Natural Molecules, Nutraceuticals, and Engineered Nanosystems: A Comprehensive Strategy for Combating Gardnerella vaginalis-Induced Bacterial Vaginosis
Abstract
1. Introduction
2. Methodology
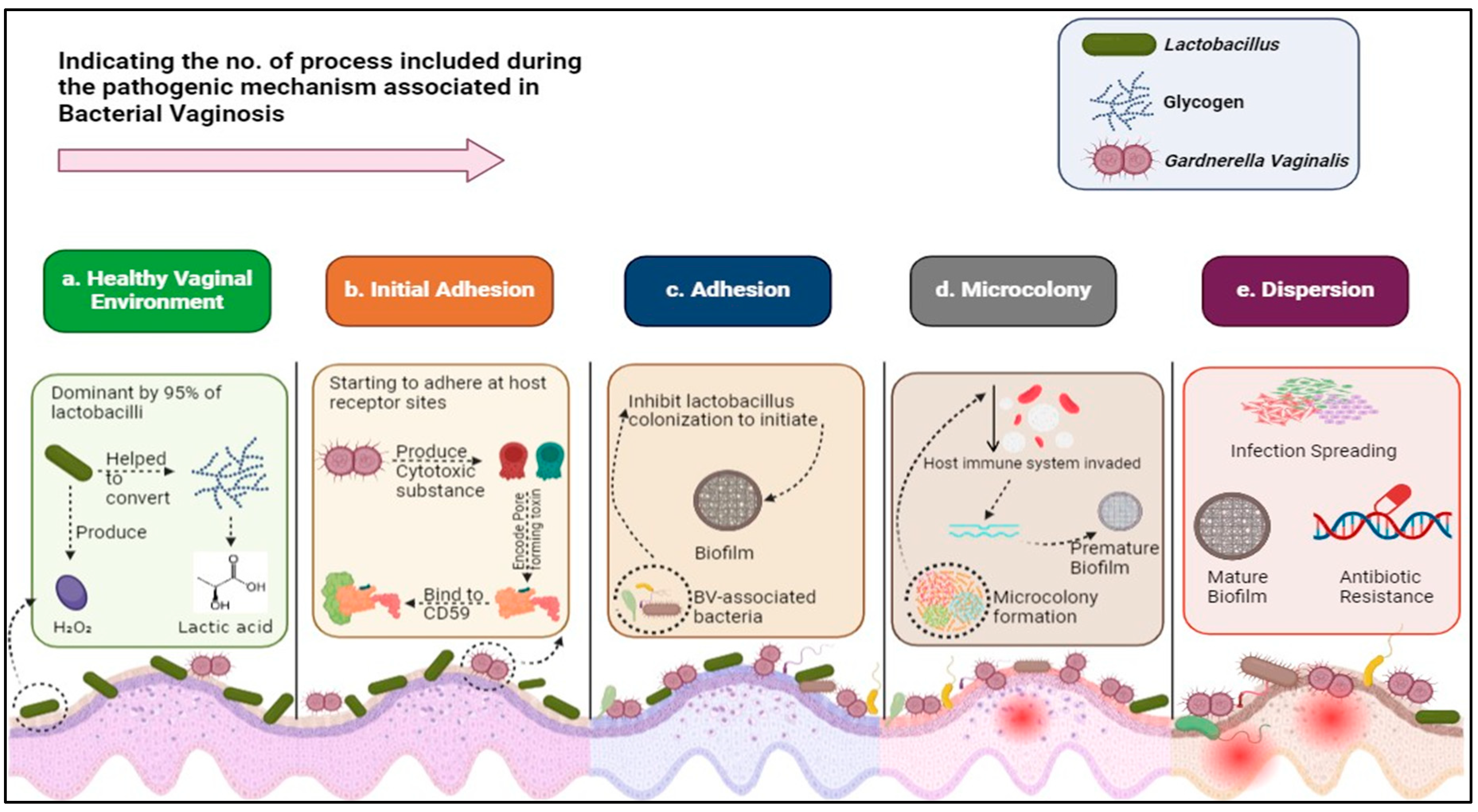
3. Mechanisms of Gardnerella vaginalis Pathogenicity
4. Characterizing the Resistance Profile of Gardnerella vaginalis
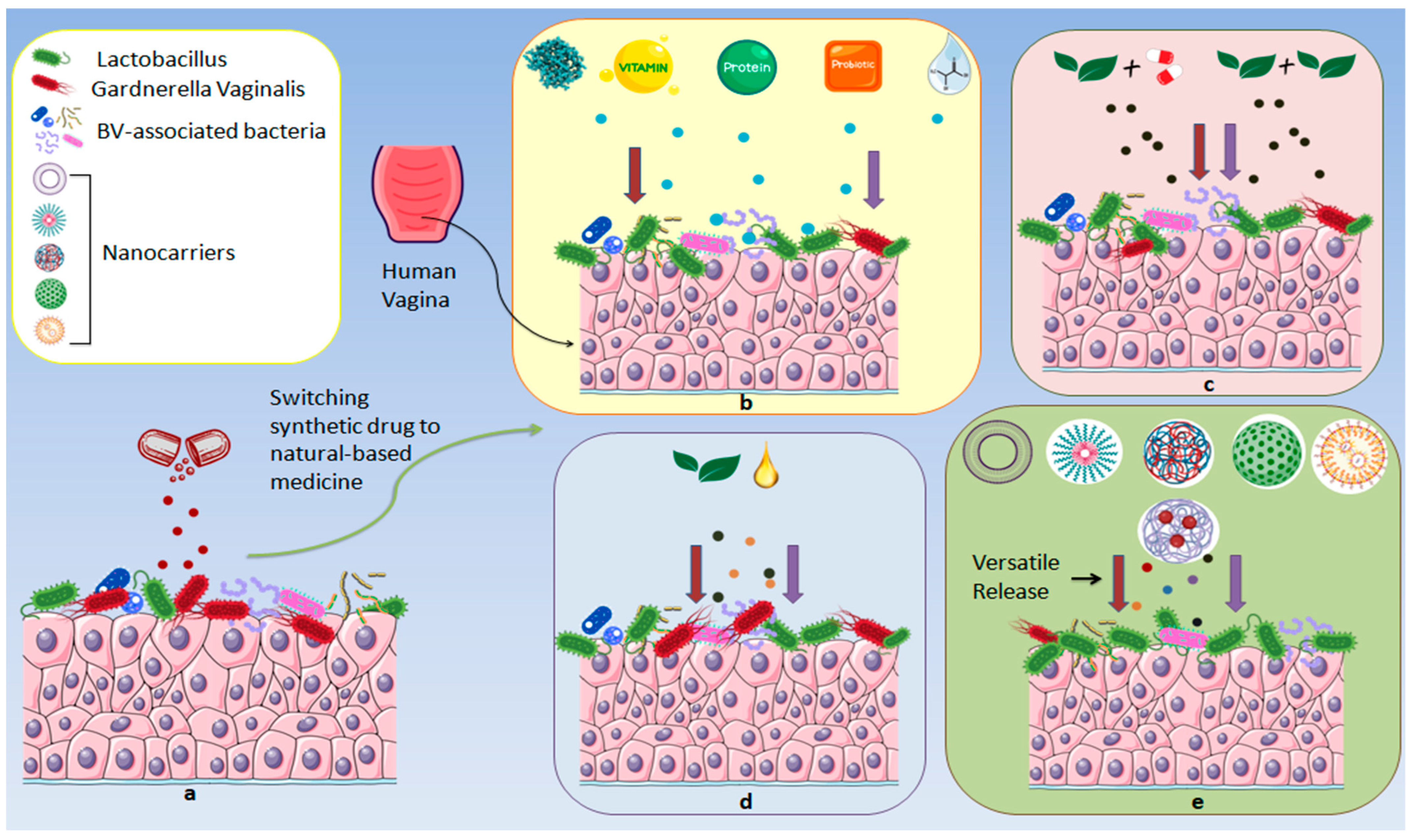
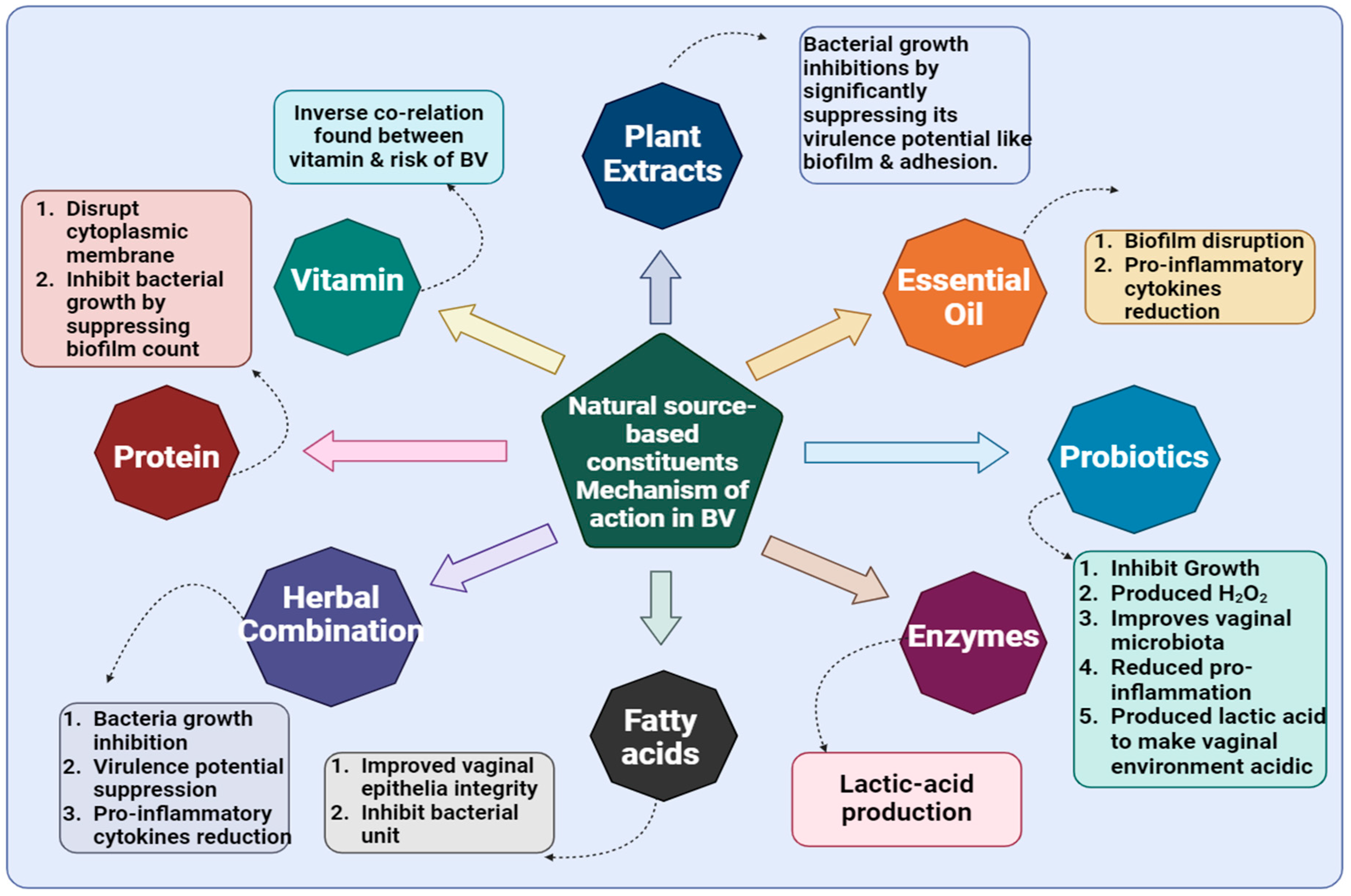
5. Naturally Derived Agents Against BV
5.1. Synergistic Applications of Herbal Therapies
5.1.1. Live Bacteria
5.1.2. Natural Molecules
| Sl. No. | Combination | Performed Assay/Method | Strains Used | Types of Study | Outcomes | References |
|---|---|---|---|---|---|---|
| Live Bacteria | ||||||
| 1. | Ligilactobacillus salivarius MG242, Limosilactobacillus fermentum MG901, Lactiplantibacillus plantarum MG989, Lacticaseibacillus paracasei MG4272, and Lacticaseibacillus rhamnosus MG4288 | Detection of H2O2 Production Study, Analysis of LA Production, Antibacterial Effects of Lactobacilli Strains on G. vaginalis Growth, e Cytotoxic Effect Assay, Adhesion Ability of Lactobacilli Strains to HeLa, MPO Activity in Vaginal Lysates | G. vaginalis (KCTC5096) | In vitro (HeLa cell line used) and In Vivo (C57BL/6) | Synergistic potential against G. vaginalis and significant inhibition of vaginal epithelial exfoliation were observed. | [64] |
| 2. | S. cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 probiotic | Inhibition and Disaggregation of G. vaginalis Biofilm Study, Co-Aggregation Assay, Anti-Biofilm Activity of MTZ and Clindamycin | Streptomycin-resistant G. vaginalis | In vitro | Promising inhibition and disruption of bacterial biofilm were observed. | [65] |
| 3. | L. rhamnosus HN001 and L. acidophilus GLa-14 | Assay for the Inhibitory Effects of Probiotics Against the Growth of G. vaginalis and AV, Assay for the Antagonistic Effects of Probiotics on the Adherence of G. vaginalis to HeLa Cells, MPO Activity Assay, ELISA | G. vaginalis KCTC5096 and Atopobium vaginae KCTC15240 | In vitro (HeLa cells) and In vivo (C57BL/6) | Probiotics comprising a combination of this spp. exhibited desired killing activity against G. vaginalis with anti-inflammatory characteristics. | [66] |
| 4. | MTZ and oral probiotics (prOVag) | Antibacterial Study | G. vaginalis | Multicentre, randomized, double-blind, placebo controlled trial (NCT01993524) | Clinical and microbiological parameters of BV were improved by this combination. | [67] |
| Natural Molecules | ||||||
| 1. | Pea protein, Grape seed extract, and LA | CFU Evaluation, MPO Activity, SLD Activity Assay, MucoadhesionStudy | G. vaginalis (KCTC5096) | In vivo (C57BL/6 mice model) | This combination improved vaginal tissue architecture while suppressing bacterial invasion. | [68] |
| 2. | Senna alata, Ricinus communis, and Lannea barteri | Antimicrobial Bioassay, Synergy Analysis | E. coli | In vitro | Additive antibacterial activity was demonstrated. | [69] |
| 3. | Carvacrol, α-terpinene, γ-terpinene, ρ-cymene, and linalool | Vaginal Irritation Test, Biofilm Biomass Quantification by Crystal Violet Staining Method, Checkerboard Method for Fractional Inhibitory Concentration | G. vaginalis UM137, G. piotii UM035, G. leopoldii UGent 09.48, and G. swidsinskii GS 9838-1 | In vitro (Human Vaginal Epithelium (HVESkinEthic) | No cytotoxicity was observed in the tested vaginal tissue. | [70] |
| 4. | Acetic acid and LA (intravaginal combination therapy) | Antimicrobial Study | Patients with vaginal infection | Double blinded randomized controlled feasibility trial (ACTRN 12620001084976) | The BV recurrence rate had drastically reduced. | [71] |
| 5. | Subtilosin and glycerol monolaurate | Checkerboard Assays, Antimicrobial Assay | Gardnerella vaginalis ATCC 14018, Peptostreptococcus anaerobius ATCC 27337, and Mobiluncus curtisii ATCC 35241 | In vitro (first time performed) | From MIC 4.6–25 μg/mL, this herbal combination showed 4-fold growth inhibition of G. vaginalis. | [72] |
| 6. | Cranberry extract, Bacillus coagulans, and Turmeric extract | Antibacterial Study, Recurrence Assay | Patients with BV | Observational/descriptive study. | 76.9% of patients improved after exposure to combination therapy. | [73] |
| 7. | Prangos ferulacea vaginal cream with MTZ | Antibacterial Study | Patients suffered from BV | Randomized controlled clinical trial (IRCT2016042327534N1) | Recovery of BV in patients was observed. | [74] |
| 8. | Subtilosin and LAE | Bacterial Biofilm Formation Assay, Time Bactericidal Activity of Antimicrobials Against Biofilm-Associated G. vaginalis, Plate Counting Method, Checkerboard Assay | G. vaginalis ATCC 14018 | In vitro | Effective inhibition of G. vaginalis biofilm observed without affecting vaginal Lactobacilli at minimum bactericidal concentrations for biofilm cells (MBCs B) of 50 µg mL−1 (LAE), 69.5 µg mL−1 (subtilosin). | [75] |
| 9. | LAE, e-poly-L-lysine, clindamycin phosphate, and MTZ | Determination of Minimal Inhibitory Concentration (MICs), Checkerboard Assays, and Antibacterial Study | Gardnerella vaginalis ATCC 14018, clinical isolates in healthy women (Lactobacillus gasseri ATCC 33323 and L. plantarum ATCC 39268), and those with recurrent (L. acidophilus ATCC 4356 and L. vaginalis ATCC 49540) | In vitro | Potential antibacterial activity was noticed. | [76] |
5.2. Comparative Study of Natural vs. Synthetic Drugs for BV
6. Nutraceutical Approaches to BV: Focus on Probiotic Strains (L. gasseri, L. helveticus, L. rhamnosus, L. acidophilus, L. plantarum, L. delbrueckii, L. fermentum, and L. salivarius)
| Sl. No. | Lactobacillus Strains (Probiotics) | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References | |
|---|---|---|---|---|---|---|---|
| 1. | L. gasseri CCFM1201 | 1. AntiSLD assay 2. Antibiofilm study 3. Histopathological examination | In vitro (HeLa) and in vivo (murine model) | G. vaginalis | Potential improvement of Vaginal epithelial cell exfoliation observed. | [89] | |
| 2. | L. helveticus HY7801 | 1. Measurement of cytokines in vaginal tissues 2. Histopathological examination 3. Adhesion and biofilm assay 4. Antibacterial study 5. Determination of H2O2 and organic acid production 6. Effect of HY7801 on G. vaginalis virulence gene expression | In vitro (HeLaand in vivo (C57BL/6) | G. vaginalis ATCC14018 | This probiotics strain provided the desired anti-G. vaginalis activity. | [90] | |
| 3. | Serum carotenoids | Antibacterial study | Cross-sectional | Patients (1252) suffered from BV | Serum carotenoids were found to be negatively associated with BV. | [93] | |
| 4. | VagiBIOM Lactobacillus | 1. Vaginal swabs, DNA isolation, and microbiome analysis 2. Statistical and bioinformatics analysis | Randomized, double-blind, placebo-controlled pilot study (NCT05060029) | Patients (92) | Beneficial effects on the vaginal microbiome are demonstrated. | [94] | |
| 5. | S. cerevisiae | 1. SLD activity assay 2. Adhesion and displacement assays 3. Co-aggregation assay | In vitro (A-431 and HeLa cell lines) andin vivo (C57/Bl6 female mice) | G. vaginalis clinical isolates | Significant inhibition of G. vaginalis was investigated. | [95] | |
| 7. | L. rhamnosus, L. acidophilus, L. rhamnosus and L. plantarum | 1. Antibacterial activity of Lactobacilli 2. Adherence of bacteria to HeLa 3. Clinical study protocol and sample collection 4. RNA Extraction and quantitative reverse transcription polymerase chain reaction (qRT PCR) analysis of inflammatory cytokines 5. RNA extraction and qRT PCR analysis of inflammatory cytokines 6. DNA extraction and vaginal microbiota analysis | In vitro (HeLa cell line), a randomized, double-blinded trial (NCT 03116789) | G. vaginalis (BCRC 17040), E. coli (BCRC 11634) | Promising bacterial adhesion inhibition observed. | [96] | |
| 8. | L. delbrueckii DM8909, L. plantarum ATCC14917, and L. plantarum ZX27 | 1. Co-aggregation assay 2. Co-culture assay 3. Evaluation of Lactobacillus sp. for antagonism of G. vaginalis adhesion and biofilm formation and preformation 4. Impact of Lactobacillus sp. CFS on G. vaginalis gene expression 5. Anti-inflammatory Effects of Probiotic Bacteria on HeLa | In vitro (HeLa cell line) | G. vaginalis. ATCC49145 | This study demonstrated that gene expression related to G. vaginalis biofilm has been potentially downregulated. | [97] | |
| 9. | L. paracasei CH88 | 1. Evaluation of the ameliorative effect of L. paracasei CH88 and the <3 kDa LCFSP on BV-induced mice 2. Evaluation of G. vaginalis CFU in vaginal fluid 3. Histopathological examination | In vivo (Female C57BL/6 mice) | G. vaginalis KCTC 5097 | G. vaginalis biofilm properties were inhibited. | [98] | |
| 10. | Probiotic | L. salivarius MG242, L. fermentum MG901, and L. plantarum MG989 | 1. Histopathological analysis 2. Adhesion 3. Antibiotic susceptibility 4. Assessment of enzyme production 5. Analysis of the LA level using the HPLC UV method 6. Hemolysis activity 7. Bile salt hydrolase activity | In vivo (seven-week-old 57BL/6J female mice) | G. vaginalis KCTC5096 | G. vaginalis-related vaginal colonies were significantly reduced. | [99] |
| L. fermentum 5.2, L. plantarum 6.2, and L. plantarum 7.1 | 1. Auto-aggregation and co-aggregation assays 2. Microbial hydrophobicity assay 3. Lactobacillus adhesion to HMVII cells 4. Antimicrobial activity of Lactobacillus culture supernatants | In vitro (HMVII, a vaginal epithelial cell line (BCRJ 0316)) | G. vaginalis ATCC 49154 | Vaginal microbiome improved. | [100] | ||
| L. plantarum strains (ZX1, ZX2, ZX27, and ZX69) | 1. Antibacterial tests in vitro by agar spot and well diffusion tests 2. Antibacterial testing of untreated cell-free supernatant (CFS) and CFN 3. Antibiotic susceptibility testing 4. Detection of plantaricin-related genes 5. Virulence genes in G. vaginalis affected by Lactobacillus | In vitro | G. vaginalis (ATCC49145) | Upregulation of the transcription levels of antimicrobial resistance genes in G. vaginalis was demonstrated. | [101] | ||
| 11 | LA | 1. Lactate dehydrogenase (LDH) assay 2. HIV-leakage assay 3. TNF-α ELISA 4. qRT-PCR 5. Air–liquid interface (ALI) cultures | In vitro (VK2 E6/E7 (Vk2) vaginal epithelial cell line) | G. vaginalis | Vaginal microbiome improved. | [91] | |
| 1. LA determination 2. Killing activity in co-culture conditions 3. Characterization of the killing activity of Lactobacillus CF-culture CSs 4. Killing activity of LA and H2O2 | In vitro | G. vaginalis DSM 4944 | Vaginal pathogens, including G. vaginalis, were negatively affected by LA. | [92] | |||
| 1. Histopathological examination 2. ELISA for cytokine detection 3. qPCR 4. Vaginal microbiota analysis | In vivo (Female BALB/c) | G. vaginalis ATCC 14018 | Significant growth inhibitions of the tested bacteria were observed. | [93] | |||
7. Protein Roles in BV Pathogenesis and Management
8. Fatty Acid Roles in BV Pathogenesis and Management
9. Natural Compounds and Essential Oils in BV
10. Vitamins’ Roles in BV Pathogenesis and Management
11. Miscellaneous Factors in BV Pathogenesis and Management
12. Herbal Nanoformulations for BV
13. Underlying Mechanisms of BV
14. Critical Analysis of Patent Literature on BV
15. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BV | Bacterial vaginosis |
| MDR | Multidrug resistance |
| MTZ | MTZ |
| SLD | Sialidase |
| ECM | Extracellular matrix |
| MPO | Myeloperoxidase |
| CFU | Colony-forming unit |
| AV | Aerobic vaginitis |
| BVAV | Bacterial vaginosis–aerobic vaginitis |
| MIC | Minimum inhibitory concentration |
| FICI | Fractional inhibitory concentration index |
| LAE | Lauramide arginine ethyl ester |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum Fungicidal Concentration |
| GML | Glycerol monolaurate |
| LAB | Lactic acid-producing bacteria |
| HCY | Homocysteine |
| IZ | Inhibition zone |
| EO | Essential oil |
| NPs | Nanoparticles |
References
- Zeng, X.; An, R.; Li, H. Risk factors of recurrent bacterial vaginosis among women of reproductive age: A cross-sectional study. Open Med. 2023, 18, 20230743. [Google Scholar] [CrossRef]
- Wnorowska, U.; Piktel, E.; Daniluk, T.; Paprocka, P.; Savage, P.B.; Durnaś, B.; Bucki, R. Ceragenins Prevent the Development of Murine Vaginal Infection Caused by Gardnerella vaginalis. Pharmaceuticals 2024, 17, 1445. [Google Scholar] [CrossRef] [PubMed]
- Dalabehera, M.; Subudhi, R.N.; Boateng, J.; Choonara, Y.E.; Chaudhari, S.; Chellappan, D.K.; Kanojia, N.; Mnqiwu, K.; Singh, T.G.; Negi, P. Metallic and lipid nanoparticles against multidrug resistant candida: Advances and translational hurdles. Expert Opin. Drug Deliv. 2025; accepted. [Google Scholar] [CrossRef]
- Mitra, A.; Gultekin, M.; Ellis, L.B.; Bizzarri, N.; Bowden, S.; Taumberger, N.; Bracic, T.; Vieira-Baptista, P.; Sehouli, J.; Kyrgiou, M. Genital tract microbiota composition profiles and use of prebiotics and probiotics in gynaecological cancer prevention: Review of the current evidence, the European Society of Gynaecological Oncology prevention committee statement. Lancet Microbe 2024, 5, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Vodstrcil, L.A.; Muzny, C.A.; Plummer, E.L.; Sobel, J.D.; Bradshaw, C.S. Bacterial vaginosis: Drivers of recurrence and challenges and opportunities in partner treatment. BMC Med. 2021, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 2021, 11, 672429. [Google Scholar] [CrossRef]
- Savicheva, A.M. Molecular Testing for the Diagnosis of Bacterial Vaginosis. Int. J. Mol. Sci. 2023, 25, 449. [Google Scholar] [CrossRef]
- Khedkar, R.; Pajai, S. Bacterial Vaginosis: A Comprehensive Narrative on the Etiology, Clinical Features, and Management Approach. Cureus 2022, 14, e31314. [Google Scholar] [CrossRef]
- Laniewski, P.; Herbst-Kralovetz, M.M. Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. npj Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef]
- Reiter, S.; Kellogg Spadt, S. Bacterial vaginosis: A primer for clinicians. Postgrad. Med. 2019, 131, 8–18. [Google Scholar] [CrossRef]
- Kifilie, A.B.; Mengist, A.; Belew, H.; Aschale, Y.; Terefe, A.R. The Prevalence, Antibiotic Resistance Pattern, and Associated Factors of Bacterial Vaginosis Among Women of the Reproductive Age Group from FelegeHiwot Referral Hospital, Ethiopia. Infect. Drug Resist. 2021, 14, 2685–2696. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Asymptomatic Bacterial Vaginosis: To Treat or Not to Treat? Curr. Infect. Dis. Rep. 2020, 22, 32. [Google Scholar] [CrossRef]
- Ziogou, A.; Ziogos, E.; Giannakodimos, I.; Giannakodimos, A.; Sifakis, S.; Ioannou, P.; Tsiodras, S. Bacterial Vaginosis and Post-Operative Pelvic Infections. Healthcare 2023, 11, 1218. [Google Scholar] [CrossRef]
- Donders, G.G.; Zodzika, J.; Rezeberga, D. Treatment of bacterial vaginosis: What we have and what we miss. Expert Opin. Pharmacother. 2014, 15, 645–657. [Google Scholar] [CrossRef]
- Tomás, M.; Palmeira-De-Oliveira, A.; Simões, S.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef]
- Redelinghuys, M.J.; Geldenhuys, J.; Jung, H.; Kock, M.M. Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities. Front. Cell. Infect. Microbiol. 2020, 10, 354. [Google Scholar] [CrossRef]
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yu, Y.; Zhou, Q. Bacterial Vaginosis: Effects on reproduction and its therapeutics. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102174. [Google Scholar] [CrossRef] [PubMed]
- Celeste, C.; Ming, D.; Broce, J.; Ojo, D.P.; Drobina, E.; Louis-Jacques, A.F.; Gilbert, J.E.; Fang, R.; Parker, I.K. Ethnic disparity in diagnosing asymptomatic bacterial vaginosis using machine learning. npj Digit Med. 2023, 6, 211. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Cheu, R.K.; Lemke, M.M.; Gustin, A.T.; France, M.T.; Hampel, B.; Thurman, A.R.; Doncel, G.F.; Ravel, J.; Klatt, N.R.; et al. Quantitative modeling predicts mechanistic links between pre-treatment microbiome composition and MTZ efficacy in bacterial vaginosis. Nat. Commun. 2020, 11, 6147. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Balkus, J.; Mitchell, C.; Sobel, J.D.; Workowski, K.; Marrazzo, J.; Schwebke, J.R. Diagnosis and Management of Bacterial Vaginosis: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74 (Suppl. S2), S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Usyk, M.; Schlecht, N.F.; Pickering, S.; Williams, L.; Sollecito, C.C.; Gradissimo, A.; Porras, C.; Safaeian, M.; Pinto, L.; Herrero, R.; et al. molBV reveals immune landscape of bacterial vaginosis and predicts human papillomavirus infection natural history. Nat. Commun. 2022, 13, 233. [Google Scholar] [CrossRef]
- Eschenbach, D.A. History and review of bacterial vaginosis. Am. J. Obstet. Gynecol. 1993, 169 Pt 2, 441–445. [Google Scholar] [CrossRef]
- Dalabehera, M.; Rathore, C.; Rathee, A.; Lal, U.R. From plants to particles: Herbal solutions and nanotechnology combating resistant vulvovaginal candidiasis. Ther. Deliv. 2024, 15, 371–392. [Google Scholar] [CrossRef]
- Pybus, V.; Onderdonk, A.B. A commensal symbiosis between Prevotellabivia and Peptostreptococcus anaerobius involves amino acids: Potential significance to the pathogenesis of bacterial vaginosis. FEMS Immunol. Med. Microbiol. 1998, 22, 317. [Google Scholar] [CrossRef]
- Wu, S.; Hugerth, L.W.; Schuppe-Koistinen, I.; Du, J. The right bug in the right place: Opportunities for bacterial vaginosis treatment. npj Biofilms Microbiomes 2022, 8, 34. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Smith, S.E.; Muzny, C.A. Bacterial Vaginosis in Postmenopausal Women. Curr. Infect. Dis. Rep. 2023, 25, 7–15. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Xiao, B. The role of SLDs in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell. Infect. Microbiol. 2024, 14, 1367233. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Muzny, C.A.; Josey, W.E. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 2014, 210, 338–343. [Google Scholar] [CrossRef]
- Ignacio, M.A.d.O.; Buesso, T.S.; Morales, J.A.P.; Silva, M.d.C.; da Silva, M.G.; Duarte, M.T.C. Factors associated with bacterial vaginosis in women with homosexual, bisexual and heterosexual practices. Braz. J. Infect. Dis. 2023, 27, 102760. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses. J. Infect. Dis. 2016, 214 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Muzny, C.A.; Blanchard, E.; Taylor, C.M.; Aaron, K.J.; Talluri, R.; E Griswold, M.; Redden, D.T.; Luo, M.; A Welsh, D.; Van Der Pol, W.J.; et al. Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J. Infect. Dis. 2018, 218, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Ehlers, M.M.; Lombaard, H.; Redelinghuys, M.J.; Kock, M.M. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 2017, 43, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Laniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Sobel, J.D.; Vempati, Y.S. Bacterial Vaginosis and Vulvovaginal Candidiasis Pathophysiologic Interrelationship. Microorganisms 2024, 12, 108. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Mechanistic Insights into Immune Suppression and Evasion in Bacterial Vaginosis. Curr. Microbiol. 2022, 79, 84. [Google Scholar] [CrossRef]
- Castro, J.; Machado, D.; Cerca, N. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: The impact of other vaginal pathogens living as neighbors. ISME J. 2019, 13, 1306–1317. [Google Scholar] [CrossRef]
- Muzny, C.A.; Sobel, J.D. Understanding and Preventing Recurring Bacterial Vaginosis: Important Considerations for Clinicians. Int. J. Womens Health 2023, 15, 1317–1325. [Google Scholar] [CrossRef]
- Mondal, A.; Sharma, R.; Trivedi, N. Bacterial vaginosis: A state of microbial dysbiosis. Med. Microecol. 2023, 16, 100082. [Google Scholar] [CrossRef]
- Joseph, R.J.; Ser, H.-L.; Kuai, Y.-H.; Tan, L.T.-H.; Arasoo, V.J.T.; Letchumanan, V.; Wang, L.; Pusparajah, P.; Goh, B.-H.; Ab Mutalib, N.-S.; et al. Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Antibiotics 2021, 10, 719. [Google Scholar] [CrossRef]
- Roselletti, E.; Sabbatini, S.; Perito, S.; Mencacci, A.; Vecchiarelli, A.; Monari, C. Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci. Rep. 2020, 10, 1978. [Google Scholar] [CrossRef]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.L.; Stull-Lane, A.; Girerd, P.H.; Jefferson, K.K. Analysis of adherence, biofilm formation and cytotoxicity suggests a higher virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology 2010, 156 Pt 2, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Singh, J.; Kaur, M. Immunopathology of Recurrent Vulvovaginal Infections: New Aspects and Research Directions. Front. Immunol. 2019, 10, 2034. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Pal, K.; Kumar, N.; Behera, B.; Sagiri, S.S.; Ray, S.S.; Roy, S. Bacterial vaginosis: Etiology and modalities of treatment-A brief note. J. Pharm. Bioallied Sci. 2011, 3, 496–503. [Google Scholar] [CrossRef]
- Abbe, C.; Mitchell, C.M. Bacterial vaginosis: A review of approaches to treatment and prevention. Front. Reprod. Health 2023, 5, 1100029. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Tarin-Pello, A.; Suay-Garcia, B.; Perez-Gracia, M.T. Antibiotic resistant bacteria: Current situation and treatment options to accelerate the development of a new antimicrobial arsenal. Expert Rev. Anti Infect. Ther. 2022, 20, 1095–1108. [Google Scholar] [CrossRef]
- Verwijs, M.C.; Agaba, S.K.; Darby, A.C.; van de Wijgert, J. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am. J. Obstet. Gynecol. 2020, 222, 157.e1–157.e13. [Google Scholar] [CrossRef]
- Menard, J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Womens Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Thulkar, J.; Kriplani, A.; Agarwal, N. A comparative study of oral single dose of metronidazole, tinidazole, secnidazole and ornidazole in bacterial vaginosis. Indian J. Pharmacol. 2012, 44, 243–245. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Brotman, R.M. Making inroads into improving treatment of bacterial vaginosis—Striving for long-term cure. BMC Infect. Dis. 2015, 15, 292. [Google Scholar] [CrossRef]
- Armstrong, N.R.; Wilson, J.D. Tinidazole in the treatment of bacterial vaginosis. Int. J. Womens Health 2010, 1, 59–65. [Google Scholar] [CrossRef]
- Joesoef, M.R.; Hillier, S.L.; Wiknjosastro, G.; Sumapouw, H.; Linnan, M.; Norojono, W.; Idajadi, A.; Utom, B. Intravaginal clindamycin treatment for bacterial vaginosis: Effects on preterm delivery and low birth weight. Am. J. Obstet. Gynecol. 1995, 173, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- SOLOSEC®. (Secnidazole) Oral Granules; Full Prescribing Information; Lupin Pharmaceuticals, Inc.: Somerset, NJ, USA, 2022; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209363Orig1s014s016lbl.pdf (accessed on 16 September 2025).
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA): 2021 focused update on management of Clostridioides difficile infection in adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Stanford Health Care. Antimicrobial Dosing Guide. Stanford Health Care (Bugs & Drugs); Stanford Health Care: Stanford, CA, USA, 2023; Available online: https://med.stanford.edu/content/dam/sm/bugsanddrugs/documents/antimicrobial-dosing-protocols/SHC%20Antimicrobial%20Dosing%20Guide.pdf (accessed on 16 September 2025).
- Clindamycin. Johns Hopkins ABX Guide. Johns Hopkins Medicine. Available online: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540131/all/Clindamycin (accessed on 16 September 2025).
- Chaudhari, S.; Dalabehera, M.; Subudhi, R.N.; Dua, K.; Kaur, M.; Paudel, K.R.; Kumar, J. From Nature to Nanotech: Unlocking Berberine’s Therapeutic Approaches. J. Drug Deliv. Sci. Technol. 2025, 108, 106924. [Google Scholar] [CrossRef]
- Choi, S.-I.; Won, G.; Kim, Y.; Kang, C.-H.; Kim, G.-H. Lactobacilli Strain Mixture Alleviates Bacterial Vaginosis through Antibacterial and Antagonistic Activity in Gardnerella vaginalis-Infected C57BL/6 Mice. Microorganisms 2022, 10, 471. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Decherf, A.C.; Bozza, S.; Camilloni, B.; Perito, S.; Vecchiarelli, A. AntiBiofilm Properties of Saccharomyces cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 Probiotics against G. vaginalis. Microorganisms 2020, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Jeong, J.-J.; Choi, S.-Y.; Kim, H.; Han, M.J.; Kim, D.-H. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 Attenuate Gardnerella vaginalis-Infected Bacterial Vaginosis in Mice. Nutrients 2017, 9, 531, Correction in Nutrients 2017, 9, 715. [Google Scholar] [CrossRef]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Womens Health 2015, 15, 115. [Google Scholar] [CrossRef]
- Lanza, M.; Scuderi, S.A.; Capra, A.P.; Casili, G.; Filippone, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Effect of a combination of pea protein, grape seed extract and lactic acid in an in vivo model of bacterial vaginosis. Sci. Rep. 2023, 13, 2849. [Google Scholar] [CrossRef] [PubMed]
- Donkor, M.N.; Donkor, A.M.; Mosobil, R. Combination therapy: Synergism among three plant extracts against selected pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic effects of carvacrol, alpha-terpinene, gamma-terpinene, rho-cymene and linalool against Gardnerella species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Strydom, M.B.; Khan, S.; Walpola, R.L.; Testa, C.; Ware, R.S.; Tiralongo, E. Intravaginal Combination Therapy of Acetic and Lactic Acid in premenopausal women with recurrent vulvovaginal candidiasis: A randomized, double-blind placebo-controlled feasibility trial. Womens Health 2023, 19, 17455057231194138. [Google Scholar] [CrossRef]
- Noll, K.S.; Prichard, M.N.; Khaykin, A.; Sinko, P.J.; Chikindas, M.L. The natural antimicrobial peptide subtilosin acts synergistically with glycerol monolaurate, lauric arginate, and epsilon-poly-L-lysine against bacterial vaginosis-associated pathogens but not human lactobacilli. Antimicrob. Agents Chemother. 2012, 56, 1756–1761. [Google Scholar] [CrossRef]
- Mazhar, S.; Munim, T.; Yasmin, H.; Ara, J.; Humayun, S.; Najmi, R.; Noman, Y.; Ikram, M.; Khatoon, K.; Maheshwary, N. Efficacy of Cranberry Extract Bacillus Coagulans and Turmeric Extract in Patients With Bacterial Vaginosis. Med. Forum Mon. 2017, 28, 59–63. [Google Scholar]
- Motlagh, A.A.; Dolatian, M.; Mojab, F.; Nasiri, M.; Ezatpour, B.; Sahranavard, Y.; Shakiba, H.; Rahimy, B.; Ghanati, K. The Effect of Prangos ferulacea Vaginal Cream on Accelerating the Recovery of Bacterial Vaginosis: A Randomized Controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery 2018, 6, 100–110. [Google Scholar] [CrossRef]
- Algburi, A.; Volski, A.; Chikindas, M.L. Natural antimicrobials subtilosin and lauramide arginine ethyl ester synergize with conventional antibiotics clindamycin and MTZ against biofilms of Gardnerella vaginalis but not against biofilms of healthy vaginal lactobacilli. Pathog. Dis. 2015, 73, ftv018. [Google Scholar] [CrossRef] [PubMed]
- van de Wijgert, J.H.H.M.; Verwijs, M.C.; Agaba, S.K.; Bronowski, C.; Mwambarangwe, L.; Uwineza, M.; Lievens, E.; Nivoliez, A.; Ravel, J.; Darby, A.C. Intermittent Lactobacilli-containing Vaginal Probiotic or MTZ Use to Prevent Bacterial Vaginosis Recurrence: A Pilot Study Incorporating Microscopy and Sequencing. Sci. Rep. 2020, 10, 3884. [Google Scholar] [CrossRef]
- Khazaeian, S.; Navidian, A.; Navabi-Rigi, S.-D.; Araban, M.; Mojab, F.; Khazaeian, S. Comparing the effect of sucrose gel and MTZ gel in treatment of clinical symptoms of bacterial vaginosis: A randomized controlled trial. Trials 2018, 19, 585. [Google Scholar] [CrossRef]
- Baig, K.; Sultana, A.; Rahman, K. A randomized comparative study of Kakrasingi (Pistacia integerrima J.L. Stewart ex Brandis) and MTZ in bacterial vaginosis. J. Herb. Med. 2022, 36, 100609. [Google Scholar] [CrossRef]
- Afzali, E.; Siahposh, A.; Haghighi-Zadeh, M.H.; Farajzadeh, A.; Abbaspoor, Z. The effect of Quercus (Oak Gal) vaginal cream versus MTZ vaginal gel on bacterial vaginosis: A double-blind randomized controlled trial. Complement. Ther. Med. 2020, 52, 102497. [Google Scholar] [CrossRef]
- Zare, A.; Moshfeghy, Z.; Zarshenas, M.M.; Jahromi, B.N.; Akbarzadeh, M.; Sayadi, M. Quercus brantiiLindl. Vaginal cream versus placebo on Bacterial Vaginosis: A randomized clinical trial. J. Herb. Med. 2019, 16, 100247. [Google Scholar] [CrossRef]
- Durić, K.; Hadžiabdić, S.K.; Durić, M.; Nikšić, H.; Uzunović, A.; Čančar, H.D. Efficacy and safety of three plant extracts based formulations of vagitories in the treatment of vaginitis: A randomized controlled trial. Med. Glas. (Zenica) 2021, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Miraj, S.; Rafieian-Kopaei, M. Comparison of the Effects of Myrtus communis L., Berberis Vulgaris and MTZ Vaginal Gel alone for the Treatment of Bacterial Vaginosis. J. Clin. Diagn. Res. 2016, 10, QC04–QC07. [Google Scholar] [CrossRef]
- Shabanian, S.; Ghahfarrokhi, S.; Lotfizadeh, M. Comparative study of the effects of MTZ gel and Berberis vulgaris gel on the treatment of bacterial vaginosis. J. Appl. Hortic. 2019, 21, 244–248. [Google Scholar] [CrossRef]
- Mohammadzadeh, F.; Dolatian, M.; Jorjani, M.; Majd, H.A.; Borumandnia, N. Comparing the therapeutic effects of garlic tablet and oral MTZ on bacterial vaginosis: A randomized controlled clinical trial. Iran. Red Crescent Med. J. 2014, 16, e19118. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, S.; Farhan, F.; Farshbaf-Khalili, A.; Dehghan, P.; Javadzadeh, Y.; Abbasalizadeh, S.; Khalvati, B. The effect of prebiotic vaginal gel with adjuvant oral MTZ tablets on treatment and recurrence of bacterial vaginosis: A triple-blind randomized controlled study. Arch. Gynecol. Obstet. 2018, 297, 109–116. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Q.; Cui, S.; Zhao, J.; Chen, W.; Zhang, H. Inhibitory effect of Lactobacillus gasseri CCFM1201 on Gardnerella vaginalis in mice with bacterial vaginosis. Arch. Microbiol. 2022, 204, 315. [Google Scholar] [CrossRef]
- Kim, J.Y.; Moon, E.C.; Kim, J.-Y.; Kim, H.J.; Heo, K.; Shim, J.-J.; Lee, J.-L. Lactobacillus helveticus HY7801 ameliorates bacterial vaginosis by inhibiting biofilm formation and epithelial cell adhesion of Gardnerella vaginalis. Food Sci. Biotechnol. 2023, 32, 507–515. [Google Scholar] [CrossRef]
- Atassi, F.; Servin, A.L. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 2010, 304, 29–38. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Jiang, Y.; Lessing, D.J.; Chu, W. Synthetic bacterial consortia transplantation for the treatment of Gardnerella vaginalis-induced bacterial vaginosis in mice. Microbiome 2023, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Z.; Feng, Y.X.; Hong, D.Y.; Guo, X.G. Association between serum carotenoids and bacterial vaginosis infection among American women. BMC Infect. Dis. 2024, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, V.; Khan, Z.H.; Venugopal, G.; Musunuru, B.; Mishra, P.; Srivastava, S.; Ramadass, B.; Subhadra, B. VagiBIOM Lactobacillus suppository improves vaginal health index in peri-menopausal women with bacterial vaginosis: A randomized control trial. Sci. Rep. 2024, 14, 3317. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Mosci, P.; Decherf, A.C.; Pélerin, F.; Perito, S.; Scarpelli, P.; Vecchiarelli, A. Saccharomyces cerevisiae-based probiotic as novel antimicrobial agent for therapy of bacterial vaginosis. Virulence 2018, 9, 954–966. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsu, I.-L.; Tsai, W.-H.; Chu, Y.-C.; Kuan, L.-C.; Huang, M.-S.; Yeh, W.-L.; Chen, Y.-H.; Hsu, S.-J.; Chang, W.-W. Improvement of bacterial vaginosis by oral Lactobacillus supplement: A randomized, double-blinded trial. Appl. Sci. 2021, 11, 902. [Google Scholar] [CrossRef]
- Qian, Z.; Zhu, H.; Zhao, D.; Yang, P.; Gao, F.; Lu, C.; Yin, Y.; Kan, S.; Chen, D. Probiotic Lactobacillus sp. Strains Inhibit Growth, Adhesion, Biofilm Formation, and Gene Expression of Bacterial Vaginosis-Inducing Gardnerella vaginalis. Microorganisms 2021, 9, 728. [Google Scholar] [CrossRef]
- Moon, E.C.; Park, M.S.; Lim, T.; Kim, R.H.; Ji, G.E.; Kim, S.Y.; Hwang, K.T. Antibacterial effect of cell-free supernatant fraction from Lactobacillus paracasei CH88 against Gardnerella vaginalis. Sci. Rep. 2022, 12, 4763. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Kang, C.H. In vivo Confirmation of the Antimicrobial Effect of Probiotic Candidates against Gardnerella vaginalis. Microorganisms 2021, 9, 1690. [Google Scholar] [CrossRef]
- Pessoa, W.F.B.; Melgaço, A.C.C.; de Almeida, M.E.; Ramos, L.P.; Rezende, R.P.; Romano, C.C. In vitro Activity of Lactobacilli with Probiotic Potential Isolated from Cocoa Fermentation against Gardnerella vaginalis. Biomed Res. Int. 2017, 2017, 3264194. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhao, D.; Yin, Y.; Zhu, H.; Chen, D. Antibacterial Activity of Lactobacillus Strains Isolated from Mongolian Yogurt against Gardnerella vaginalis. Biomed Res. Int. 2020, 2020, 3548618. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Giunta, G.; Randazzo, C.L.; Caruso, S.; Caggia, C.; Cianci, A. Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial. Microb. Ecol. Health Dis. 2017, 28, 1357417. [Google Scholar] [CrossRef]
- Alanwar, A.M.; Ezzat, R.S.; Gerges, M.A.; Barhami, K.A.M.A.A.; Ibrahim, S.A.E.S. Role of Oral Lactoferrin in Prevention of Recurrent Bacterial Vaginosis in Third Trimester of Pregnancy. Egypt. J. Hosp. Med. 2023, 91, 4368–4372. [Google Scholar] [CrossRef]
- Pino, A.; Mazza, T.; Matthews, M.-A.H.; Castellana, S.; Caggia, C.; Randazzo, C.L.; Gelbfish, G.A. Antimicrobial activity of bovine lactoferrin against Gardnerella species clinical isolates. Front. Microbiol. 2022, 13, 1000822. [Google Scholar] [CrossRef]
- Johnston, W.; Ware, A.; Kuiters, W.F.; Delaney, C.; Brown, J.L.; Hagen, S.; Corcoran, D.; Cummings, M.; Ramage, G.; Kean, R. In vitro bacterial vaginosis biofilm community manipulation using endolysin therapy. Biofilm 2023, 5, 100101. [Google Scholar] [CrossRef]
- Arroyo-Moreno, S.; Cummings, M.; Corcoran, D.B.; Coffey, A.; McCarthy, R.R. Identification and characterization of novel endolysins targeting Gardnerella vaginalis biofilms to treat bacterial vaginosis. npj Biofilms Microbiomes 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, T.; Chen, Y.; Huang, Z.-M.; Li, X.-J.; Chen, H.-K.; Huang, Y.-Q.; Guo, X.-G. The association between homocysteine and bacterial vaginosis: Results from NHANES 2001-2004. Sci. Rep. 2023, 13, 21388. [Google Scholar] [CrossRef]
- Aroutcheva, A.A.; Simoes, J.A.; Faro, S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect. Dis. Obstet. Gynecol. 2001, 9, 33–39. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Ludescher, R.D.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Lactocin 160, a Bacteriocin Produced by Vaginal Lactobacillus rhamnosus, Targets Cytoplasmic Membranes of the Vaginal Pathogen, Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2009, 1, 67–74. [Google Scholar] [CrossRef]
- Strandberg, K.L.; Peterson, M.L.; Lin, Y.-C.; Pack, M.C.; Chase, D.J.; Schlievert, P.M. Glycerol monolaurate inhibits Candida and Gardnerella vaginalis in vitro and in vivo but not Lactobacillus. Antimicrob. Agents Chemother. 2010, 54, 597–601. [Google Scholar] [CrossRef]
- Schwecht, I.; Nazli, A.; Gill, B.; Kaushic, C. Lactic acid enhances vaginal epithelial barrier integrity and ameliorates inflammatory effects of dysbiotic short chain fatty acids and HIV-1. Sci. Rep. 2023, 13, 20065. [Google Scholar] [CrossRef] [PubMed]
- Hymes, S.R.; Randis, T.M.; Sun, T.Y.; Ratner, A.J. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J. Infect. Dis. 2013, 207, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Sarabia, K.; Rodríguez-Nava, C.; Medina-Flores, Y.; Mata-Ruíz, O.; López-Meza, J.E.; Gómez-Cervantes, M.D.; Parra-Rojas, I.; Illades-Aguiar, B.; Flores-Alfaro, E.; Vences-Velázquez, A. Production and characterization of a monoclonal antibody against the SLD of Gardnerella vaginalis using a synthetic peptide in a MAP8 format. Appl. Microbiol. Biotechnol. 2020, 104, 6173–6183. [Google Scholar] [CrossRef]
- Pezantes-Orellana, C.; Bermúdez, F.G.; De la Cruz, C.M.; Montalvo, J.L.; Orellana-Manzano, A. Essential oils: A systematic review on revolutionizing health, nutrition, and omics for optimal well-being. Front. Med. 2024, 11, 1337785. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, Z.; Zhang, Z.; Bai, H. Antimicrobial Effects of Sophora flavescens Alkaloids on MTZ-Resistant Gardnerella vaginalis in Planktonic and Biofilm Conditions. Curr. Microbiol. 2023, 80, 263. [Google Scholar] [CrossRef]
- Nord, C.; Levenfors, J.J.; Bjerketorp, J.; Sahlberg, C.; Guss, B.; Öberg, B.; Broberg, A. Antibacterial Isoquinoline Alkaloids from the Fungus Penicillium Spathulatum Em19. Molecules 2019, 24, 4616. [Google Scholar] [CrossRef]
- Askari, S.F.; Jahromi, B.N.; Dehghanian, A.; Zarei, A.; Tansaz, M.; Badr, P.; Azadi, A.; Mohagheghzadeh, A. Effect of a novel herbal vaginal suppository containing myrtle and oak gall in the treatment of vaginitis: A randomized clinical trial. DARU 2020, 28, 603–614. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jain, G.K.; Mazumder, A.; Khar, R.K. Antimicrobial activity of a novel polyherbal combination for the treatment of vaginal infection. J. Adv. Pharm. Technol. Res. 2019, 10, 190–194. [Google Scholar] [CrossRef]
- Selis, N.d.N.; de Oliveira, H.B.M.; dos Anjos, Y.B.; Leão, H.F.; Sampaio, B.A.; Correia, T.M.L.; Reis, M.M.; Brito, T.L.S.; Almeida, C.F.; Pena, L.S.C.; et al. Gardnerella vaginalis and Neisseria gonorrhoeae Are Effectively Inhibited by Lactobacilli with Probiotic Properties Isolated from Brazilian Cupuacu (Theobroma grandiflorum) Fruit. Biomed Res. Int. 2021, 2021, 6626249. [Google Scholar] [CrossRef]
- Machado, D.; Gaspar, C.; Palmeira-De-Oliveira, A.; Cavaleiro, C.; Salgueiro, L.; Martinez-De-Oliveira, J.; Cerca, N. Thymbra capitata essential oil as potential therapeutic agent against Gardnerella vaginalis biofilm-related infections. Future Microbiol. 2017, 12, 407–416. [Google Scholar] [CrossRef]
- Rosca, A.S.; Castro, J.; Sousa, L.G.V.; França, A.; Cavaleiro, C.; Salgueiro, L.; Cerca, N. Six Bacterial Vaginosis-Associated Species Can Form an in vitro and Ex Vivo Polymicrobial Biofilm That Is Susceptible to Thymbra capitata Essential Oil. Front. Cell. Infect. Microbiol. 2022, 12, 824860. [Google Scholar] [CrossRef]
- Bogavac, M.A.; Karaman, M.A.; Suđi, J.J.; Radovanović, B.B.; Janjušević, L.N.; Ćetković, N.B.; Tešanović, K.D. Antimicrobial Potential of Rosmarinus officinalis Commercial Essential Oil in the Treatment of Vaginal Infections in Pregnant Women. Nat. Prod. Commun. 2017, 12, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.T.; Lee, I.A.; Hyun, Y.J.; Kim, D.H. Artemisia princeps Pamp. Essential oil and its constituents eucalyptol and alpha-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-kappaB activation. Planta Med. 2011, 77, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Duttke, C.; Hild, J.; Müller, H.J. In vitro activity of essential oils on microorganisms isolated from vaginal infections. Int. J. Aromather. 2006, 16, 169–174. [Google Scholar] [CrossRef]
- Mojtahedi, S.F.; Mohammadzadeh, A.; Mohammadzadeh, F.; Shahri, J.J.; Bahri, N. Association between bacterial vaginosis and 25-Hydroxy vitamin D: A case-control study. BMC Infect. Dis. 2023, 23, 208. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Li, L.; Zhang, L.; Lin, Z.; Qin, H. Vitamin D deficiency increases the risk of bacterial vaginosis during pregnancy: Evidence from a meta-analysis based on observational studies. Front. Nutr. 2022, 9, 1016592. [Google Scholar] [CrossRef]
- Cui, T.-T.; Luo, J.; Deng, R.-L.; Yang, Y.-T.; Yin, Y.-W.; Chen, X.-F.; Chen, H.-K.; Liao, W.-Z.; Huang, Z.-M.; Deng, X.-Y.; et al. Negative associations between folate and bacterial vaginosis in the NHANES 2001 to 2004. BMC Infect. Dis. 2023, 23, 483. [Google Scholar] [CrossRef]
- Mohammad-Alizadeh, S.; Dokhanchi, T.; Hakimi, S.; Javadzadeh, Y.; Takallu, L.; Gharabaghi, P.M. The addition of vitamin C vaginal tablets to oral MTZ and its effect on the treatment and recurrence of bacterial vaginosis: A randomized triple-blind clinical trial. Int. J. Women’s Health Reprod. Sci. 2017, 5, 193–199. [Google Scholar] [CrossRef][Green Version]
- Krasnopolsky, V.N.; Krasnopolsky, V.N.; Prilepskaya, V.N.; Polatti, F.; Zarochentseva, N.V.; Bayramova, G.R.; Caserini, M.; Palmieri, R. Efficacy of vitamin C vaginal tablets as prophylaxis for recurrent bacterial vaginosis: A randomised, double-blind, placebo-controlled clinical trial. J. Clin. Med. Res. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Nanomedicines for the topical treatment of vulvovaginal infections: Addressing the challenges of antimicrobial resistance. Adv. Drug Deliv. Rev. 2021, 178, 113855. [Google Scholar] [CrossRef] [PubMed]
- Chanaj-Kaczmarek, J.; Rosiak, N.; Szymanowska, D.; Rajewski, M.; Wender-Ozegowska, E.; Cielecka-Piontek, J. The Chitosan-Based System with Scutellariae baicalensis radix Extract for the Local Treatment of Vaginal Infections. Pharmaceutics 2022, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Chanaj-Kaczmarek, J.; Romaniuk-Drapała, A.; Rubiś, B.; Szymanowska, D.; Kobus-Cisowska, J.; Szymańska, E.; Winnicka, K.; Cielecka-Piontek, J. Mucoadhesive Chitosan Delivery System with Chelidonii herba Lyophilized Extract as a Promising Strategy for Vaginitis Treatment. J. Clin. Med. 2020, 9, 1208. [Google Scholar] [CrossRef]
- Arrue, A.; Olivas, O.; Erkoreka, L.; Alvarez, F.J.; Arnaiz, A.; Varela, N.; Bilbao, A.; Rodríguez, J.-J.; Moreno-Calle, M.T.; Gordo, E.; et al. Development of Nanotechnology-Based Drug Delivery Systems for Controlling Clinical Multidrug-Resistant Staphylococcus aureus and Escherichia coli Associated with Aerobic Vaginitis. Pharmaceutics 2023, 15, 2134. [Google Scholar] [CrossRef]
- Bouaouina, S.; Aouf, A.; Touati, A.; Ali, H.; Elkhadragy, M.; Yehia, H.; Farouk, A. Effect of Nanoencapsulation on the Antimicrobial and Antibiofilm Activities of Algerian Origanum glandulosum Desf. against Multidrug-Resistant Clinical Isolates. Nanomaterials 2022, 12, 2630. [Google Scholar] [CrossRef]
- Tomás, M.; Sousa, L.G.V.; Oliveira, A.S.; Gomes, C.P.; Palmeira-De-Oliveira, A.; Cavaleiro, C.; Salgueiro, L.; Cerca, N.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal Sheets with Thymbra capitata Essential Oil for the Treatment of Bacterial Vaginosis: Design, Characterization and In vitro Evaluation of Efficacy and Safety. Gels 2023, 9, 293. [Google Scholar] [CrossRef]
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022, 12, 963868. [Google Scholar] [CrossRef] [PubMed]
- Fialová, S.B.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, L.M.; Vassallo, A.; Mengoni, A.; Miceli, E.; Bogani, P.; Firenzuoli, F.; Fani, R.; Maggini, V. Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health. Pathogens 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Wegener, P.; Hara, Y. Compositions and Treatments of Bacterial Vaginosis. U.S. Patent WO/2006/047590, 4 May 2006. [Google Scholar]
- Xiaoyan, Z.; Meihua, X.; Weiqiang, X.; Jun, X.; Yinjie, L.; Weile, G.; Yongguo, X.; Yanhong, L.; Xun, C.; Xiuzhi, W.; et al. Traditional Chinese Medicine for Treating Bacterial Vaginosis and Preparation Method of Traditional Chinese Medicine. China Patent 201410265286.6, 3 September 2014. [Google Scholar]
- Xinsuo, W. Chinese Traditional Medicine Preparations for Treating Bacterial Vaginitis. China Patent 200710120906.7, 29 October 2007. [Google Scholar]
- Nash, J.R. Bioactive Phytochemicals in Zizyphus and Guarana. Indian Patent WO/2020/115475, 11 June 2020. [Google Scholar]
- Solecki, B.; Urban Juve Provisions Inc. Cannabis Root Extract, Method of Manufacture, Method of Use. U.S. Patent PCT/CA2019/050451, 17 October 2019. [Google Scholar]
- Chonglan, X. Chinese Herbal Preparation for Treating Bacterial Vaginosis and Preparing Method. China Patent 102016000121541, 29 June 2016. [Google Scholar]
- Adisovna, A.; Fuatovna, B.; Akdasovich, N. Method of Treating Bacterial Vaginosis. Russian Patent 2009110700/14, 10 July 2010. [Google Scholar]
- Young, S. Herbal Medicine Composition for Treating Vaginitis and Preventing Uterine Cervical Dysplasia. Korean Patent 1020200095649, 5 February 2021. [Google Scholar]
- Evgenevich, S. Agent for Vaginal Douche in Phase of Vaginal Microflora Recovery Following Treatment of Bacterial Vaginosis and Vaginal Thrush (Vaginal Yeast). Russian Patent 2012126560/15, 10 July 2013. [Google Scholar]
- Peian, Z.; Jianmin, W.; Kamp Pharmaceuticals Co., Ltd. Antiinflammatory Leucorrhoea Arresting Washing Liquor. China Patent 201310618673.9, 3 June 2015. [Google Scholar]
- Evgenevich, S. Agent for Vaginal Douche in First Stage of Treatment Of Bacterial Vaginosis. Russian Patent 2012126562/15, 10 July 2013. [Google Scholar]
- Xiaoming, L. Vagina-Cleaning Sanitary Lotion. China Patent 102014000533282, 20 April 2016. [Google Scholar]
- Alberto, C.; Maurizio, S.; Velleja Res Srl. Topical Compositions for the Prevention and Treatment of Inflammatory and/or Infective Conditions of the Genital Area. European Patent 08009684, 28 May 2008. [Google Scholar]
- Papa, F.; Colognato, R.; Organicare LLC. Natural Composition for Use in Gynecology. U.S. Patent 17827504, 27 May 2022. [Google Scholar]
- Feifei, Z.; Hongxin, L.; Guangzhou Jiexika Biological Technology Co., Ltd. Medicinal Composition for Repairing Female Vaginal Microecological Balance. China Patent 201610664574.8, 16 November 2016. [Google Scholar]
- Gang, C.; Peixin, D.; Qiuqiu, C.; Lunaler Health Technology Co., Ltd. Private Part Antibacterial Composition, Private Part Nursing Product and Preparation Method and Application of Private Part Antibacterial Composition and Private Part Nursing Product. China Patent 202311350774.2, 11 November 2023. [Google Scholar]
- Sunil, G.; Grace, N. Neem Seed Oil Based Vaginal Capsule for Treatment of Abnormal Vaginal Discharge. Indian Patent 202141047207, 26 November 2021. [Google Scholar]
- Viktorovich, C.; Alexandrovna, K.; Cherkasova, J.; Alexandrovna, K.; Leonidovna, J. Federal State Budgetary Institutions of Aukiorenburg Federal Research Centre of Russian Academy of Sciences. Method of Treatment of Bacterial Vaginosis. Russian Patent 2022121049, 30 September 2022. [Google Scholar]
- Komorowski, J.; Sylla, S.; Bernsley, D.; Bonafide Health, LLC. Probiotic and Folic Acid Compositions and Methods of Using. U.S. Patent 17958310, 4 April 2024. [Google Scholar]
- Kort, R.; Veer, C. Nederlandse Organisatievoor Toegepast-Natuurwetenschappelijk Onderzoek TNO. U.S. Patent 16628762, 7 May 2020. [Google Scholar]
- Kort, R.; Veer, V. Nederlandse Organisatievoor Toegepast-Natuurwetenschappelijk Onderzoek TNO. New Probiotic Composition for Prevention of BACTERIAL Vaginosis. Netherlands Patent WO/2019/013637, 17 January 2019. [Google Scholar]
- Kompella, P.V.; Soman, L.J. Probiotic Layered Condom. Indian Patent WO/2014/020613, 6 February 2014. [Google Scholar]
- Mogna, G.; Strozzi, G.P.; Mogna, L. Effervescent Composition in Solid Form for Use in Vaginal Applications for Treating Vaginal Infections. Russian Patent 2013137656/15, 20 June 2016. [Google Scholar]
- Milyausha, A.; Rafaelevich, B.R.; Akdasovich, N.M. Remedy for Treating Bacterial Vaginosis and Method of Bacterial Vaginosis Treatment. Russian Patent 2008112493/15, 10 May 2009. [Google Scholar]
- Qingjun, W.; Wan, G.; Nanjing Shengnuo Bio-Technology Industry. Probiotics Composition for Improving Health of Uro-Genital System of Women and Preparation Method of Composition. China Patent 201910680707.4, 10 September 2019. [Google Scholar]
- Weibin, Y.; Jiajia, G.; Songzhi, C.; Mingxin, G.; Weiping, G.; Henan ZhongwenYuxiu Health Management Co., Ltd. Female Vagina Tightening Capsule and Preparation Process Thereof. China Patent 202110362861.4, 28 May 2021. [Google Scholar]
- Tingtao, C.; Puyuan, T.; Nanchang University. Construction of Lactobacillus crispatus bt1386 and Application in Treatment of Bacterial Vaginosis. China Patent 201810873678.9, 18 December 2018. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv, 2020; preprint. [Google Scholar] [CrossRef]


| No. | Formulation | Dose | Administration | Company Name (Example) | Typical Indication/Organisms | Adverse Effects | Pregnancy Note | References |
|---|---|---|---|---|---|---|---|---|
| 1 | MTZ (metronidazole) | 500 mg | Orally (twice daily for a week) | Pfizer (Pfizer Inc., New York, NY, USA) | BV; anaerobes; T. vaginalis (alternative regimens) | Difficulty in breathing, stomach pain, headaches, back pain, blurred vision. | Commonly used in pregnancy when indicated (discuss in text and follow local guidance). | [52] |
| 2 | Clindamycin (cream 2.0%) | 2.0% cream | Intravaginally (once daily for a week) | Alkem laboratories (Alkem Laboratories Ltd., Mumbai, India) | BV (topical/local therapy) | Redness, burning, itching at the site of application. Stomach pain or cramping. | Topical use generally considered when needed; discussed in text (see pregnancy studies). | [53] |
| 3 | MTZ (0.75% gel) | 0.75% gel | Intravaginally (once daily for a week) | Galderma laboratories (Galderma Laboratories, LP, Dallas, TX, USA) | BV; local therapy for vaginal anaerobes | Vaginal itching, redness, vaginal discharge may occur. | Topical therapy often used in pregnancy as alternative—discussed evidence in text. | [54] |
| 4 | Clindamycin (oral) | 300 mg | Orally (twice daily for a week) | Pfizer | BV (oral alternative), skin/soft-tissue infections (depending on context) | Abdominal pain, heartburn, diarrhea, nausea, and vomiting. | Oral clindamycin used in pregnancy in some situations; weigh benefits/risks. | [55] |
| 5 | Tinidazole | 1 g | Orally (once daily for 2 days) | Farlex Pharmaceuticals Pvt Ltd. (Farlex Pharmaceuticals Pvt. Ltd., Panchkula, India) | T. vaginalis, protozoa (also used in BV in some regimens) | Headache, constipation, dizziness, stomach pain, cramps. | Generally avoid in pregnancy unless necessary; check label. | [56] |
| 6 | Clindamycin (ovule) | 100 mg | Intravaginally (once daily for 3 days) | Pfizer | BV (intravaginal ovule) | Redness, burning, itching at the site of application. | Topical intravaginal clindamycin studied in pregnancy (see Joesoef et al.). | [57] |
| 7 | Tinidazole | 2 g | Orally (once daily for 5 days) | Pfizer | T. vaginalis; protozoal infections | Upset stomach, nausea, vomiting, diarrhea. | As above (avoid in pregnancy unless indicated). | [54] |
| 8 | Metronidazole (single-dose option) | 2 g | Oral—single dose (alternative to 7-day regimen for T. vaginalis) | Generic (Flagyl®) Pfizer Inc. New York, NY, USA | T. vaginalis (single-dose therapy possible); BV alternative regimens | Metallic taste, nausea, disulfiram-like reaction with alcohol, GI upset. | Metronidazole has pregnancy data; use per guideline and local practice. | [54,58] |
| 9 | Secnidazole (oral granules—single dose) | 2 g | Single oral packet (sprinkle on food) | SOLOSEC® Lupin Pharmaceuticals, Inc., Baltimore, MD, USA | Single-dose option for BV; also indicated for trichomoniasis in some labels | Nausea; vulvovaginal candidiasis; alcohol interaction advice on label | Check product label and regional approvals for pregnancy guidance. | [54,59] |
| 10 | Metronidazole (IV) | 500 mg | IV q8 h (or q6–8 h per severity) | Generic hospital IV formulation | Severe/complicated anaerobic infections, intra-abdominal sepsis, severe gynecologic infections | Same systemic effects as oral; IV-local infusion reactions; neurotoxicity with prolonged use | Use in pregnancy only if clinically indicated; follow obstetric/infectious-disease guidance. | [58,60] |
| 11 | Clindamycin (IV) | 600–900 mg | IV q6–8 h depending on diagnosis | Cleocin® Pfizer Inc.—New York, NY, USA | Severe pelvic infections, necrotizing soft-tissue infection, alternative for anaerobes | Diarrhea, risk of C. difficile colitis, hypersensitivity | Clindamycin used in pregnancy in certain contexts (e.g., gynecologic infections)—see refs. | [60,61] |
| 12 | Vancomycin (oral) | 125 mg | Oral q6 h × 10 days | Vancocin® Capsules (U.S.) ANI Pharmaceuticals, Inc. Baudette, MN, USA | First-line for initial Clostridioides difficile infection (PO) | Local GI effects; poor systemic absorption (benefit for GI infection) | Use in pregnancy per guideline (benefit > risk for severe CDI). | [60] |
| 13 | Fidaxomicin (oral) | 200 mg | Oral BID × 10 days | Dificid® (fidaxomicin) E. Lincoln Avenue, Rahway, NJ, USA | Alternative/often preferred agent for initial C. difficile (lower recurrence) | Nausea, GI upset; higher cost/limited availability | Considered acceptable in pregnancy when indicated (consult local guidance). | [60,62] |
| 14 | Clindamycin (vaginal ovule/supp.) | 100 mg | Intravaginally at bedtime × 3 days | Cleocin® Vaginal Ovules. Pharmacia & Upjohn Company LLC, New York, NY, USA | BV; local therapy | Local burning/irritation; oleaginous base can weaken latex condoms | Label/clinical notes recommend condom caution; pregnancy evidence discussed separately. | [57] |
| Sl. No. | Comparison | Type of Study | Trial Registration No. | No. of Patients | Dose | Observation | References |
|---|---|---|---|---|---|---|---|
| 1. | Sucrose gel with MTZ gel | Triple-blind, parallel, randomized clinical trial | IRCT2016112631105N1 | 70 | MTZ gel 0.75% and Sucrose gel 9% | Sucrose vaginal gel comparatively improved vaginal microflora. | [77] |
| 2. | Kakrasingi with MTZ | Randomized (1:1), standard controlled, single-center | 100609 | 62 | Kakrasingi (1 g) and MTZ (400 mg) | No side effects were demonstrated in herbal-based medicine. | [78] |
| 3. | Quercus (Oak Gal) cream with MTZ gel | Double-blind, randomized controlled trial | IRCT20180122038477N1 | 84 | Oak Gall vaginal cream and MTZ vaginal gel (5 g) | Promising antibacterial activity was demonstrated. | [79] |
| 4. | Quercus brantiilindl vaginal cream with placebo and MTZ tablet | Randomized clinical trial | IRCT2016071428929N1 | 176 | Q. brantii and MTZ (500 mg) | Clinical symptoms of BV were suppressed after herbal cream treatment. | [80] |
| 5. | Calendulae extractumoleosum, Bursae pastoris extractumoleosum, Matricariae extractumoleosum, Hyperici extractumoleosum, and Millefolii extractumoleosum withtea tree oil (TTO) | Randomized controlled clinical, in vitro | NCT04558697 | 210 | TTO (200 mg) | The herbal treatment showed potential anti-G. vaginalis activity. | [81] |
| 6. | Myrtus communis L. and Berberis vulgaris with MTZ vaginal gel | Randomized clinical trial, in vitro and in vivo | IRCT201411102085N13 | 120 | M. communis (77.1 ug/mL), B. vulgaris (157 mg/g) | Desired antibacterial and anti-inflammatory properties were observed. | [82] |
| 7. | Berberis vulgaris gel with MTZ gel | Double-blind clinical trial | IRCT201411102085N13 | 80 | B. vulgaris gel 5% and MTZ gel 0.75% | Herbal-based vaginal gel exhibited higher effective activity across BV. | [83] |
| 8. | Garlic tablet with oral MTZ | Single-blind randomized controlled clinical trial | IRCT201207153226N4 | 120 | Garlic tablet (500 mg) and MTZ tablet (250 mg) | Fewer side effects were observed in the garlic group of patients. | [84] |
| 9. | Probiotics with MTZ | Randomized clinical trial Triple-blind randomized clinical trial | NCT02459665 215121721917N5 | 68 100 | MTZ (500 mg), prebiotic vaginal gel (5 mg), and MTZ (250 mg) | Beneficial antimicrobial efficacy was observed in probiotics. Probiotic vaginal gel improved clinical symptoms of BV and refined the vaginal microbiome. | [76] |
| Sl. No. | Proteins Used | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References |
|---|---|---|---|---|---|---|
| 1. | Lactoferrin | 1. Microbiological analysis 2. RNA isolation from vaginal swabs 3. Ion Torrent 16S rRNA gene-based analysis 4. Taxonomic identification | Open prospective randomized trial (SHI-EVE-2014.01) | Sixty women with BV | Improvement of the vaginal ecosystem was observed after lactoferrin administration. | [102] |
| 1. Antibacterial study | Randomized controlled clinical trial | 66 cases with a history of recurrent BV | Beneficial effects were investigated. | [103] | ||
| Bovine Lactoferrin (MTbLF) | 1. Antimicrobial susceptibility testing 2. Antimicrobial activity of bovine lactoferrin against presumptive G. vaginalis clinical isolates 3. Antibiotic interference/synergy test | In vitro antimicrobial study, Clinical trial (ACTRN12619000295145) | G. vaginalis clinical isolates | Promising anti-G. vaginalis efficacy was demonstrated. | [104] | |
| 2. | Endolysin | 1. Monospecies biofilm formation and treatment 2. Polymicrobial biofilm treatment 3. DNA extraction and PMAxx treatment 4. SEM | In vitro | G.vaginalis (ATCC 14018) | Suppression of bacterial load was observed. | [105] |
| 1. Endolysin stability assays 2. MIC assays 3. Resistance assays using serial passages 4. Biofilm prevention and disruption assays 5. Reproducibility and statistical analysis | In vitro | G. vaginalis UG860107 and A. vaginae UG71161 | Maximum biofilm inhibitions were observed by CCB2M94_8 and CCB7.1 (best endolysin candidate). | [106] | ||
| 3. | Homocysteine | 1. Antibacterial study | Association study | G. vaginalis | G. vaginalis growth was inhibited significantly. | [107] |
| 4. | Bacteriocin | 1. Demonstration of antimicrobial activity | In vitro | G. vaginalis | The desired antibacterial activity of this protein was investigated. | [108] |
| Lactocin 160 | 1. The Membrane Disruption (Ethidium Bromide) Assay 2. The ATP Assay 3. ΔpH Dissipation Assay 4. ΔΨ Dissipation Assay | In vitro | G. vaginalis ATCC 14018 and L. rhamnosus 160 | The cytoplasmic membrane of G. vaginalis was altered. | [109] |
| Sl. No. | Fatty Acids Used | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References |
|---|---|---|---|---|---|---|
| 1. | GML | 1. Antimicrobial assay | In vitro experiments and a single-center, double-blind, randomized study | Clinical isolate of G. vaginalis | Significant reductions were seen in G. vaginalis counts | [110] |
| Sl. No. | ConstituentsUsed | Types of Performed Study | Experimental Types | Pathogen | Key Findings | References |
| 1. | DNase (Enzyme) | 1. Biofilm Assays | In vitro and in vivo (eight-week-old female C57BL/6J mice) | This enzyme potentially inhibits bacterial biofilm. | [112] | |
| 2. | Monoclonal Antibody | 1. G. vaginalis culture and growth kinetics 2. SLD production during the growth of G. vaginalis | In vitro (HeLa cells) | G. vaginalis ATCC 14018 | The virulence potential of the tested pathogens was significantly suppressed. | [113] |
| Sl. No. | Used Constituents | Active Molecules | Souce | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Sophora favescens (alkaloid) | Matrine and oxymatrine | Plant | 1. HPLC 2.Antimicrobial Susceptibility Testing 3. Bacterial Biofilm Formation Assay | In vitro | G. vaginalis. B. fragilis ATCC 25285 | Significant bacterial growth inhibitions were observed at MIC 0.3125–1.25 mg/mL. | [115] |
| 2. | Penicillium spathulatum Em19 | 6,7-dihydroxy-5,10-dihydropyrrolo[1,2-b]isoquinoline-3-carboxylic acid and 5,10-dihydropyrrolo[1,2-b]isoquinoline-6,7-diol | Isoquinolinealkaloids from the fungus Penicillium Spathulatum Em19 | 1. MIC Determination 2. Determination of Toxicity Huh 7 Cells (Hepatocellular Carcinoma Cells) | In vitro bioassay | E. coli LMG15862 | The MIC range between 0.05 and 50 µg/mL was found. | [116] |
| 3. | Myrtus communis L. (myrtle) and Quercus infectoria G. Olivier (oak gall) | Citric acid, gallotannicacid, gallic acid, andellagic acid | Plant | 1. Naranjo Scale for Adverse Drug Reaction (ADR) 2. Antibacterial Study | Parallel randomized clinical trial (IRCT2016030526917N1) | 120 women with vaginitis | Desired antibacterial effects were demonstrated. | [117] |
| 4. | Azadirachta indica (AI), Cichorium intybus (CI), and Trigonella foenum-graecum (TFG) | beta.dMannofuranoside and O-geranyl | Plant | 1. Antimicrobial Screening Using Agar Well Diffusion Method | In vitro | E. coli (Strain No. 8739-8/03/15) | Broad-spectrum antimicrobial activities were investigated. | [118] |
| 5. | Theobroma grandiflorum (Cupuaçu) | Linoleic acid and oleic acid | Plant (fruit) | 1. Heat Tolerance Assay 2. pH Tolerance Assay 3. Antibiotic Susceptibility Assay 4. Hemolytic Activity Assay 5. Biofilm Formation Assay 6. Hydrophobicity Assay 7. Auto-aggregation Assay 8. Co-aggregation Assay 9. Inhibition Assay by Coculture | In vitro | G. vaginalis ATCC 49154 | Vaginal microbiota improved. | [119] |
| Sl. No. | EO Used | Active Molecules | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References |
|---|---|---|---|---|---|---|---|
| 1. | Thymbra capitata EO | Carvacrol and thymol | 1. MIC and minimal lethal concentration determination 2. Flow cytometry analysis 3. Biofilm formation and quantification 4. Confocal laser scanning microscopy analysis of G. vaginalis biofilms | In vitro | G. vaginalis | Bacterial biofilm was potentially inhibited. | [70] |
| 1. MIC and MLC determination 2. In vitro biofilm formation and quantification 3. Formation and characterization of polymicrobial biofilms on the reconstructed human vaginal epithelium 4. Characterization of in vitro and ex vivo polymicrobial biofilms | In vitro and ex vivo (vaginal epithelial tissue) | G. vaginalis ATCC 14018 | High antibacterial effect against polymicrobial biofilms of the tested bacteria was observed. | [71] | |||
| 2. | Rosmarinus officinalis EO | Tricyclene, α-pinene, camphene | 1. Antimicrobial assay 2. Brine shrimp toxicity assay | In vitro | E. coli I, E. coli II | Significant suppression of bacterial load was demonstrated. | [122] |
| 3. | Artemisia princeps Pamp. EO | Eucalyptol and α-terpineol | 1. Antimicrobial activity assay 2. Histopathologic examinations 3. Assay of MPO activity 4. ELISA and immunoblot analysis | In vitro and in vivo (Male and female ICR mice) | G. vaginalis KCTC5096 | No cytotoxicity effect was noticed in the murine model. | [123] |
| 4. | Lemongrass, Palmarosa, Tea tree, Neroli, Manuka, Lavender, Rosemary, Thyme linalool, Geranium and Clary sage EO | Geranial, terpinen-4-ol, Linalool, Leptospermone, 1,8-cineole, Citronellol, myrcene | 1. MIC, MBC/MFC 2. Antibacterial assay | In vitro | G. vaginalis | Restoration of the natural vaginal flora after exposure to EO. | [124] |
| Sl. No. | Vitamins Used | Types of Performed Study | Experimental Types | Antibacterial Against | Key Findings | References |
|---|---|---|---|---|---|---|
| 1. | 25-Hydroxy vitamin D | 1. Diagnosis of BV 2. Evaluation of serum 25-hydroxy vitamin D 3. Antibacterial study | Case–control study (IR.GMU.REC.1398.150) | 250 confirmed BV cases | The inverse association between vitamin and BV was investigated. | [125] |
| 2. | Vitamin D | 1. Antibacterial study | Observation study | Patients with BV | Vitamin D supplementation exhibited effective actions. | [126] |
| 3. | Folate | 1. Determination of serum folate concentrations 2. Determination of RBC folate concentrations 3. Antibacterial assay | Cross-sectional study | Patients diagnosed with BV | Serum folate was inversely associated with the risk of BV. | [127] |
| 4. | Vitamin C | 1. Antibacterial study | Triple-blind randomized clinical trial (IRCT2015042521917N3) | 160 women with BV | Vaginal environment recovery was demonstrated. | [128] |
| 1. Antimicrobial study | Randomized, double blind, placebo-controlled, parallel-group clinical trial | 144 Patients with BV | A lower recurrence risk of BV was found. | [129] |
| Sl. No. | Natural-Based Constituents | Nanoformulation | No. of Performed Studies | Experimental Types | Outcomes | References |
|---|---|---|---|---|---|---|
| 1. | SBE | Chitosan-based system | 1. ATR FTIR and DFT 2. XRPD, Thermogravimetric Analysis (TG), DSC 3. Anti-Hyaluronidase Activity 4. Ferrous Ion Chelating Activity | In Vitro | Promising antibacterial activity was observed. | [132] |
| 2. | Chelidonii herba | Mucoadhesive chitosan system | 1. MTT Test 2. Permeability Studies 3. Microbiological Activity | In Vitro Release Studies and Ex Vivo Mucoadhesive Properties (Porcine Vaginal Mucosa) | Enhanced bioavailability observed in the chitosan system. | [133] |
| 3. | Probiotics | Nanoemulsion | 1. Antimicrobial Susceptibility Assay 2. MIC and MBC 3. Antibiofilm Assay 4. Cell Surface Hydrophobicity 5. Bacterial Cell Membrane Disintegration Test | In Vitro | Potential disintegration of the bacterial cell was observed. | [134] |
| 4. | Origanum glandulosum Desf | Nanoemulsion system | 1. Antibiotic Susceptibility Test 2. Broth Microdilution Assay and Agar Diffusion Method 3. Antimicrobial Assay | In Vitro | This formulation could be an alternative in BV treatment options. | [135] |
| 5. | Thymbra capitata EO | Vaginal sheets | 1. Bioadhesion 2. Cellular Toxicity 3. Cytotoxicity Test (MTT Assay) 4. Vaginal Irritation—Hen’s Egg TestChorioallantoic Membrane Assay (HET CAM) | HeLa and HEC 1A Cell Lines Were Used | Efficient antibacterial activity observed at 0.03 g/mL. | [136] |
| Sl. No | Inventors | Application No. | Title of Inventive Appellation | Plant Used | Country | Publication Date | Outcomes | References |
|---|---|---|---|---|---|---|---|---|
| Plants | ||||||||
| 1 | Wegener et al. | WO/2006/047590 | Compositions and treatments ofBV. | Epigallocatechin gallate, epicatechin gallate, epigallocatechin, epicatechin | US Patent | 4 May 2006 | With the help of plant polyphenols, treatment was provided for BV. | [140] |
| 2 | Xiaoyan et al. | 201410265286.6 | Traditional Chinese medicine for treatingBV and the preparation methods of traditional Chinese medicine. | Osbeckia herb, horseweed herb, hairy euphorbia, wandering jew, Amaranthus viridis, wideleafwideleafosbeckia root, herb of sword brake, herba Fibraureae recisae, Dictamni cortex, Lantana camara, Cryptotaenia japonica hassk, Senecio dianthus franch | China Patent | 28 April 2005 | A suppository of traditional Chinese medicine was administered once a day continuously for 15 days. No recurrence was seen while following up for 1 year. | [141] |
| 3 | Wang Xinsuo | 200710120906.7 | Chinese traditional medicine preparations for treatingBV. | Anemarrhena rhizome, Amur corktree bark, radix Rehmanniae preparata, common yam rhizome | China Patent | 30 January 2008 | The present intervention was only able to treat BV, but it was also able to prevent bacterial infection, had no toxicity or side effects, and had better patient compliance. | [142] |
| 4 | Nash et al. | WO/2020/115475 | Bioactive phytochemicals in zizyphus and guarana | Cucurbitaceae extract | Indian Patent | 11 June 2020 | It can adhere to biofilm and has less salidase activity. | [143] |
| 5 | Soleck et al. | PCT/CA2019/050451 | Cannabis root extract, method of manufacture, and method of use. | Cannabis root | American Patent | 17 October 2019 | Therapeutic efficacy against BV with fewer side effects. | [144] |
| 6 | Xu Chonglan | 102016000121541 | Chinese herbal preparation for treating BV and the preparation method. | Roots of Chinese pulsatilla, broom cypress fruits, seeds of feather cockscomb, small fruit fig aerial roots, all grass of Pilea japonica (maxim.) | China Patent | 29 June 2016 | The preparation method of the traditional Chinese method is appropriate in compatibility, can reduce inflammation, and has no adverse or toxic effects. | [145] |
| 7. | Aдиcoвнa et al. | 2009110700/14 | Method of treating BV. | Herbal tincture containing camomileflowers, pine buds, birch buds, celandine herb, bird cherry, oak bark, and alder collective fruits at an equal ratio, dosed 250.0 mL | Russia Patent | 10 July 2010 | This method has a very good therapeutic effect on vaginal dysbiosis and helps in restoring hormonal imbalance. | [146] |
| 8. | Jung, So Young | 1020200095649 | Herbal medicine composition for treating vaginitis and preventing uterine cervical dysplasia. | Coix lachrymajobi var. Mayuen, Atracty lodeslancea, Dictamnus dasycarpus, and Sophora flavescens | Korea Patent | 5 February 2021 | This herbal combination of medicine has an efficacy of preventing cervical dysplasia by effective treatment and prevention of BV and candida vaginitis. | [147] |
| 9. | Eвгeньeвич et al. | 2012126560/15 | Agent for vaginal douche in phase of vaginal microflora recovery following treatment ofBVand vaginal thrush (vaginal yeast). | Anemarrhena rhizome, Amur corktree bark, radix Rehmanniae preparata, common yam rhizome | RussianPatent | 10 July 2013 | Use of an aqueous solution of vaginal douche creates favorable conditions for the therapeutic efficacy of medicinal herbs, which decreases the chances of developing any allergic reaction. | [148] |
| 10. | Peian et al. | 201310618673.9 | Anti-inflammatory leukorrhea, arresting washing liquor. | Climbing groundsel herb, Folium isatidis, mint, wild chrysanthemum flower, and Cortex dictamni | China Patent | 3 June 2015 | Prepared formulation has all the natural ingredients, has no toxicity or side effects, no irritation, and also reduces inflammation. | [149] |
| 11. | Eвгeньeвич et al. | 2012126562/15 | Agent for vaginal douche in first stage of treatment ofBV. | Matricaria chamomilla L. | RussianPatent | 10 July 2013 | Duration of treatment was reduced with the help of the douching procedure, and restoration of natural vaginal flora has high therapeutic efficacy. | [150] |
| Essential Oil (EO) and Oil | ||||||||
| EO and Natural Oil Used | ||||||||
| 1. | Luan Xiaoming | 102014000533282 | Vagina cleaning sanitary lotion. | Rosemary, menthol EO | China Patent | 20 April 2016 | Prepared formulation has a cleaning effect on HPV, fungi, and cocci and has a therapeutic efficacy against BV. | [151] |
| 2. | Alberto et al. | 08009684 | Topical compositions for prevention and treatment of inflammatory and/or infective conditions of genital area. | Matricaria chamomilla and Melaleuca alternifolia EO | European Patent | 14 January 2009 | Recurrence of genital infections like BV can be prevented with the help of topical herbal formulations. | [152] |
| 3. | Papa et al. | 17827504 | Natural composition for use in gynecology. | Olive oil | USAPatent | 17 November 2022 | Total negativizationof the vaginal swab was observed at the end of treatment, and relapse was only observed in 2 patients out of 10. | [153] |
| 4. | Feifei et al. | 201610664574.8 | Medicinal composition for repairing female vaginal microecological balance. | TTO | China Patent | 16 November 2016 | Growth of probiotics was present, but growth of harmful bacteria was not present due to a lack of food. | [154] |
| 5. | Gang et al. | 202311350774.2 | Vaginal antibacterial composition, private part nursing product, and preparation method, and application of private part antibacterial composition and private part nursing product. | Rape oil amide propyl dimethylamine, aspartic acid, and valerian extract | China Patent | 24 November 2023 | Herbal extracts have antibacterial, antifungal, and anti-inflammatory effects and can be used for the treatment of BV. | [155] |
| 6. | Gupta Sunil | 202141047207 | Neem seed oil-based vaginal capsule for treatment of abnormal vaginal discharge. | Neem seed oil | Indian Patent | 26 November 2021 | It has 93.33% efficacy against BV and has no toxic effects. | [156] |
| Probiotics | ||||||||
| Strains Used | ||||||||
| 1. | Bиктopoвичet al. | 2022121049 | Method of treatment of BV. | L. acidophilus isolated from Laktonorm, Ecofemin, Gynoflor, and one strain of L. casei subsp. Rhamnosus Lcr35 isolated from Laktozhina | Russia Patent | 30 September 2022 | Current drug intervention has high therapeutic efficacy against BV. | [157] |
| 2. | Komorowski et al. | 17958310 | Probiotic and folic acid compositions and methods of use. | Lactobacillus and folic acid | USAPatent | 4 April 2024 | The current treatment has been proven to be efficacious in clinical trials. Evaluation treatment was also proven to be safe. | [158] |
| 3. | Kort et al. | 16628762 | Probioticcomposition for prevention ofBV. | Probiotics | USA Patent | 7 May 2020 | A total of 25 strains were tested for glycogen degradation, of which 5 strains were less efficient in degrading glycogen, and one strain did not show glycogen degradation at all. | [159] |
| 4. | Kort et al. | WO/2019/013637 | New probiotic composition for prevention of BV. | Lactobacillus crispatus | Netherland Patent | 17.01.2019 | Five strains were less efficient in glycogen degradation, and one strain was not able to degrade glycogen at all. | [160] |
| 5. | Kompella et al. | WO/2014/020613 | Probiotic-layered condom. | LA | Indian Patent | 6 February 2014 | Maintenance of a normal pH range for the vagina to maintain normal vaginal flora to prevent BV and other urogenital infections in females of reproductive age. | [161] |
| 6. | Джoвaнни et al. | 2013137656/15 | Effervescent composition in solid form for use in vaginal applications for treating vaginal infections. | L. plantarum, L. pentosus, L. casei, L. casei ssp. paracasei, L. rhamnosus, Lactobacillus acidophilus, L. delbrueckii, L. delbrueckii ssp. bulgahcus, L. delbrueckii ssp. delbrueckii, L. fermentum, L. gasseri, L. reuteri, Bifidobacterium longum, B. bifidum, B. breve, B. animalis ssp. lactis, B. adolescentis, B. pseudocatenulatum, B. eatenulatum or B. infantis | Russian Patent | 20 June 2016 | Improved stability and a decrease in the capping effect of the tablet while maintaining the distribution of probiotics in the vaginal cavity. | [162] |
| Vitamin | ||||||||
| Vitamins Used | ||||||||
| 1. | Aдиcoвнa et al. | 2008112493/15 | Remedy for treatingBVand method fortreatment. | Ascorbic acid, pantothenic acid, riboflavin, thiamine, vitamin B12 | RussianPatent | 10 May 2009 | Recovery of normal microflora of the vagina and cleaning. | [163] |
| 2. | Qingjun et al. | 201910680707.4 | Probiotics composition for improving health of urogenital system of women and preparation method of composition. | Probiotics (cranberries, Hibiscus sabdariffa, Lactobacillus, collagen peptide) and vitamin C | China Patent | 10 September 2019 | With the help of vitamin C and probiotics, immunity has been increased; the ecological balance of the vagina can be maintained with the help of freeze-dried powder of Lactobacillus. | [164] |
| 3. | Weibin et al. | 202110362861.4 | Female vagina-tightening capsule and preparation process thereof. | Olive, radix Sophorae flavescentis, Fructus cnidii, Saffron crocus, radix Arnebiaeseulithospermi, radix stemonae, Fructus kochiae, a cortex Phellodendri extract, Resina draconis extract, Stiff silkworm extract, Borneol extract, and vitamin E | China patent | 28 May 2021 | The intervention had an antibacterial and anti-inflammatory effect, helped in repairing vaginal tissues, and had no toxicity and/or irritation. | [165] |
| Protein | ||||||||
| Proteins Used | ||||||||
| 1. | Tingtao et al. | 201810873678.9 | Construction of L. crispatus BT1386 and application in treatment. | CXCL12 (human and mouse chemokine) | China Patent | 18 December 2018 | Improvement in the BV treatment by restoration of normal vaginal flora helps in tissue repair and decreases inflammation | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalabehera, M.; Alhudhaibi, A.M.; Abdallah, E.M.; Taha, T.H.; Chaudhari, S.; Kumari, A.; Subudhi, R.N.; Rathore, C. Natural Molecules, Nutraceuticals, and Engineered Nanosystems: A Comprehensive Strategy for Combating Gardnerella vaginalis-Induced Bacterial Vaginosis. Microorganisms 2025, 13, 2411. https://doi.org/10.3390/microorganisms13102411
Dalabehera M, Alhudhaibi AM, Abdallah EM, Taha TH, Chaudhari S, Kumari A, Subudhi RN, Rathore C. Natural Molecules, Nutraceuticals, and Engineered Nanosystems: A Comprehensive Strategy for Combating Gardnerella vaginalis-Induced Bacterial Vaginosis. Microorganisms. 2025; 13(10):2411. https://doi.org/10.3390/microorganisms13102411
Chicago/Turabian StyleDalabehera, Manoj, Abdulrahman Mohammed Alhudhaibi, Emad M. Abdallah, Tarek H. Taha, Shubham Chaudhari, Alka Kumari, Rudra Narayan Subudhi, and Charul Rathore. 2025. "Natural Molecules, Nutraceuticals, and Engineered Nanosystems: A Comprehensive Strategy for Combating Gardnerella vaginalis-Induced Bacterial Vaginosis" Microorganisms 13, no. 10: 2411. https://doi.org/10.3390/microorganisms13102411
APA StyleDalabehera, M., Alhudhaibi, A. M., Abdallah, E. M., Taha, T. H., Chaudhari, S., Kumari, A., Subudhi, R. N., & Rathore, C. (2025). Natural Molecules, Nutraceuticals, and Engineered Nanosystems: A Comprehensive Strategy for Combating Gardnerella vaginalis-Induced Bacterial Vaginosis. Microorganisms, 13(10), 2411. https://doi.org/10.3390/microorganisms13102411












