Plastid Phosphatidylglycerol Homeostasis Is Required for Plant Growth and Metabolism in Arabidopsis thaliana
Abstract
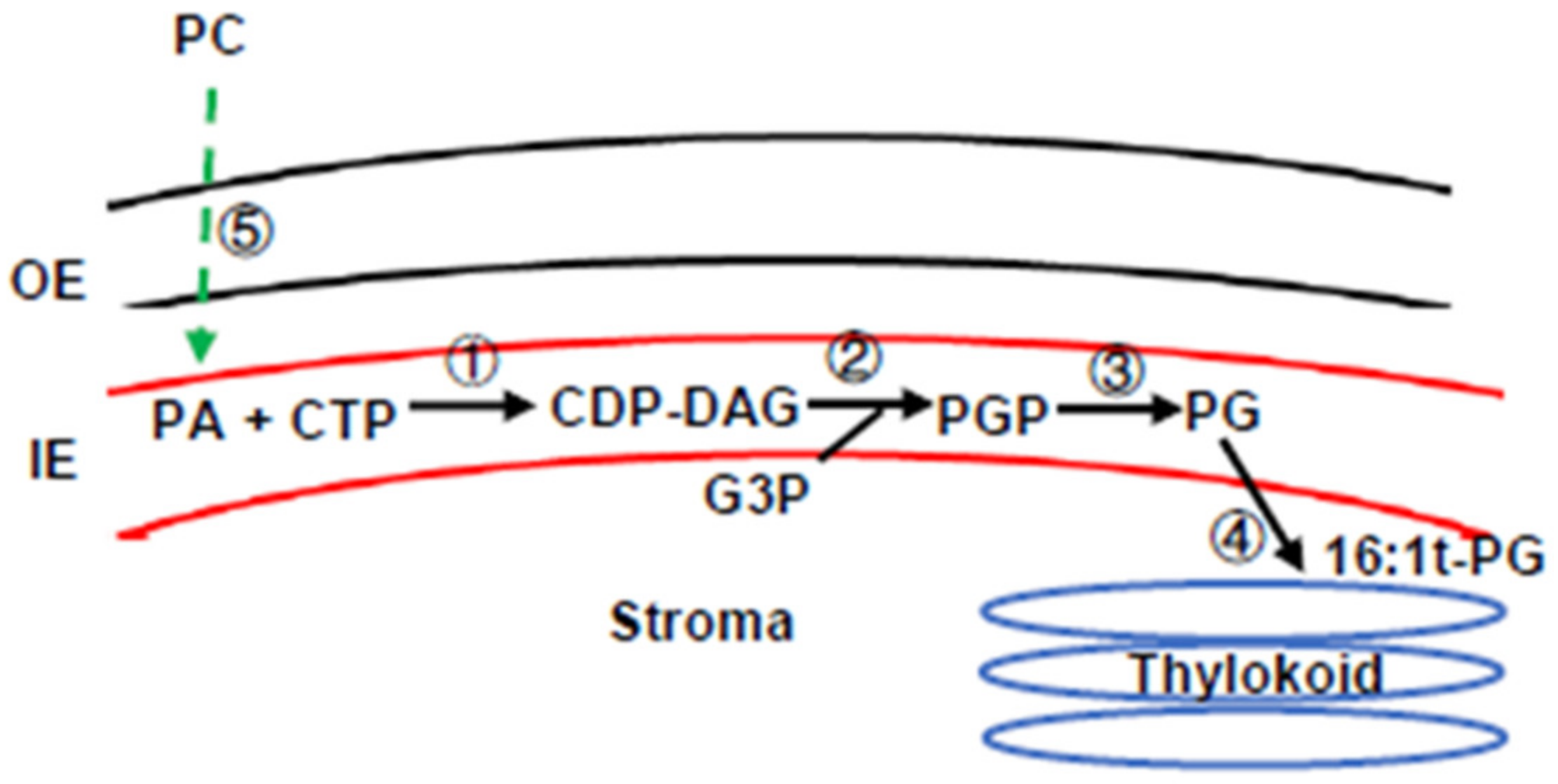
1. Introduction
2. Materials and Methods
2.1. Plant Growth
2.2. Overexpression of the FAD4 Gene in Arabidopsis
2.3. RT-qPCR
2.4. Plant Growth Parameter Measurement
2.5. Total Lipid Extraction
2.6. Lipidomic Profiling
2.7. Statistical Analysis
3. Results
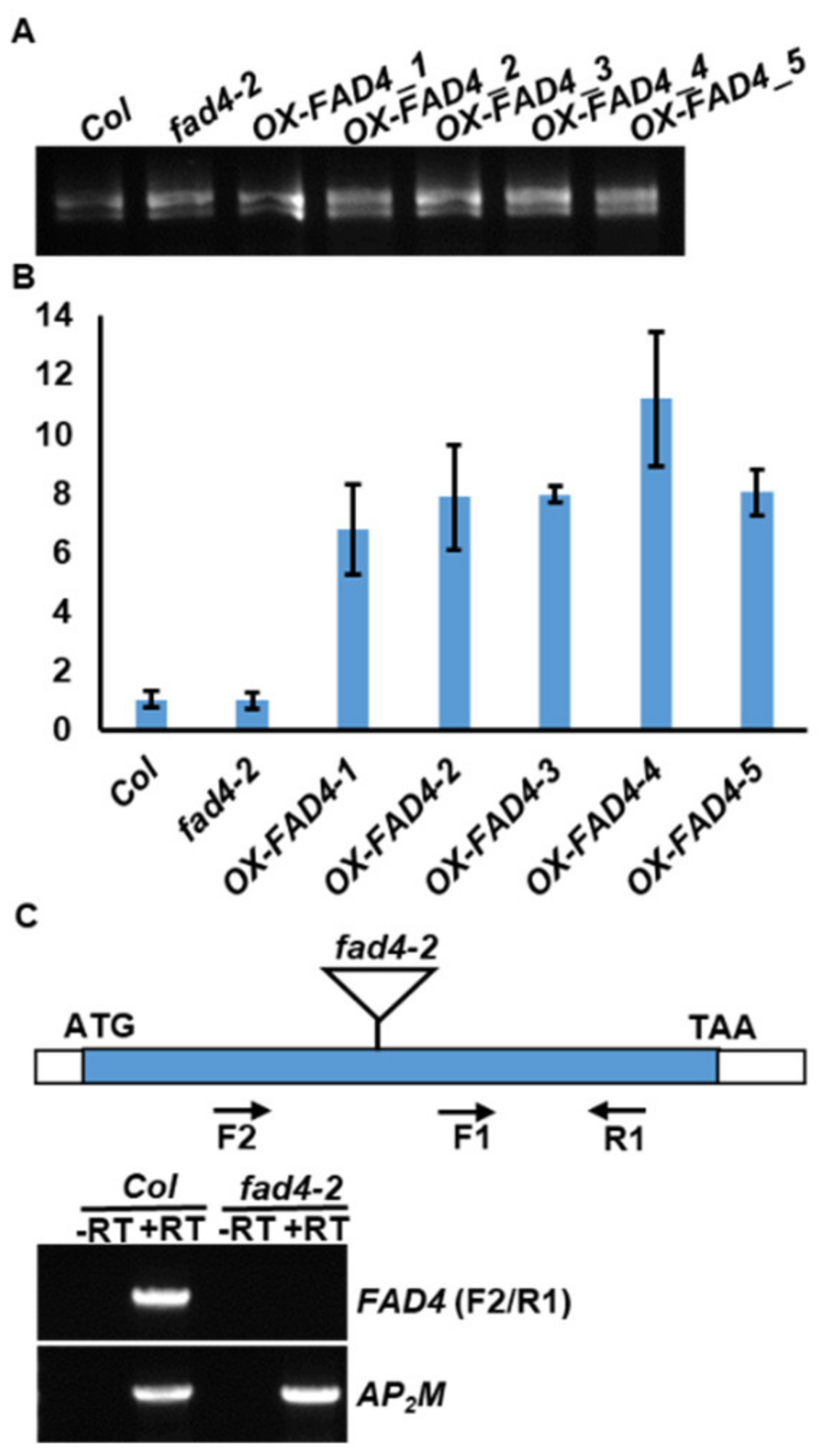
3.1. Confirmation of FAD4 Overexpression and Knockout lines by RT-qPCR
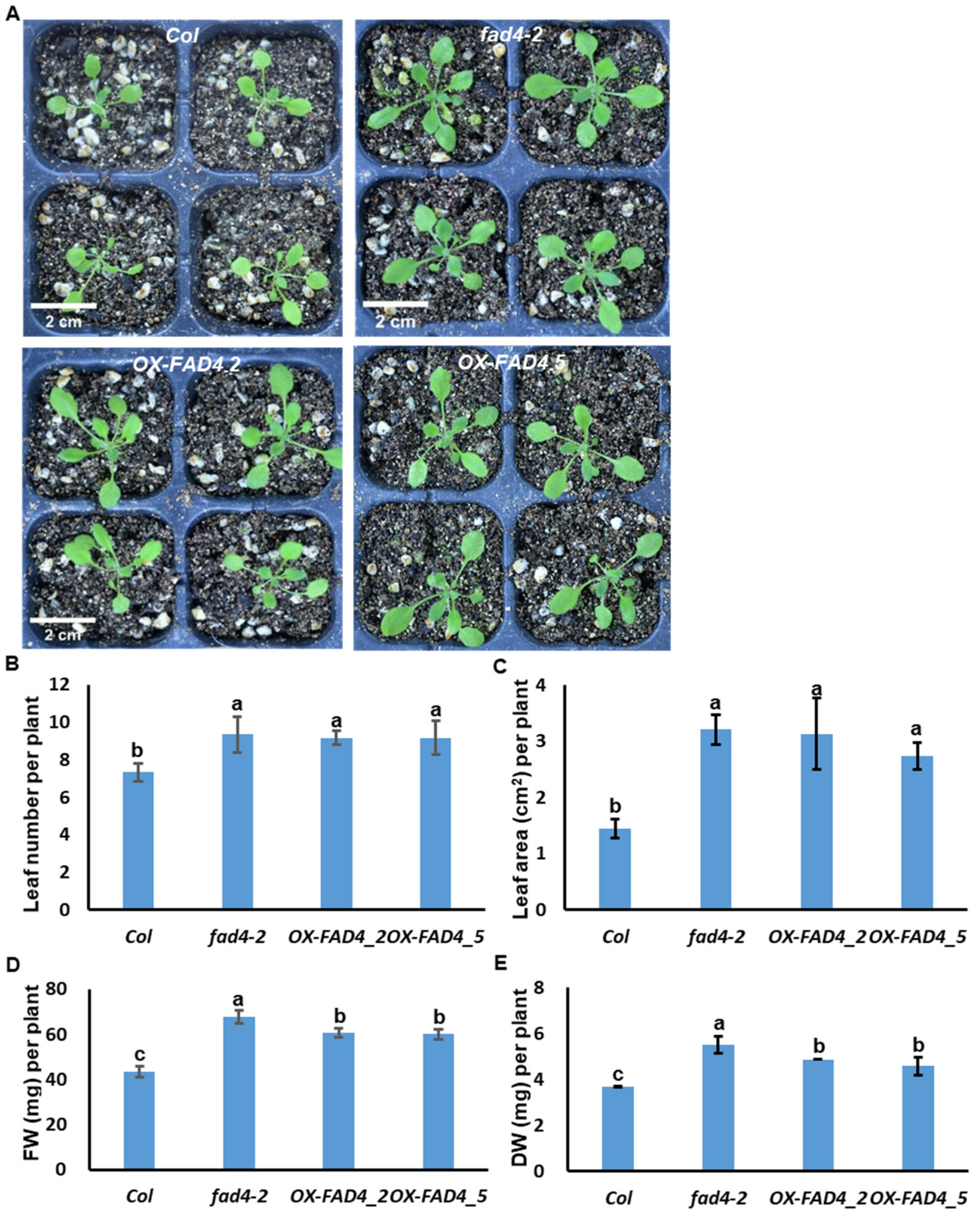
3.2. Overexpression or Knockout of FAD4 Enhances Plant Growth
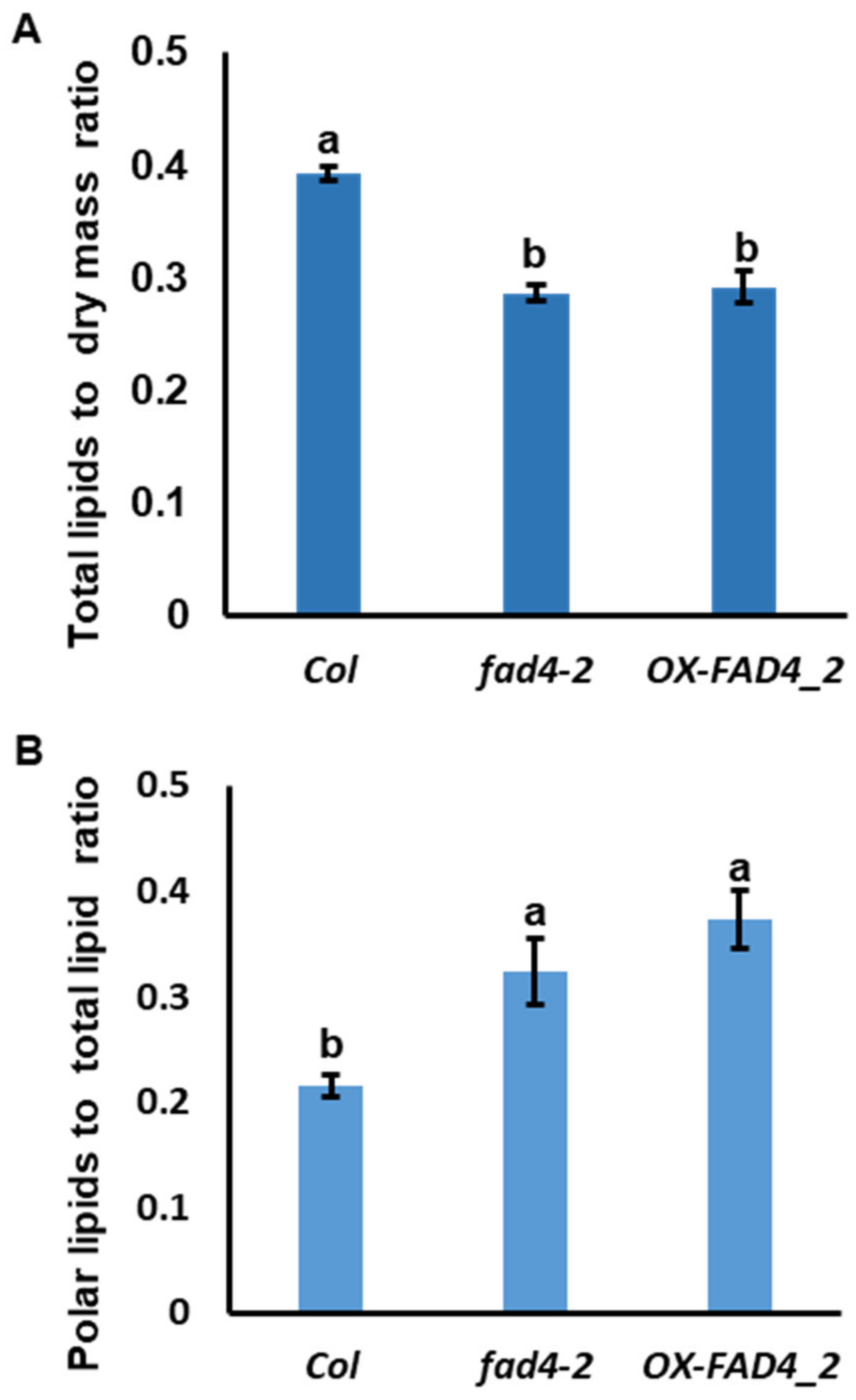
3.3. Disrupting FAD4 Homeostasis Reduced Total Lipid Content but Increased Polar Lipid Proportion
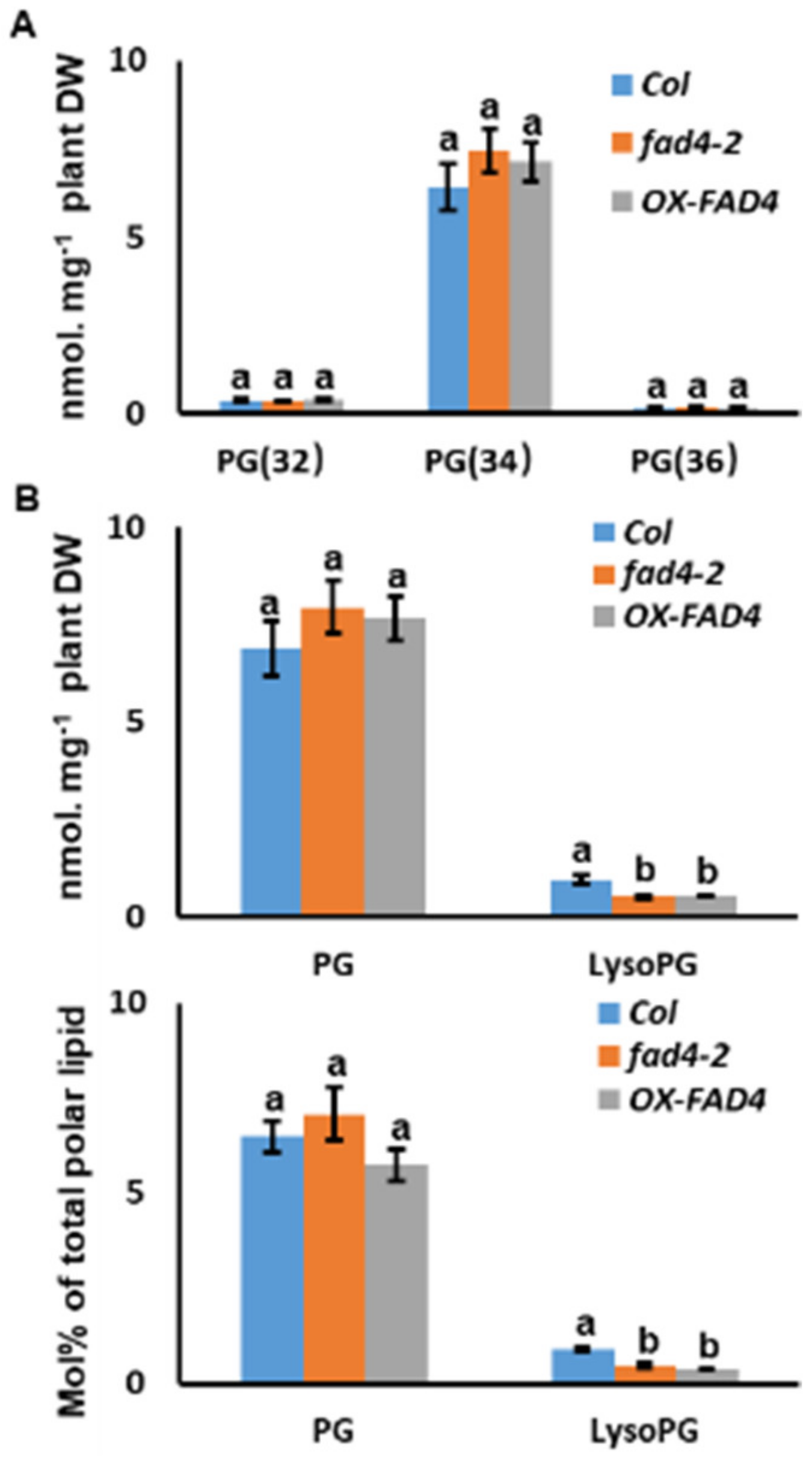
3.4. Lipidomic Profiling Confirmed FAD4 Enzyme Activities for Prokaryotic PG Synthesis
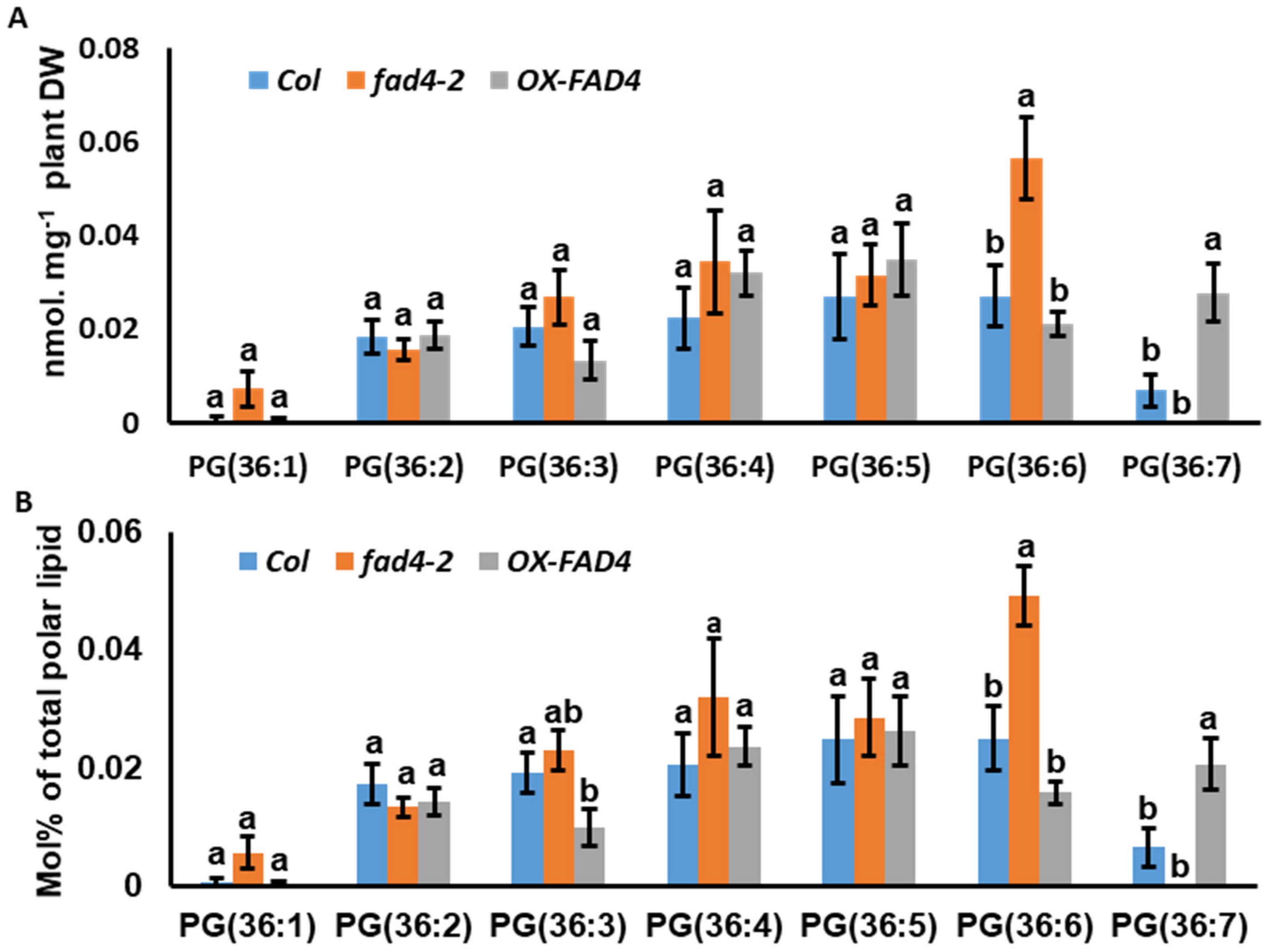
3.5. Lipidomic Profiling Uncovered a Potential New Substrate for FAD4
3.6. Disruption of FAD4 Expression Reduced LysoPG Contents
3.7. Over-expression of FAD4 Slightly Enhanced Prokaryotic and Eukaryotic MGDG Synthesis
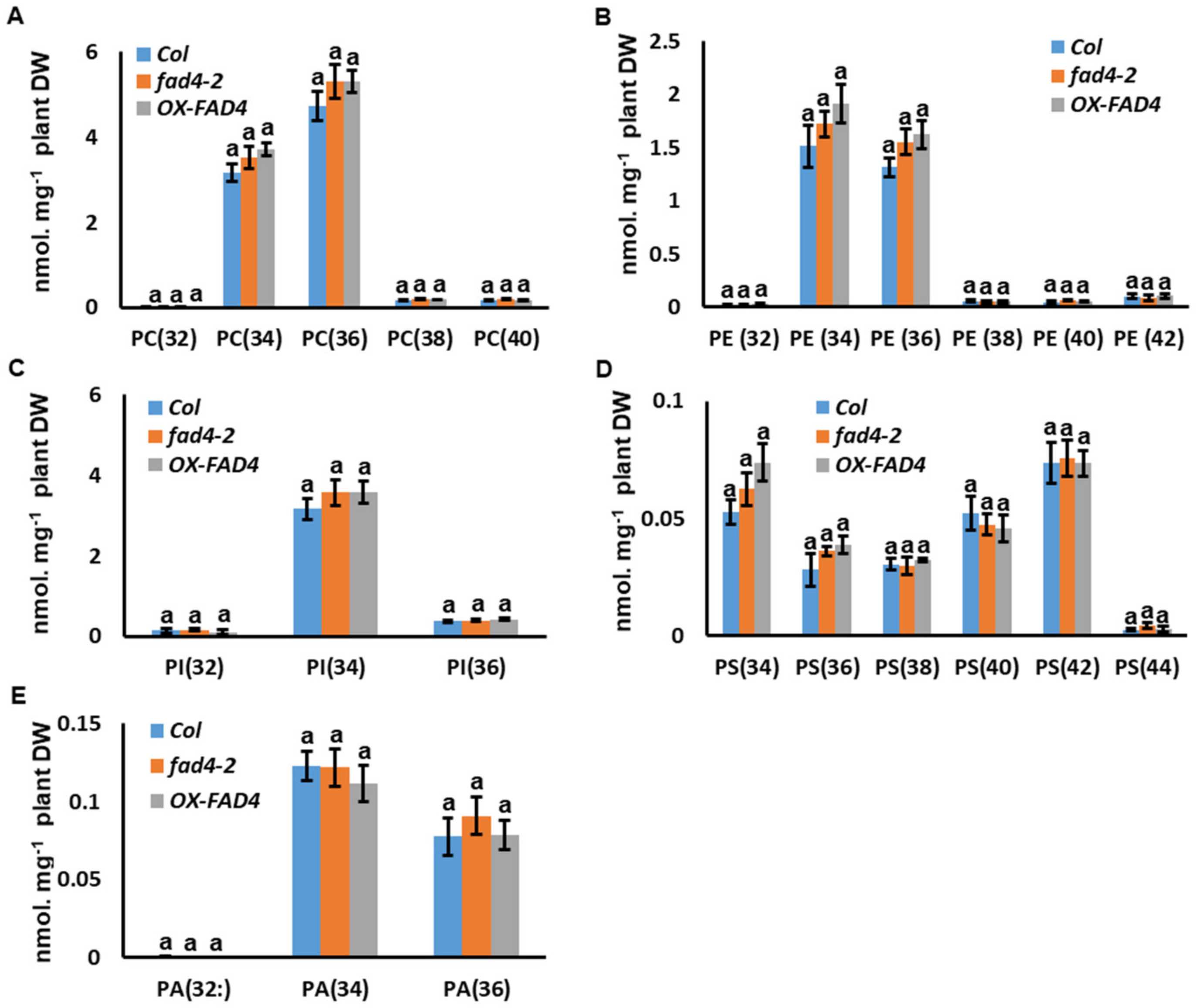
3.8. Disruption of FAD4 Expression Did Not Affect PC, PE, PI, PS and PA Contents or Compositions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohlrogge, J.B.; Kuhn, D.N.; Stumpf, P.K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc. Natl. Acad. Sci. USA 1979, 76, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 2013, e0161. [Google Scholar] [CrossRef] [PubMed]
- Roughan, P.G.; Holland, R.; Slack, C.R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C] acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem. J. 1980, 188, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, M.; Heinz, E.; McKeon, T.A.; Stumpf, P.K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur. J. Biochem. 1983, 129, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Heinz, E.; Roughan, G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983, 72, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Block, M.A.; Dorne, A.-J.; Joyard, J.; Douce, R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts—Part II: Biochemical characterization. J. Biol. Chem. 1983, 258, 13281–13286. [Google Scholar] [CrossRef]
- Moore, T.S. Phospholipid biosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 235–259. [Google Scholar] [CrossRef]
- Xu, C.; Härtel, H.; Wada, H.; Hagio, M.; Yu, B.; Eakin, C.; Benning, C. The pgp1 locus of Arabidopsis encodes a phosphatidylglycerol synthase with impaired activity. Plant Physiol. 2002, 129, 594–604. [Google Scholar] [CrossRef]
- Andrews, J.; Mudd, J.B. Phosphatidylglycerol synthesis in pea chloroplasts: Pathway and localization. Plant Physiol. 1985, 79, 259–265. [Google Scholar] [CrossRef]
- Zhou, Y.; Weth, A.; Peisker, H.; Baumgartner, W.; Dörmann, P.; Frentzen, M. Extraplastidial cytidinediphosphate diacylglycerol synthases of Arabidopsis thaliana. Plant J. 2013, 75, 867–879. [Google Scholar] [CrossRef]
- Schlame, M.; Rüstow, B.; Kunze, D.; Rabe, H.; Reichmann, G. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem. J. 1986, 240, 247–252. [Google Scholar] [CrossRef]
- Muller, F.; Frentzen, M. Phosphatidylglycerophosphate synthases from Arabidopsis thaliana. FEBS Lett. 2001, 509, 298–302. [Google Scholar] [CrossRef]
- Babiychuk, E.B.; Draeger, A. Annexins in cell membrane dynamics: Calcium regulated association of lipidmicrodomains. J. Cell Bio. 2000, 150, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ajjawi, I.; Manoli, A.; Sawin, A.; Xu, C.; Froehlich, J.E.; Last, R.L.; Benning, C. FATTY ACID DESATURASE4 of Arabidopsis encodes a protein distinct from characterized fatty acid desaturases. Plant J. 2009, 60, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hölzl, G.; vom Dorp, K.; Peisker, H.; Melzer, M.; Frentzen, M.; Dörmann, P. Identification and characterization of a plastidial phosphatylglycerophosphate phosphatase in Arabidopsis thaliana. Plant J. 2016, 89, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.J.; Smith, M.D.; Clark, T.R.; Froehlich, J.E.; Benning, C. PEROXIREDOXIN Q stimulates the activity of the chloroplast 16:1Δ3trans FATTY ACID DESATURASE4. Plant J. 2020, 102, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, R.; van Klompenburg, W.; Pilon, M.; Kozubek, A.; de Korte-Kool, G.; Demel, R.A.; Weisbeek, P.J.; de Kruijff, B. The transit sequence mediates the specific interaction of the precursor of ferredoxin with chloroplast envelope membrane lipids. J. Biol. Chem. 1993, 268, 4037–4042. [Google Scholar] [CrossRef]
- Schmid, V.H.R.; Cammarata, K.V.; Bruns, B.U.; Schmidt, G.W. In vitro reconstruction of the photosystem I light harvesting complex LHCI-730: Heterodimerization is required for antenna pigment organization. Proc. Natl. Acad. Sci. USA 1997, 94, 7667–7672. [Google Scholar] [CrossRef]
- Kruse, O.; Hankammer, B.; Konczak, C.; Gerle, C.; Morris, E.; Radunz, A.; Schmid, G.H.; Barber, J. Phosphatidylglycerol is involved in the dimerization of photosystem II. J. Biol. Chem. 2000, 275, 6509–6514. [Google Scholar] [CrossRef]
- Yu, B.; Benning, C. Anionic lipids are required for chloroplast structure and function in Arabidopsis. Plant J. 2003, 36, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Savage, L.J.; Larson, M.D.; Wilkerson, C.G.; Last, R.L. Chloroplast 2010: A database for large-scale phenotypic screening of Arabidopsis mutants. Plant Physiol. 2011, 155, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Lamkemeyer, P.; Laxa, M.; Collin, V.; Li, W.X.; Finkemeier, I.; Schöttler, M.A.; Holtkamp, V.; Tognetti, V.B.; Issakidis-Bourguet, E.; Kandlbinder, A.; et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 2006, 45, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Falconet, D.; Bastien, O.; Maréchal, E. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim. Biophys. Acta 2014, 1837, 470–480. [Google Scholar] [CrossRef]
- McCourt, P.; Browse, J.; Watson, J.; Arntzen, C.J.; Somerville, C.R. Analysis of photosynthetic antenna function in a mutant of Arabidopsis thaliana (L.) lacking trans-hexadecenoic acid. Plant Physiol. 1985, 78, 853–858. [Google Scholar] [CrossRef]
- Lam, S.M.; Shui, G. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genom. 2013, 40, 375–390. [Google Scholar] [CrossRef]
- Chen, M.J.; Thelen, J.J. The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. Plant Cell 2010, 22, 77–90. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Chen, M.J.; Thelen, J.J. Acyl-lipid desaturase 2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 1430–1444. [Google Scholar] [CrossRef]
- Chen, M.J.; Thelen, J.J. Acyl-lipid desaturase 1 primes cold acclimation response in Arabidopsis. Physiol. Plant 2016, 158, 11–22. [Google Scholar] [CrossRef]
- Shiva, S.; Enninful, R.; Roth, M.R.; Tamura, P.; Jagadish, K.; Welti, R. An efficient modified method for plant leaf lipid extraction results in improved recovery of phosphatidic acid. Plant Methods 2018, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses. J. Bio. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Shiva, S.; Vu, H.S.; Roth, M.R.; Zhou, Z.; Marepally, S.R.; Nune, D.S.; Lushington, G.H.; Visvanathan, M.; Welti, R. Lipidomic analysis of plant membrane lipids by direct infusion tandem mass spectrometry. Methods Mol. Biol. 2013, 1009, 79–91. [Google Scholar] [PubMed]
- Hsu, F.; Turk, J.; Williams, T.; Welti, R. Electrospray ionization multiple stage quadrupole ion-trap and tandem quadrupole mass spectrometric studies on phosphatidylglycerol from Arabidopsis leaves. J. Am. Soc. Mass Spectrom. 2007, 18, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.V.; Steponkus, P.L. Plasma membrane lipid alterations associated with cold acclimation of winter rye seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987, 83, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Liu, R.M.; Huang, X.H.; Du, Z.H.; Heng, S.P.; Zeng, W. Characterization of low temperature-induced plasma membrane lipidome remodeling combined with gene expression analysis reveals mechanisms that regulate membrane lipid desaturation in Carica papaya. Sci. Hortic. 2020, 272, 109505. [Google Scholar] [CrossRef]
- Fritz, M.; Lokstein, H.; Hackenberg, D.; Welti, R.; Roth, M.; Zähringer, U.; Fulda, M.; Hellmeyer, W.; Ott, C.; Wolter, F.P.; et al. Channeling of eukaryotic diacylglycerol into the biosynthesis of plastidial phosphatidylglycerol. J. Bio. Chem. 2007, 282, 4613–4625. [Google Scholar] [CrossRef]
- Burgos, A.; Szymanski, J.; Seiwert, B.; Degenkolbe, T.; Hannah, M.A.; Giavalisco, P.; Willmitzer, L. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. Plant J. 2011, 66, 656–668. [Google Scholar] [CrossRef]
- Browse, J.; McCourt, P.; Somerville, C.R. A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science 1985, 227, 763–765. [Google Scholar] [CrossRef]
- Hurlock, A.K.; Wang, K.; Takeuchi, T.; Horn, P.J.; Benning, C. In vivo lipid ‘tag and track’ approach shows acyl editing of plastid lipids and chloroplast import of phosphatidylglycerol precursors in Arabidopsis thaliana. Plant J. 2018, 95, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Hugly, S.; Somerville, C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992, 99, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, J.; Iba, K. Characterization of a nonsense mutation in FAD7, the gene which encodes omega-3 desaturase in Arabidopsis thaliana. J. Plant Res. 1998, 111, 87–91. [Google Scholar] [CrossRef]
- Iba, K.; Gibson, S.; Nishiuchi, T.; Fuse, T.; Nishimura, M.; Arondel, V.; Hugly, S.; Somerville, C. A gene encoding a chloroplast omega-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana. J. Bio. Chem. 1993, 268, 24099–24105. [Google Scholar] [CrossRef]
- Meyer, R.C.; Steinfath, M.; Lisec, J.; Becher, M.; Witucka-Wall, H.; Törjék, O.; Fiehn, O.; Eckardt, Ä.; Willmitzer, L.; Selbig, J.; et al. The metabolic signature related to high plant growth rate in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 4759–4764. [Google Scholar] [CrossRef]
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van der Straeten, D.; Peng, J.R.; Harberd, N.P. Integration of Plant Responses to Environmentally Activated Phytohormonal Signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef]
- Hoh, D.; Horn, P.J.; Kanazawa, A.; Froehilch, J.; Cruz, J.; Tessmer, O.L.; Hall, D.; Yin, L.; Benning, C.; Kramer, D.M. Genetically-determined variations in photosynthesis indicate roles for specific fatty acid species in chilling responses. Plant Cell Environ. 2022, 45, 1682–1697. [Google Scholar] [CrossRef]
- Ye, Y.; Nikovics, K.; To, A.; Lepiniec, L.; Fedosejevs, E.T.; Van Doren, S.R.; Baud, S.; Thelen, J.J. Docking of acetyl-CoA carboxylase to the plastid envelope membrane attenuates fatty acid production in plants. Nat. Comm. 2020, 11, 6191. [Google Scholar] [CrossRef]
- Fester, T.; Fetzer, I.; Härtig, C. A core set of metabolite sink/source ratios indicative for plant organ productivity in Lotus japonicas. Planta 2013, 237, 145–160. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, S.; Zhang, Y.; Fang, D.; Thelen, J.J. Plastid Phosphatidylglycerol Homeostasis Is Required for Plant Growth and Metabolism in Arabidopsis thaliana. Metabolites 2023, 13, 318. https://doi.org/10.3390/metabo13030318
Chen M, Wang S, Zhang Y, Fang D, Thelen JJ. Plastid Phosphatidylglycerol Homeostasis Is Required for Plant Growth and Metabolism in Arabidopsis thaliana. Metabolites. 2023; 13(3):318. https://doi.org/10.3390/metabo13030318
Chicago/Turabian StyleChen, Mingjie, Shiya Wang, Yi Zhang, Dongsheng Fang, and Jay J. Thelen. 2023. "Plastid Phosphatidylglycerol Homeostasis Is Required for Plant Growth and Metabolism in Arabidopsis thaliana" Metabolites 13, no. 3: 318. https://doi.org/10.3390/metabo13030318
APA StyleChen, M., Wang, S., Zhang, Y., Fang, D., & Thelen, J. J. (2023). Plastid Phosphatidylglycerol Homeostasis Is Required for Plant Growth and Metabolism in Arabidopsis thaliana. Metabolites, 13(3), 318. https://doi.org/10.3390/metabo13030318






