Effects of Nodulation on Metabolite Concentrations in Xylem Sap and in the Organs of Soybean Plants Supplied with Different N Forms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Nitrogen Treatments
2.2. Analysis of the Principal N Metabolites
2.3. Statistics
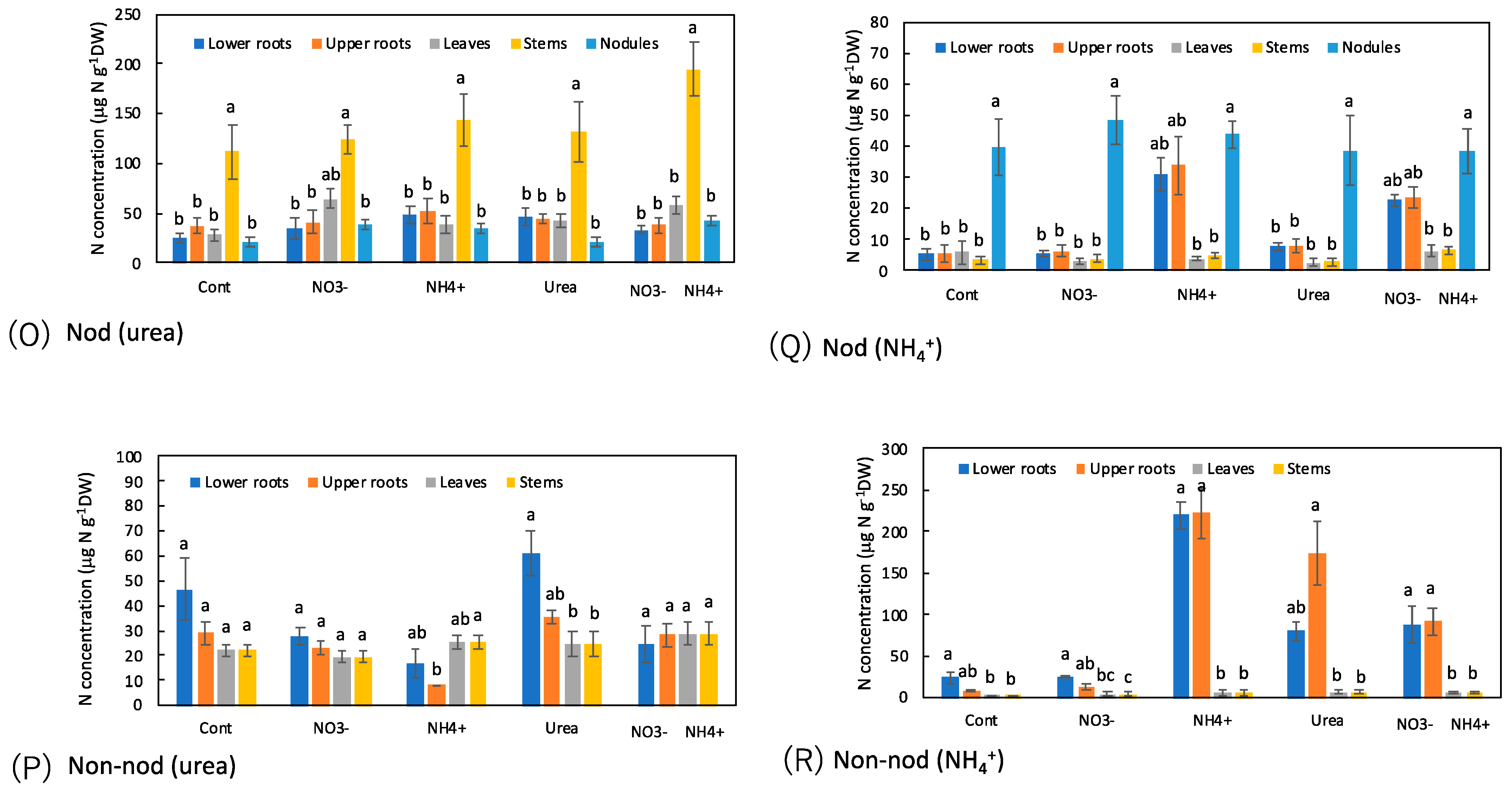
3. Results
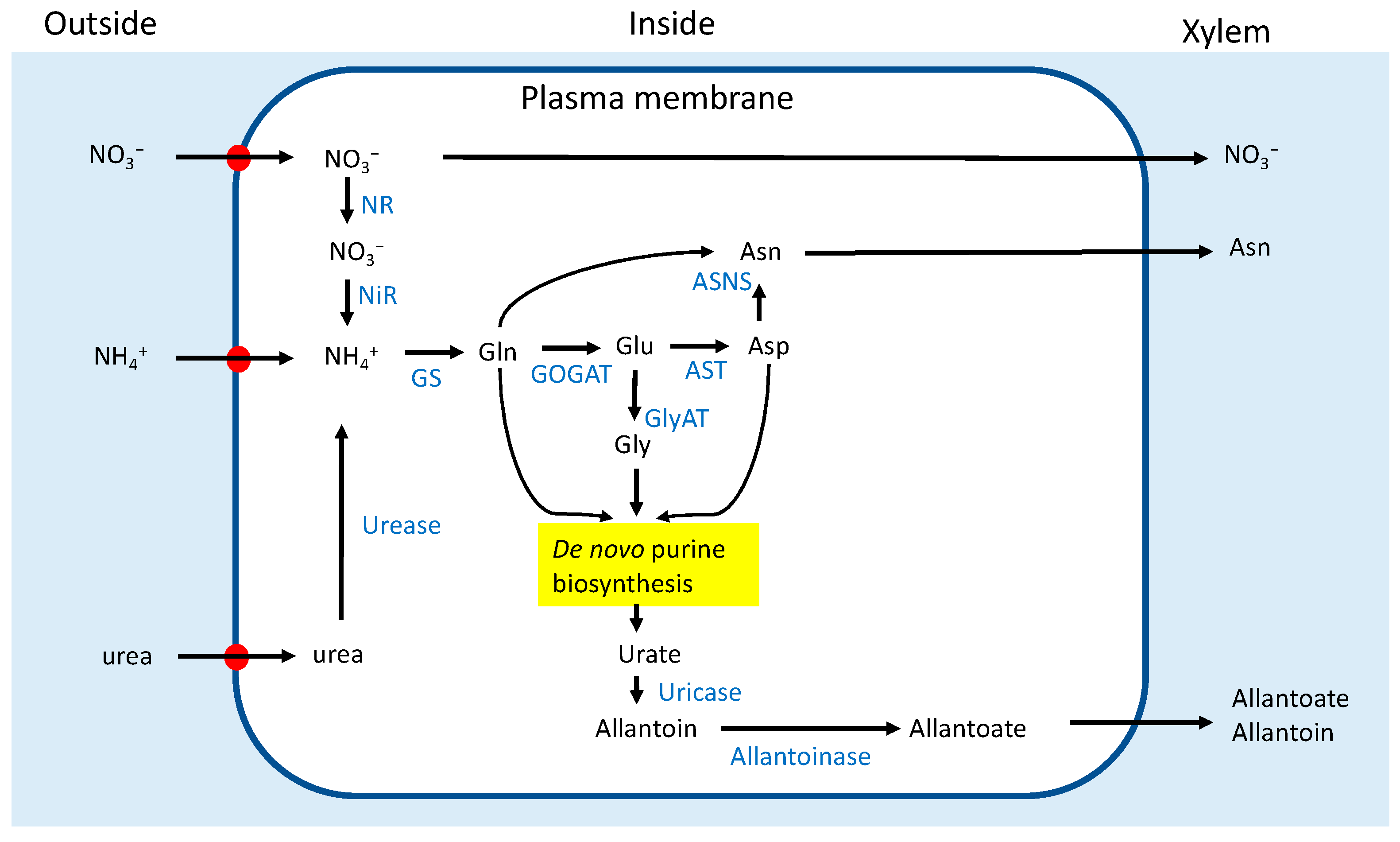
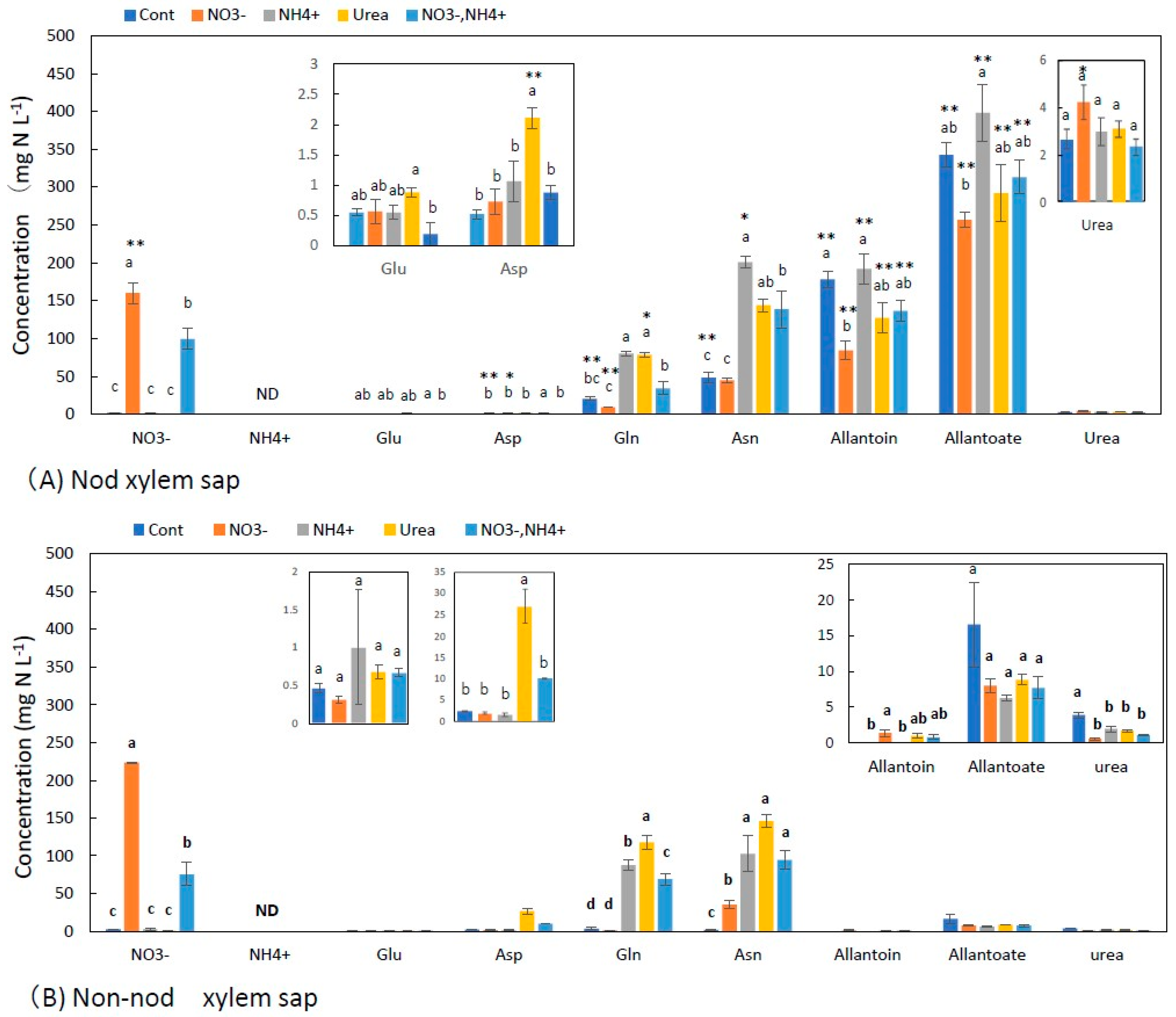
3.1. Concentrations of N Metabolites in the Xylem Sap
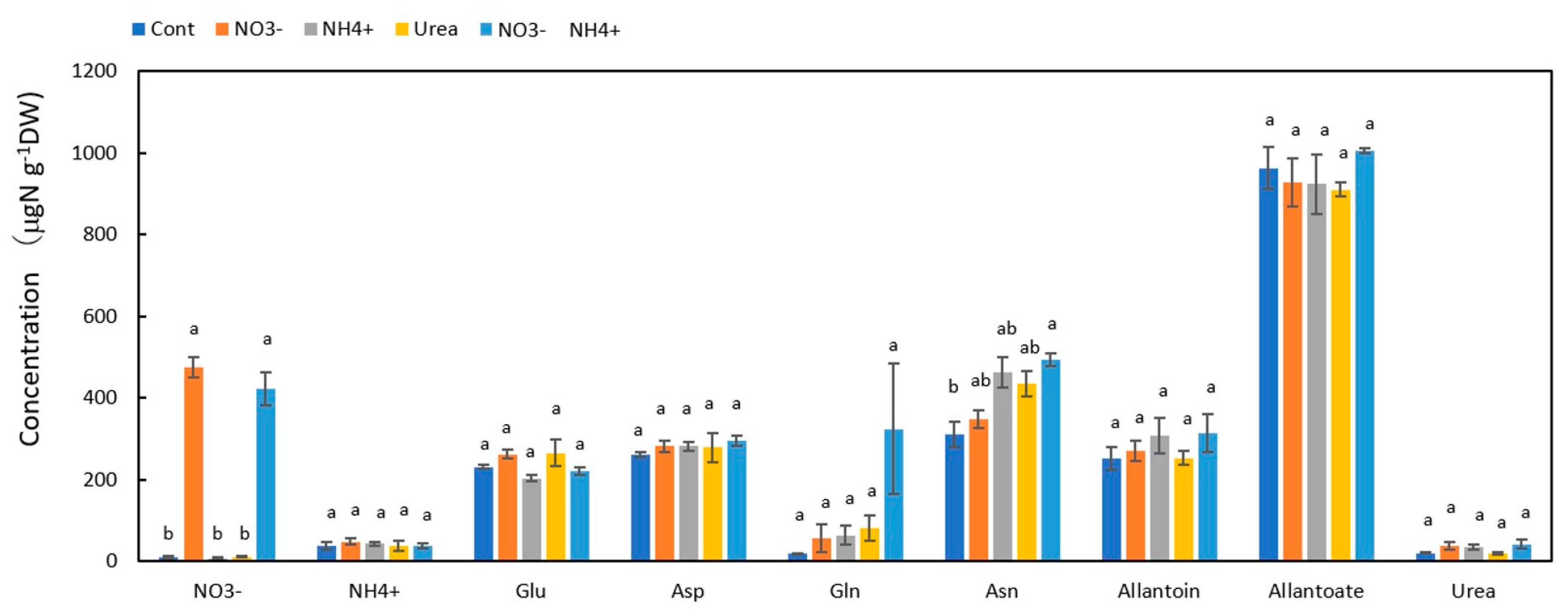
3.2. Concentrations of N Metabolites in the Nodules
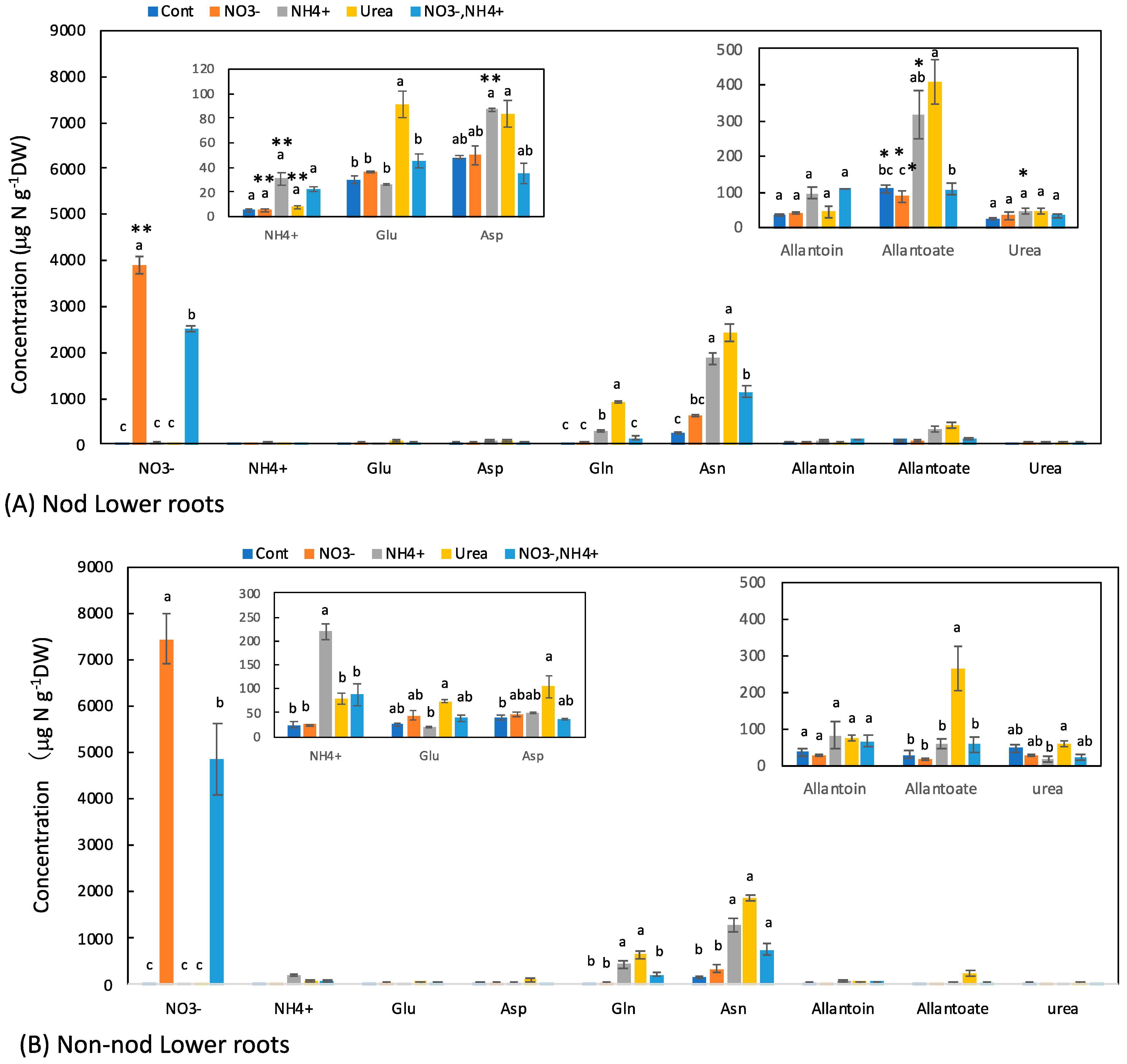
3.3. Concentrations of N Metabolites in the Lower Roots
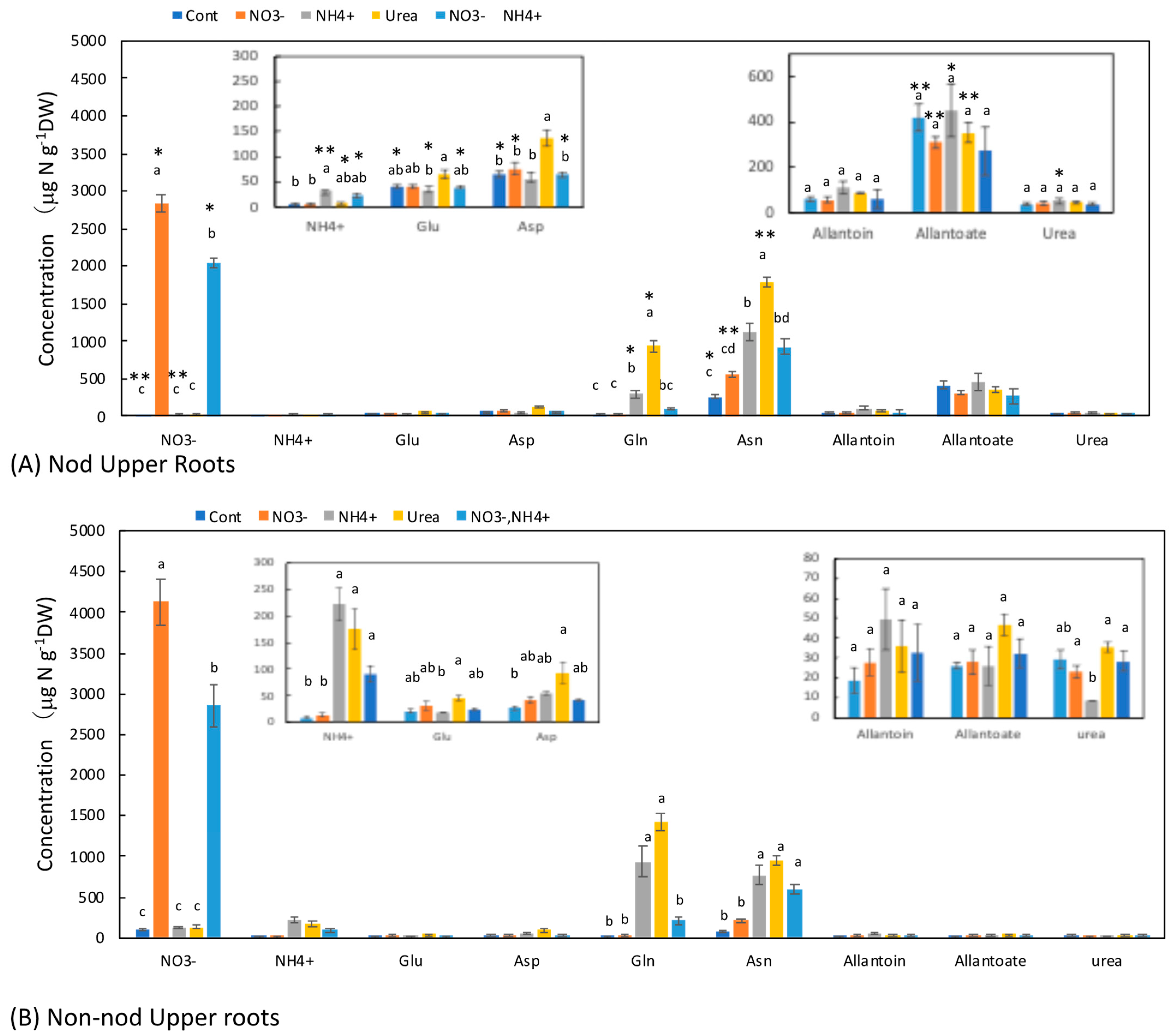
3.4. Concentrations of N Metabolites in the Upper Roots
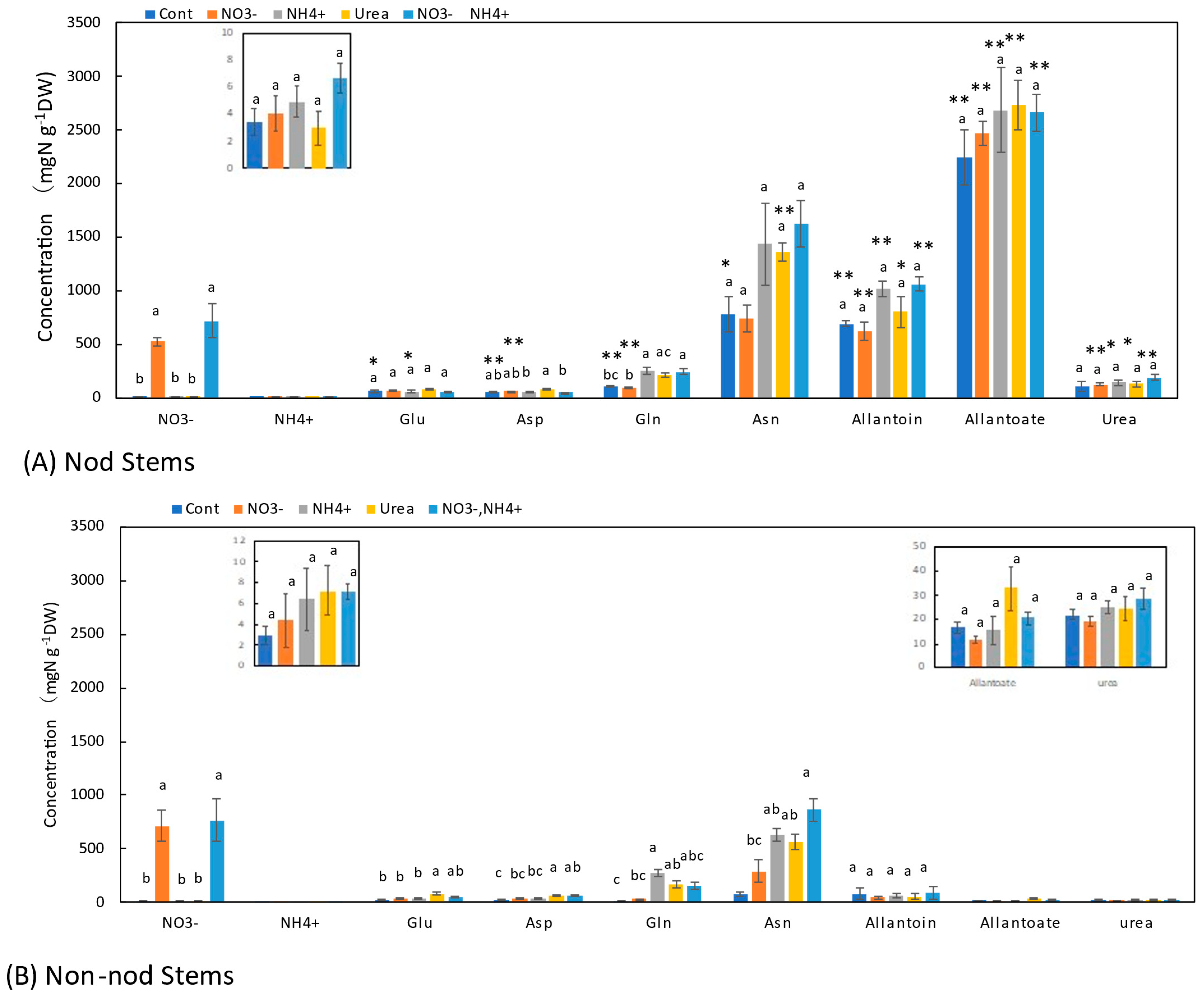
3.5. Concentrations of N Metabolites in the Stems
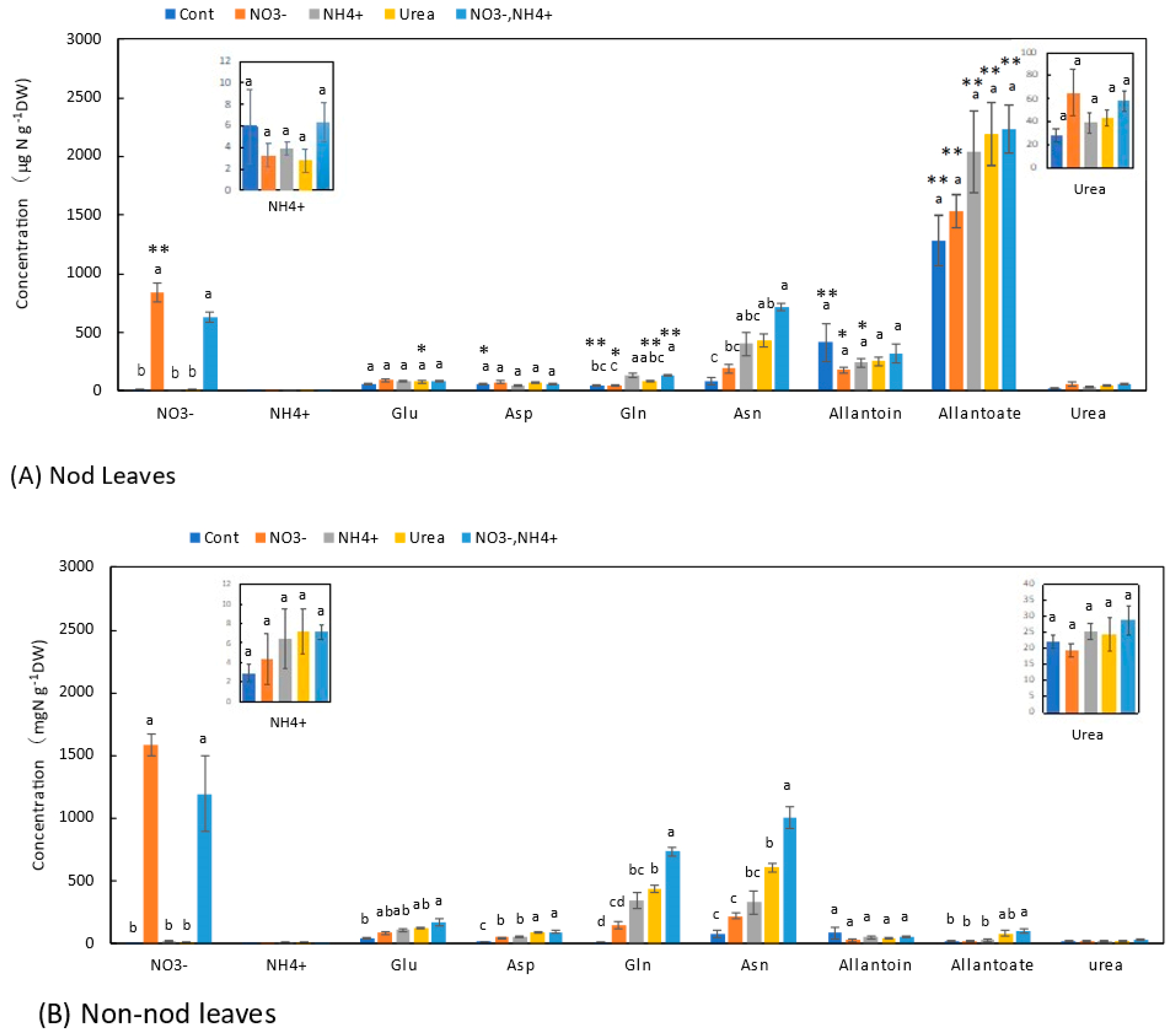
3.6. Concentrations of N Metabolites in the Leaves
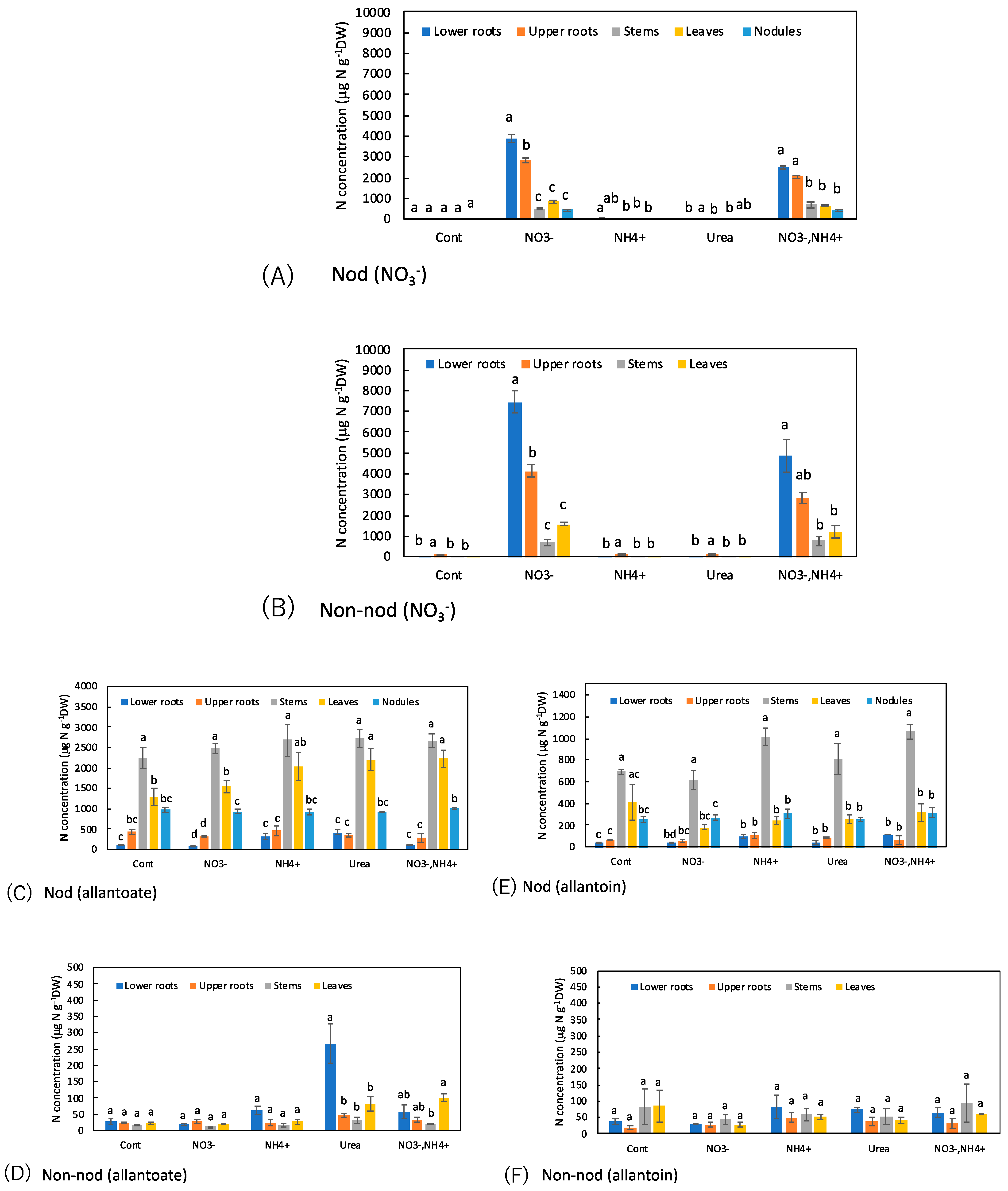
3.7. Comparison of the Concentrations of N Metabolites among the Organs with N Treatments
4. Discussion
4.1. N Metabolites in the Non-Nodulated Plants
4.2. N Metabolites in Nodulated Plants
4.3. N Metabolites among Organs
4.4. Effects of Nodulation on N Absorption, Metabolism, and Transport
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sato, T.; Onoma, N.; Fujikake, H.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Changes in four leghemoglobin components in nodules of hypernodulating soybean (Glycine max [L.] Merr.) mutant and its parent in the early nodule developmental stage. Plant Soil 2001, 237, 129–135. [Google Scholar] [CrossRef]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of different chemical forms of nitrogen on the quick and reversible inhibition of soybean nodule growth and nitrogen fixation activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Kumazawa, K. Assimilation and transport of nitrogenous compounds originated from 15N2 fixation and 15NO3 absorption. Soil Sci. Plant Nutr. 1979, 25, 9–19. [Google Scholar] [CrossRef]
- Ohyama, T.; Kumazawa, K. Comparative studies on the distribution of nitrogen in soybean plants supplied with N2 and NO3− at the pod filling stage. II. Assimilation and Transport of nitrogenous constituents. Soil Sci. Plant Nutr. 1984, 30, 219–229. [Google Scholar] [CrossRef]
- Ohyama, T.; Kumazawa, K. Nitrogen assimilation in soybean nodules, I. The role of GS/GOGAT system in the assimilation of ammonia produced by N2-fixation. Soil Sci. Plant Nutr. 1978, 24, 525–533. [Google Scholar] [CrossRef]
- Ohyama, T.; Kumazawa, K. Incorporation of 15N into various nitrogenous compounds in intact soybean nodules after exposure to 15N2 gas. Soil Sci. Plant Nutr. 1980, 26, 109–115. [Google Scholar] [CrossRef]
- Tajima, S.; Nomura, M.; Kouchi, H. Ureide biosynthesis in legume nodules. Front. Biosci. 2004, 9, 1347–1381. [Google Scholar] [CrossRef]
- Huber, T.A.; Streeter, J.G. Asparagine biosynthesis in soybean nodules. Plant Physiol. 1984, 74, 605–610. [Google Scholar] [CrossRef]
- Minamisawa, K.; Arima, Y.; Kumazawa, K. Characteristics of asparagine pool in soybean nodules in comparison with ureide pool. Soil Sci. Plant Nutr. 1986, 32, 1–14. [Google Scholar] [CrossRef]
- Ohyama, T.; Kato, N.; Saito, K. Nitrogen transport in xylem of soybean plant supplied with 15NO3−. Soil Sci. Plant Nutr. 1989, 35, 131–137. [Google Scholar] [CrossRef]
- McNeil, D.L.; LaRue, T.A. Effect of nitrogen source on ureides in soybean. Plant Physiol. 1984, 74, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-G.; Nomura, M.; Sato, T.; Fujikake, H.; Ohyama, T.; Tajima, S. Effect of exogenous NH4+-N supply on distribution of ureide content in various tissues of alfalfa plants, Medicago sativa. Soil Sci. Plant Nutr. 1999, 45, 921–927. [Google Scholar] [CrossRef]
- Ohyama, T. Comparative studies on the distribution of nitrogen in soybean plants supplied with N2 and NO3− at the pod filling stage. Soil Sci. Plant Nutr. 1983, 29, 133–145. [Google Scholar] [CrossRef]
- Ono, Y.; Fukasawa, M.; Sueyoshi, K.; Ohtake, N.; Sato, T.; Tanabata, S.; Toyota, R.; Higuchi, K.; Saito, A.; Ohyama, T. Application of nitrate, ammonium, or urea changes the concentrations of ureides, urea, amino acids and other metabolites in xylem sap and in the organs of soybean plants (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2021, 22, 4573. [Google Scholar] [CrossRef] [PubMed]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsuhashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.S.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Sakazume, T.; Tanaka, K.; Aida, H.; Ishikawa, S.; Nagumo, Y.; Takahashi, Y.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Estimation of nitrogen fixation rate of soybean (Glycine max (L.) Merr,) by micro-scale relative ureide analysis using root bleeding xylem sap and apoplast fluid in stem. Bull. Facul. Agric. Niigata Univ. 2014, 67, 27–41. [Google Scholar]
- Doi, M.; Higuchi, K.; Saito, A.; Sato, T.; Ohyama, T. N absorption, transport, and recycling in nodulated soybean plants by split-root experiment using 15N-labeled nitrate. Nitrogen 2022, 3, 636–651. [Google Scholar] [CrossRef]
- Ohyama, T.; Ikebe, K.; Okuoka, S.; Ozawa, T.; Nishiura, T.; Ishiwata, T.; Yamazaki, A.; Tanaka, F.; Takahashi, T.; Umezawa, T.; et al. A deep placement of lime nitrogen reduces the nitrate leaching and promotes soybean growth and seed yield. Crop Environ. 2022, 1, 221–230. [Google Scholar] [CrossRef]
- Reinbothe, H.; Mothes, K. Urea, ureides, and guanidines in plants. Annu. Rev. Plant Physiol. 1962, 13, 129–149. [Google Scholar] [CrossRef]
- Bollard, E.G. Nitrogenous compounds in plant xylem sap. Nature 1956, 178, 1189–1190. [Google Scholar] [CrossRef]
- Tomas, R.J.; Schrader, L.S. Ureide Metabolism in higher plants. Phytochemistry 1981, 20, 361–371. [Google Scholar] [CrossRef]
- McClure, P.R.; Israel, D.W.; Volk, R.J. Evaluation of the relative ureide content of xylem sap as an indicator of N2 fixation in soybeans. Plant Physiol. 1980, 66, 720–725. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Nishiwaki, T.; Ohtake, N.; Minagawa, R.; Ikarashi, T.; Ohyama, T. Nitrate transport pathway into soybean nodules traced by tungstate and 15NO3−. Soil Sci. Plant Nutr. 1995, 41, 75–88. [Google Scholar] [CrossRef]
- Mérigout, P.; Lelandais, M.; Bitton, F.; Renou, J.-P.; Briand, X.; Meyer, C.; Daniel-Vedele, F. Physiological and Transcriptomic Aspects of Urea Uptake and Assimilation in Arabidopsis Plants. Plant Physiol. 2008, 147, 1225–1238. [Google Scholar] [CrossRef]
- Wang, W.-H.; Köhler, B.; Cao, F.-Q.; Liu, L.-H. Molecular and physiological aspects of urea transport in higher plants. Plant Sci. 2008, 175, 467–477. [Google Scholar] [CrossRef]
- Ohyama, T.; Saito, K.; Kato, N. Diurnal rhythm in nitrate absorption by roots of soybeans (Glycine max). Soil Sci. Plant Nutr. 1989, 35, 33–42. [Google Scholar] [CrossRef]
- Yoneyama, T.; Karasuyama, M.; Kouchi, H.; Ishizuka, J. Occurrence of ureide accumulation in soybean plants. Soil Sci. Plant Nutr. 1985, 31, 133–140. [Google Scholar] [CrossRef]
- Todd, C.D.; Peter, A.; Tipton, P.A.; Blevins, D.G.; Piedras, P.; Pineda, M.; Polacco, J.C. Update on ureide degradation in legumes. J. Exper. Bot. 2006, 57, 5–12. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Sato, T.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Systemic and local effects of long-term application of nitrate on nodule growth and N2 fixation in soybean (Glycine max [L.] Merr.). Soil Sci. Plant Nutr. 2003, 49, 825–834. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Yamazaki, A.; Ito, S.; Sato, T.; Tewari, K.; Ohtake, N.; Sueyoshi, K.; Takahashi, Y.; Ohyama, T. Long-term effect of nitrate application from lower part of roots on nodulation and N2 fixation in upper part of roots of soybean (Glycine max (L.) Merr.) in two-layered pot experiment. Soil Sci. Plant Nutr. 2005, 51, 981–990. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohyama, T.; Isaka, M.; Saito, A.; Higuchi, K. Effects of Nodulation on Metabolite Concentrations in Xylem Sap and in the Organs of Soybean Plants Supplied with Different N Forms. Metabolites 2023, 13, 319. https://doi.org/10.3390/metabo13030319
Ohyama T, Isaka M, Saito A, Higuchi K. Effects of Nodulation on Metabolite Concentrations in Xylem Sap and in the Organs of Soybean Plants Supplied with Different N Forms. Metabolites. 2023; 13(3):319. https://doi.org/10.3390/metabo13030319
Chicago/Turabian StyleOhyama, Takuji, Miyuki Isaka, Akihiro Saito, and Kyoko Higuchi. 2023. "Effects of Nodulation on Metabolite Concentrations in Xylem Sap and in the Organs of Soybean Plants Supplied with Different N Forms" Metabolites 13, no. 3: 319. https://doi.org/10.3390/metabo13030319
APA StyleOhyama, T., Isaka, M., Saito, A., & Higuchi, K. (2023). Effects of Nodulation on Metabolite Concentrations in Xylem Sap and in the Organs of Soybean Plants Supplied with Different N Forms. Metabolites, 13(3), 319. https://doi.org/10.3390/metabo13030319







