-Donat, P.; Font, G.; Meca, G.; Lozano, M. Bioaccessibility and bioavailability of bioactive compounds from yellow mustard flour and milk whey fermented with lactic acid bacteria. Food Funct. 2021, 12, 11250–11261. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A. In vitro antifungal, probiotic, and antioxidant functional properties of a novel Lactobacillus paraplantarum isolated from fermented dates in Saudi Arabia. J. Sci. Food Agric. 2017, 97, 5287–5295. [Google Scholar] [CrossRef]
- Bazukyan, I.; Matevosyan, L.; Toplaghaltsyan, A.; Trchounian, A. Antifungal activity of Lactobacilli isolated from Armenian dairy products: An effective strain and its probable nature. AMB Express 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal lab isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Yépez, A.; Luz, C.; Meca, G.; Vignolo, G.; Mañes, J.; Aznar, R. Biopreservation potential of lactic acid bacteria from andean fermented food of vegetal origin. Food Control 2017, 78, 393–400. [Google Scholar] [CrossRef]
- Bettera, L.; Levante, A.; Bancalari, E.; Bottari, B.; Gatti, M. Lactic Acid bacteria in cow raw milk for cheese production: Which and how many? Front. Microbiol. 2023, 13, 1092224. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef]
- Foxx, P.F.; Guineee, T.P.; Cogann, T.M.; Mcsweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Food and Agriculture Organization. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France, 2023. [Google Scholar]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Patel, A.K.; Vaisnav, N.; Mathur, A.; Gupta, R.; Tuli, D.K. Whey waste as potential feedstock for biohydrogen production. Renew. Energy 2016, 98, 221–225. [Google Scholar] [CrossRef]
- Duncan, P.I.; Aitio, O.; Heiskanen, A.; Niemelä, R.; Saarinen, J.; Helin, J.; Porta, N.; Fiaux, M.; Moënnoz, D.; Golliard, M.; et al. Structure and function of bovine whey derived oligosaccharides showing synbiotic epithelial barrier protective properties. Nutrients 2020, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sarkar, P.; Bhunia, A.K.; Yao, Y. Delivery systems of antimicrobial compounds to food. Trends Food Sci. Technol. 2016, 57, 165–177. [Google Scholar] [CrossRef]
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey. Food Biosci. 2023, 53, 102586. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Dopazo, V.; Luz, C.; Calpe, J.; Vila-Donat, P.; Rodríguez, L.; Meca, G. Antifungal properties of whey fermented by lactic acid bacteria in films for the preservation of cheese slices. Int. J. Dairy Technol. 2022, 75, 619–629. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.H. Conservation of ‘Palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef] [PubMed]
- Anelli, P.; Dall’Asta, C.; Cozzi, G.; Epifani, F.; Carella, D.; Scarpetta, D.; Brasca, M.; Moretti, A.; Susca, A. Analysis of composition and molecular characterization of mycobiota occurring on surface of cheese ripened in dossena’s mine. Food Microbiol. 2024, 123, 104587. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Broad-Spectrum Antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. 2013, 58, 291–299. [Google Scholar] [CrossRef]
- Maricic, N.; Dawid, S. Using the Overlay Assay to Qualitatively Measure Bacterial Production of and Sensitivity to Pneumococcal Bacteriocins. J. Vis. Exp. 2014, 91, e51876. [Google Scholar] [CrossRef]
- Ponzio, A.; Rebecchi, A.; Zivoli, R.; Morelli, L. Reuterin, phenyllactic acid, and exopolysaccharides as main antifungal molecules produced by lactic acid bacteria: A scoping review. Foods 2024, 13, 752. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The Lactic Acid Bacteria: A Literature Survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef]
- Soltani, S.; Biron, E.; Ben Said, L.; Subirade, M.; Fliss, I. Bacteriocin-based synergetic consortia: A promising strategy to enhance antimicrobial activity and broaden the spectrum of inhibition. Microbiol. Spectr. 2022, 10, e00406-21. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Harris, L.J.; Krochta, J.M. Antimicrobial effects of lactoferrin, lysozyme, and the lactoperoxidase system and edible whey protein films incorporating the lactoperoxidase system against Salmonella enterica and Escherichia coli O157:H7. J. Food Sci. 2005, 70, m332–m338. [Google Scholar] [CrossRef]
- Schillinger, U.; Holzapfel, W.H.; Björkroth, K.J. Lactic acid bacteria. In Food Spoilage Microorganisms, 1st ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 541–578. [Google Scholar]
- Izzo, L.; Luz, C.; Ritieni, A.; Quiles Beses, J.; Mañes, J.; Meca, G. Inhibitory effect of sweet whey fermented by Lactobacillus plantarum strains against fungal growth: A potential application as an antifungal agent. J. Food Sci. 2020, 85, 3920–3926. [Google Scholar] [CrossRef]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Riolo, M.; Villena, A.M.; Calpe, J.; Luz, C.; Meca, G.; Tuccitto, N.; Cacciola, S.O. A circular economy approach: A new formulation based on a lemon peel medium activated with lactobacilli for sustainable control of post-harvest fungal rots in fresh citrus fruit. Biol. Control 2024, 189, 105443. [Google Scholar] [CrossRef]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Shi, G.; Zeng, M.; Liu, Z. Improving 3-Phenyllactic Acid production of Lactobacillus plantarum AB-1 by enhancing its quorum-sensing capacity. J. Food Sci. Technol. 2019, 56, 2605. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, Ó.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Azhdari, S.; Moradi, M. Application of antimicrobial coating based on carboxymethyl cellulose and natamycin in active packaging of cheese. Int. J. Biol. Macromol. 2022, 209, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite, A.; Mileriene, J.; Kasparaviciene, B.; Aleksandrovas, E.; Songisepp, E.; Rud, I.; Axelsson, L.; Muizniece-Brasava, S.; Ciprovica, I.; Paskevicius, A.; et al. Screening for antifungal indigenous lactobacilli strains isolated from local fermented milk for developing bioprotective fermentates and coatings based on acid whey protein concentrate for fresh cheese quality maintenance. Microorganisms 2023, 11, 557. [Google Scholar] [CrossRef] [PubMed]





| Fungal Strain | Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | F10 | F13 | KK13 | KB2 | KB3 | KB4 | Q1 | Q3 | Q4 | |
| P. camemberti | − | ++ | + | − | − | ++ | + | + | − | + |
| P. expansum | − | ++ | + | ++ | − | − | − | ++ | − | ++ |
| P. roqueforti | − | + | − | − | − | − | − | − | − | − |
| P. digitatum | − | ++ | − | − | − | − | − | − | − | − |
| P. brevicopactum | − | ++ | + | − | + | + | + | + | + | − |
| P. nordicum | − | ++ | ++ | − | + | ++ | ++ | ++ | + | ++ |
| P. commune | − | ++ | ++ | − | ++ | − | − | ++ | + | − |
| P. solitum | − | + | − | − | − | − | − | + | ++ | ++ |
| P. verrucosum | − | ++ | − | − | − | − | − | ++ | − | + |
| (a) MRS | ||||||||||
| Fungal Strain | LAB Strain | |||||||||
| C | F10 | F13 | KK13 | KB2 | KB3 | KB4 | Q1 | Q3 | Q4 | |
| P. camemberti | − | ++ | ++ | − | − | − | − | ++ | + | ++ |
| P. expansum | − | + | + | − | − | − | − | + | − | + |
| P. roqueforti | − | − | − | − | − | − | − | − | − | − |
| P. digitatum | − | − | − | − | − | − | − | − | − | − |
| P. brevicopactum | − | ++ | ++ | − | + | + | − | ++ | + | ++ |
| P. nordicum | − | +++ | +++ | − | + | − | − | +++ | ++ | +++ |
| P. commune | − | + | + | − | + | − | − | + | + | + |
| P. solitum | − | − | − | − | − | − | − | − | − | − |
| P. verrucosum | − | +++ | +++ | − | + | ++ | − | +++ | ++ | +++ |
| (b) Whey | ||||||||||
| Fungal Strain | LAB Strain | |||||||||
| C | F10 | F13 | KK13 | KB2 | KB3 | KB4 | Q1 | Q3 | Q4 | |
| P. camemberti | − | ++ | ++ | ++ | ++ | +++ | ++ | − | +++ | ++ |
| P. expansum | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| P. roqueforti | − | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| P. digitatum | − | + | ++ | + | ++ | ++ | − | ++ | + | + |
| P. brevicopactum | − | + | ++ | + | + | ++ | + | + | + | + |
| P. nordicum | − | +++ | +++ | +++ | ++ | +++ | ++ | +++ | +++ | ++ |
| P. commune | − | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ |
| P. solitum | − | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ |
| P. verrucosum | − | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ |
| (a) | |||
| Sample | DL-3-Phenyllactic Acid | 3-4-Dihydroxyhydrocinnamic | Benzoic Acid |
| C | nd | nd | nd |
| F10 | 3.26 ± 0.44 a | 1.26 ± 0.15 b | 1.22 ± 0.15 b |
| F13 | 2.70 ± 0.20 b | nd | 0.82 ± 0.15 c |
| KK13 | 0.18 ± 0.07 d | 0.15 ± 0.03 c | nd |
| KB2 | 0.24 ± 0.08 d | nd | 0.17 ± 0.08 d |
| KB3 | 3.92 ± 0.07 a | 2.22 ± 0.02 a | 2.08 ± 0.15 a |
| KB4 | 0.79 ± 0.08 c | 0.19 ± 0.09 c | 0.11 ± 0.04 d |
| Q1 | 3.41 ± 0.21 a | 2.12 ± 0.11 a | 1.42 ± 0.51 b |
| Q3 | 0.28 ± 0.02 d | 0.30 ± 0.03 c | 0.09 ± 0.01 d |
| Q4 | 3.02 ± 0.68 a | 0.21 ± 0.09 c | 0.10 ± 0.02 d |
| (b) | |||
| Sample | Vanillic Acid | 1-2-Dihydroxybenzene | 3-(4-hydroxy-3-methoxyphenyl) Propionic |
| C | nd | nd | nd |
| F10 | nd | nd | nd |
| F13 | nd | nd | nd |
| KK13 | 0.09 ± 0.03 a | nd | nd |
| KB2 | 0.09 ± 0.03 a | nd | nd |
| KB3 | 0.04 ± 0.03 a | nd | nd |
| KB4 | 0.06 ± 0.05 a | nd | nd |
| Q1 | 0.07 ± 0.02 a | nd | nd |
| Q3 | 0.08 ± 0.02 a | 0.06 ± 0.01 a | 0.04 ± 0.01 a |
| Q4 | 0.09 ± 0.03 a | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.; Calpe, J.; Dopazo, V.; Luz, C.; Quiles, J.M.; Meca, G. Development of a Natural Coating Based on Fermented Milk Whey for Biopreservation of Cheese. Foods 2025, 14, 2149. https://doi.org/10.3390/foods14132149
Moreno A, Calpe J, Dopazo V, Luz C, Quiles JM, Meca G. Development of a Natural Coating Based on Fermented Milk Whey for Biopreservation of Cheese. Foods. 2025; 14(13):2149. https://doi.org/10.3390/foods14132149
Chicago/Turabian StyleMoreno, Ana, Jorge Calpe, Victor Dopazo, Carlos Luz, Juan Manuel Quiles, and Giuseppe Meca. 2025. "Development of a Natural Coating Based on Fermented Milk Whey for Biopreservation of Cheese" Foods 14, no. 13: 2149. https://doi.org/10.3390/foods14132149
APA StyleMoreno, A., Calpe, J., Dopazo, V., Luz, C., Quiles, J. M., & Meca, G. (2025). Development of a Natural Coating Based on Fermented Milk Whey for Biopreservation of Cheese. Foods, 14(13), 2149. https://doi.org/10.3390/foods14132149






