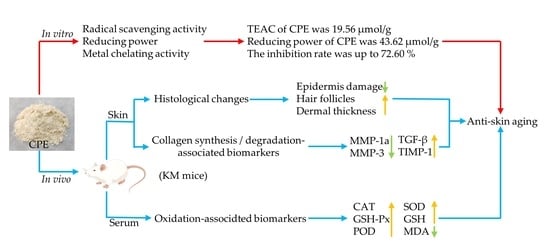
Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Placenta Collection and CPE Preparation
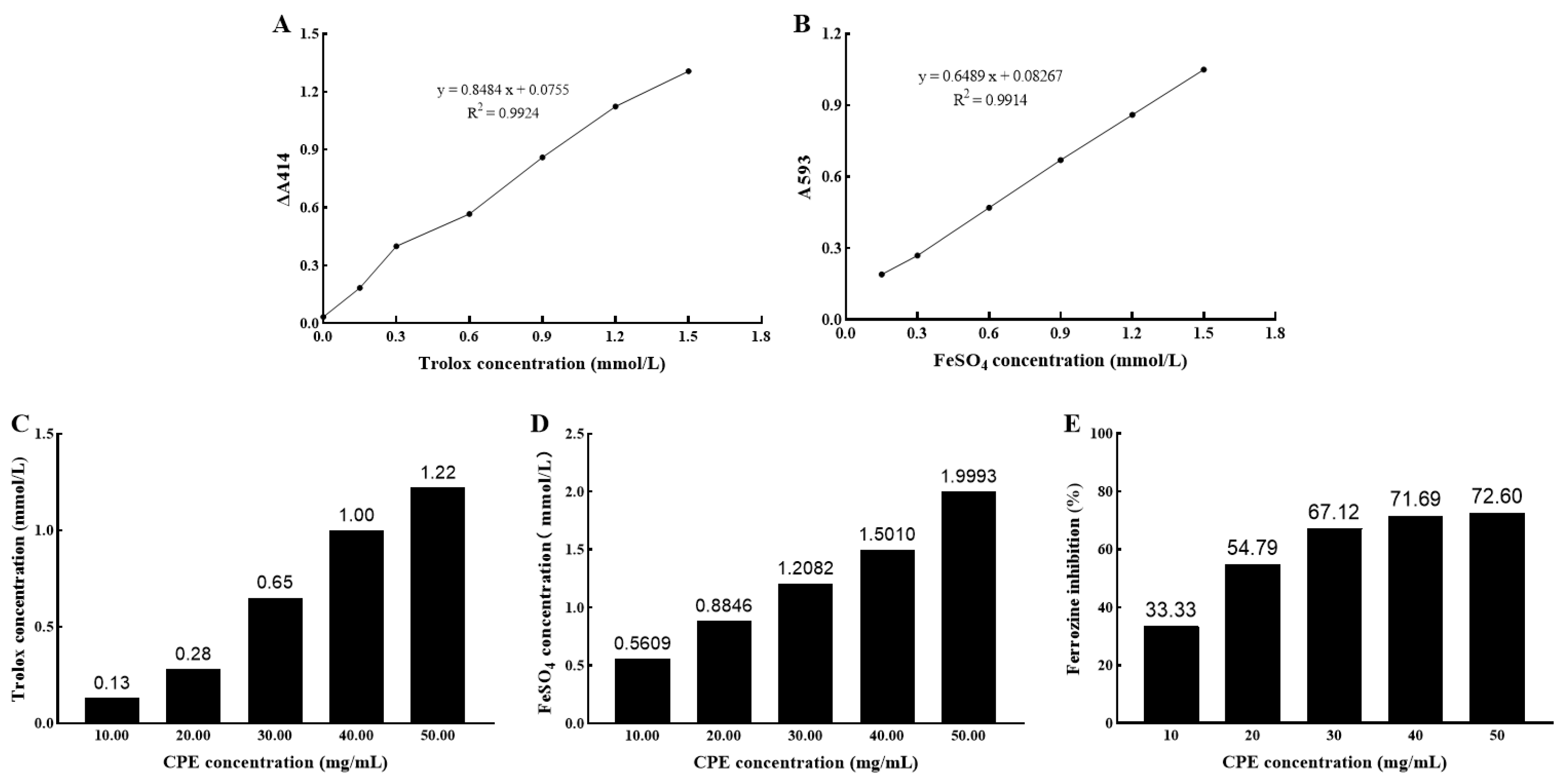
2.2. Antioxidant Capacity In Vitro
2.2.1. ABTS Radical Scavenging Activity
2.2.2. Ferric Reducing Antioxidant Power (FRAP)
2.2.3. Metal Chelating Ability
2.3. Animals and Treatment
2.4. Serum and Tissue Preparation
2.5. Oxidation-Associated Biomarkers Determination in Mouse Serum
2.6. HE and Masson Stain of Mouse Skin
2.7. MMP-1a, MMP-3, TIMP-1 and TGF-β Protein Expression in Mouse Skin
2.8. MMP-1a, MMP-3, TIMP-1 and TGF-β Gene Expression in Mouse Skin
2.9. Statistical Analysis Statistical
3. Results
3.1. Antioxidant Activity of CPE In Vitro
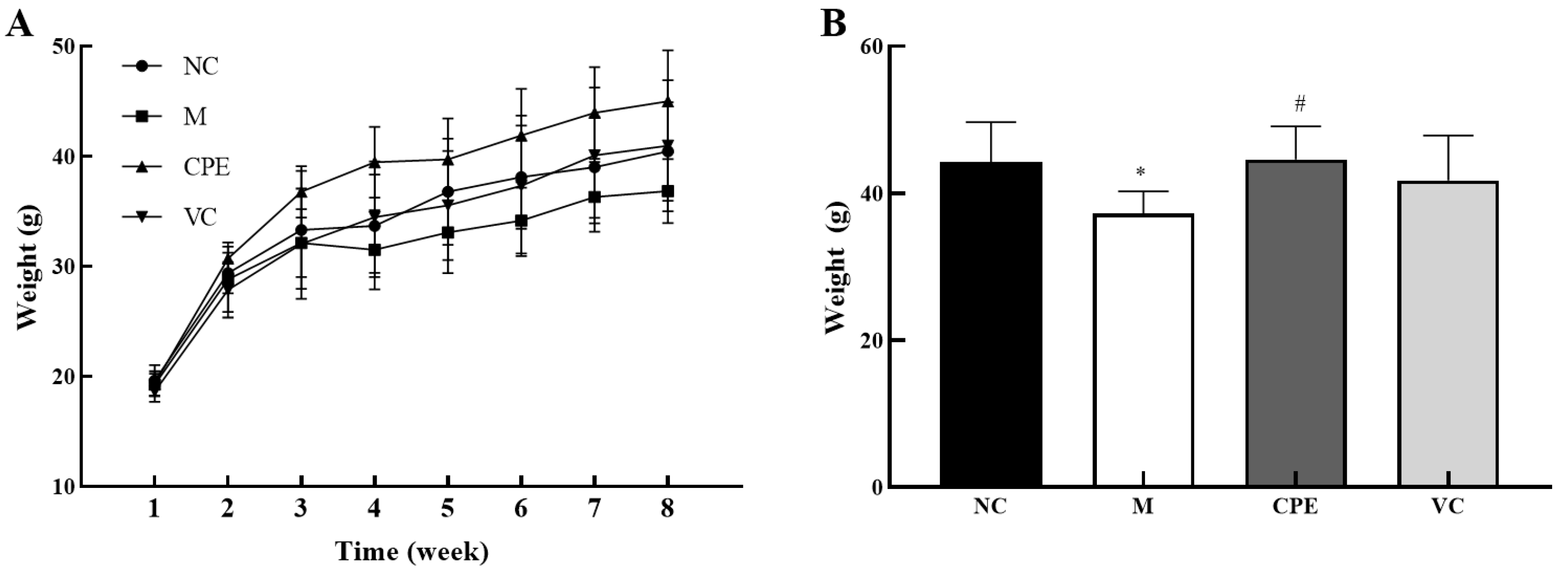
3.2. Effect of CPE on Body Weight
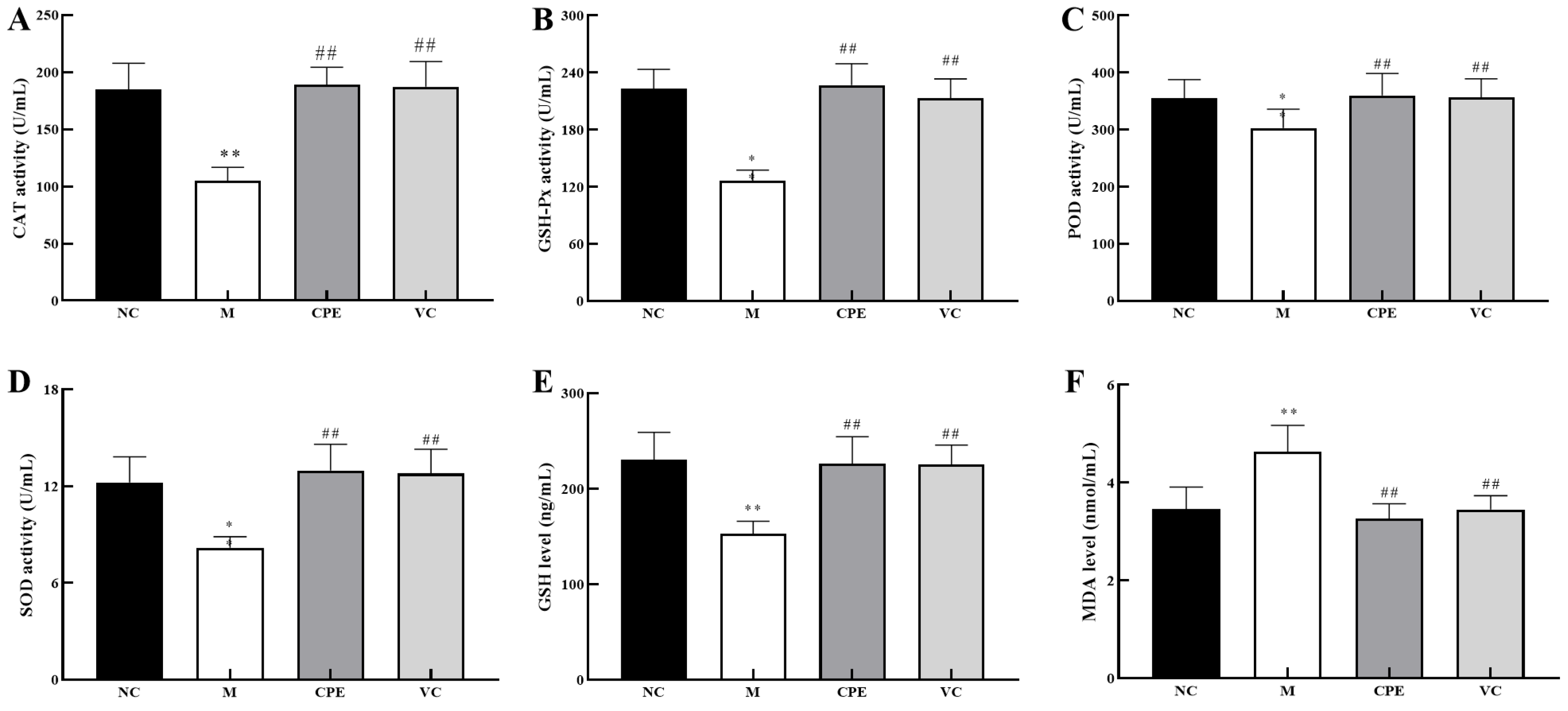
3.3. Effect of CPE on Oxidation-Associated Biomarkers in Serum
3.4. Effect of CPE on Skin Tissue Construct
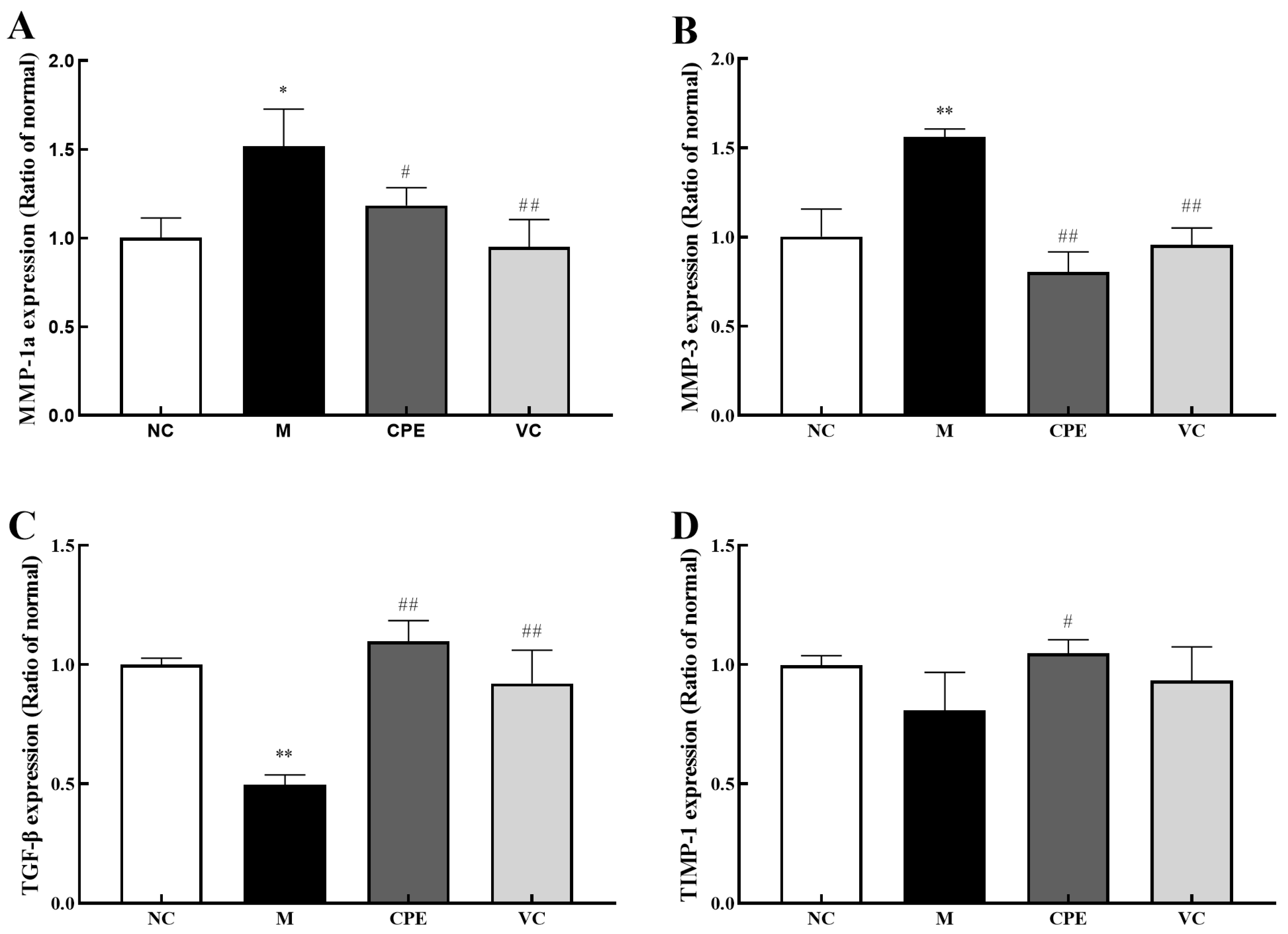
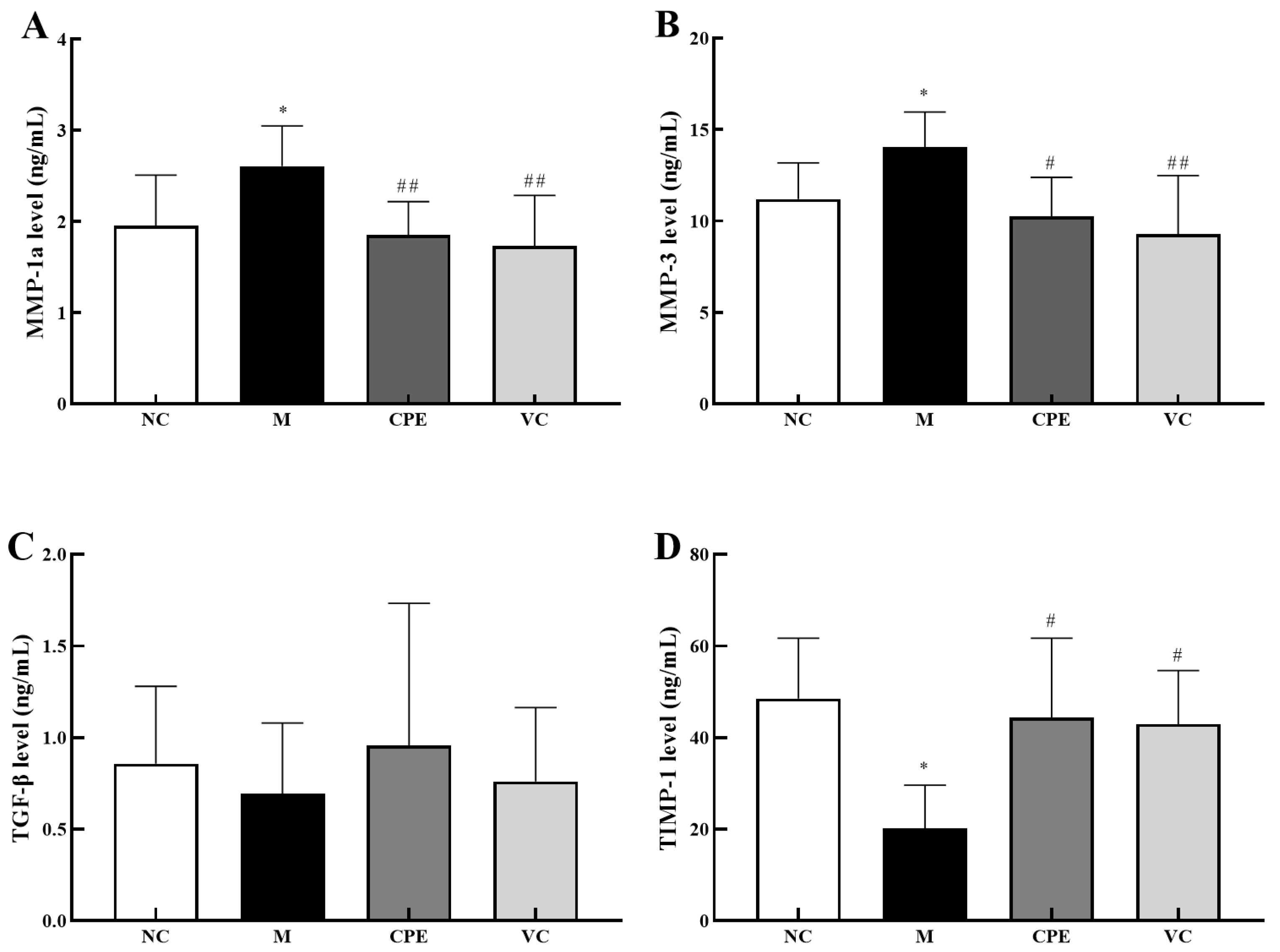
3.5. Effect of CPE on MMP-1a, MMP-3, TIMP-1 and TGF-β Gene and Protein Expression in Mice Skin
4. Discussion
4.1. Antioxidant Activity of CPE In Vitro
4.2. Effect of CPE on the Body Weight of Mice
4.3. Effect of CPE on Serum Antioxidant Indexes and in Mice
4.4. Effect of CPE on Skin Aging-Related Markers in Mice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Cazana, T.; Gonzalez, S.; Parrado, C.; Juarranz, A.; Gilaberte, Y. Influence of the Exposome on Skin Cancer. Actas Dermosifiliogr. 2020, 111, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Nagata, M.; Teramoto, M.; Yamakawa, M.; Matsuki, T.; Ohnuki, K.; Shimizu, K. Effect of Porcine Placenta Extract Supplement on Skin Condition in Healthy Adult Women: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Chin, L.C.; Kimura, K.; Nakahata, Y. Human placental extract activates a wide array of gene expressions related to skin functions. Sci. Rep. 2022, 12, 11031–11038. [Google Scholar] [CrossRef]
- Zhang, Y.; You, L.C. LC-MS/MS based analysis of dairy cow placenta hydrolysis products enzymolysis by different proteases. J. China Agric. Univ. 2021, 26, 133–141. [Google Scholar]
- Shen, L.; You, S.; Zhu, Y.; Zhang, Y.; Hussain, S.; Qian, B.; Lu, S.; Shen, Y.; Yu, S.; Zong, X.; et al. Preparation of reductive polypeptides from fresh placentas of dairy cows. Vet. Res. Forum. 2022, 13, 163–168. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Tong, H.; Liu, Y.; Wu, Y.; Ge, C. Oral Intake of Chicken Bone Collagen Peptides Anti-Skin Aging in Mice by Regulating Collagen Degradation and Synthesis, Inhibiting Inflammation and Activating Lysosomes. Nutrients 2022, 14, 1622. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhou, J.; Liu, W.; Cheng, Y.; Wu, L.; Yang, G. Preparation and Characterization of Antioxidant Peptides from Fermented Goat Placenta. Korean J. Food Sci. Anim. Resour. 2014, 34, 769–776. [Google Scholar] [CrossRef]
- Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice. Molecules 2018, 24, 28. [Google Scholar] [CrossRef]
- Tang, W.L.; Zhang, M.; Fang, Z. Optimization of ultrasound-assisted-extraction of porcine placenta water-soluble proteins and evaluation of the antioxidant activity. J. Food Sci. Technol. 2015, 52, 4042–4053. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, J.; Gao, M.; Guo, G.; Zeng, S.; Chen, X.; Wang, X.; Gong, Z.; Yan, Y. Current perspectives on the clinical implications of oxidative RNA damage in aging research: Challenges and opportunities. Geroscience 2021, 43, 487–505. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J. Cosmet. Dermatol. 2020, 19, 910–912. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Napekoski, K. New approaches to skin photodamage histology-Differentiating ‘good’ versus ‘bad’ Elastin. J. Cosmet. Dermatol. 2021, 20, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Wasityastuti, W.; Habib, N.A.; Sari, D.; Arfian, N. Effects of low and moderate treadmill exercise on liver of d-galactose-exposed aging rat model. Physiol. Rep. 2019, 7, e14279–e14286. [Google Scholar] [CrossRef]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006–110018. [Google Scholar] [CrossRef]
- Togashi, S.; Takahashi, N.; Kubo, Y.; Shigihara, A.; Higashiyama, K.; Watanabe, S.; Fukui, T. Purification and Identification of Antioxidant Substances in Human-Placenta Extracts. J. Health Sci. 2000, 46, 117–125. [Google Scholar] [CrossRef][Green Version]
- Watanabe, S.; Togashi, S.; Takahashi, N.; Fukui, T. L-tryptophan as an antioxidant in human placenta extract. J. Nutr. Sci. Vitaminol. 2002, 48, 36–39. [Google Scholar] [CrossRef]
- Togashi, S.; Takahashi, N.; Iwama, M.; Watanabe, S.; Tamagawa, K.; Fukui, T. Antioxidative collagen-derived peptides in human-placenta extract. Placenta 2002, 23, 497–502. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, Y.; You, L.; Zhang, Y.; Qian, B.; Xiao, J.; Zou, L.; Cao, S.; Peng, G.; Yu, S.; et al. Establishment of an enzymatic hydrolysis evaluation index for dairy cows’ placental hydrolysates. J. Appl. Anim. Res. 2022, 50, 666–677. [Google Scholar] [CrossRef]
- Laosam, P.; Panpipat, W.; Yusakul, G.; Cheong, L.Z.; Chaijan, M. Porcine placenta hydrolysate as an alternate functional food ingredient: In vitro antioxidant and antibacterial assessments. PLoS ONE 2021, 16, e258445. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jin, Y.; Liu, M.; Yang, Y.; Zhang, M.; Guo, Y.; Jones, G.; Liu, J.; Yin, Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and alpha-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Sun, H.; Wang, B.; Ahmad, N.; Guo, H.; Gao, H.; Gao, Y.; Liu, X.; Li, H. Hepatoprotective effects of hydroxysafflor yellow A in D-galactose-treated aging mice. Eur. J. Pharmacol. 2020, 881, 173214–173222. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Kong, S.; Li, S.; Guo, J.; Guo, M.; Cai, T.; Li, N.; Chen, R.; Luo, R.; et al. Protective effects of collagen polypeptide from tilapia skin against injuries to the liver and kidneys of mice induced by d-galactose. Biomed. Pharmacother. 2019, 117, 109204–109214. [Google Scholar] [CrossRef]
- Konig, D.; Kohl, J.; Jerger, S.; Centner, C. Potential Relevance of Bioactive Peptides in Sports Nutrition. Nutrients 2021, 13, 3997. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2 -induced oxidative damages in HepG2 cells and d -galactose-induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef]
- Xia, C.; Cao, X.; Cui, L.; Liu, H.; Wang, S.; Chen, T. Anti-aging effect of the combination of Bifidobacterium longum and B. animalis in a d-galactose-treated mice. J. Funct. Foods 2020, 69, 103938–103946. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Kalaz, E.B.; Coban, J.; Aydin, A.F.; Dogan-Ekici, I.; Dogru-Abbasoglu, S.; Oztezcan, S.; Uysal, M. Carnosine and taurine treatments decreased oxidative stress and tissue damage induced by D-galactose in rat liver. J. Physiol. Biochem. 2014, 70, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bai, F.; Liu, Y.; Yang, Y.; Yuan, Q.; Zou, D.; Qu, S.; Tian, G.; Song, L.; Zhang, T.; et al. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol. Cell. Biochem. 2015, 403, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, L.; Peng, Y.; Yuan, D.; Zhang, S.; Gong, Z.; Xiao, W. l-Theanine attenuates liver aging by inhibiting advanced glycation end products in d-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp. Gerontol. 2020, 131, 110823–110831. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by D-Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zheng, Y.; Zhang, L.; Wang, X.; Luo, Z.; Tang, J.; Lin, L.; Du, Z.; Dong, C. The effects and mechanism of collagen peptide and elastin peptide on skin aging induced by D-galactose combined with ultraviolet radiation. J. Photochem. Photobiol. B 2020, 210, 111964–111971. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Aumailley, M. The extracellular matrix of the dermis: Flexible structures with dynamic functions. Exp. Dermatol. 2011, 20, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.M.; Noh, E.M.; Kim, J.S.; Kim, J.M.; Hwang, J.K.; Kim, H.K.; Kang, J.S.; Kim, D.S.; Chae, H.J.; You, Y.O.; et al. Decursin inhibits UVB-induced MMP expression in human dermal fibroblasts via regulation of nuclear factor-kappaB. Int. J. Mol. Med. 2013, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple passion fruit seeds (Passiflora edulis f. edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab. J. Chem. 2021, 14, 102905–102924. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.L.; Yu, X.T.; Su, Z.Q.; Yuan, J.; Li, Y.C.; Su, Z.R.; Zhan, J.Y.; Lai, X.P. Protective Effect of Super-Critical Carbon Dioxide Fluid Extract from Flowers and Buds of Chrysanthemum indicum Linnen Against Ultraviolet-Induced Photo-Aging in Mice. Rejuvenation Res. 2015, 18, 437–448. [Google Scholar] [CrossRef]


| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) | Size (bp) |
|---|---|---|---|
| MMP-1a | ATAGATTCATGCCAGAACCTGA | TGCCTTTGAAATAGCGGACT | 120 |
| MMP-3 | AATCAGTTCTGGGCTATACGA | TCGATCTTCTTCACGGTTGC | 95 |
| TGF-β | AACAATTCCTGGCGTTACCTT | CTTGGTTCAGCCACTGCCGTA | 106 |
| TIMP-1 | TCCCAGAACCGCAGTGAAG | ACGCCAGGGAACCAAGAAG | 93 |
| GAPDH | GCGACTTCAACAGCAACTCCC | CACCCTGTTGCTGTAGCCGTA | 122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.-H.; Fan, L.; Zhang, Y.; Shen, Y.; Su, Z.-T.; Peng, G.-N.; Deng, J.-L.; Zhong, Z.-J.; Wu, X.-F.; Yu, S.-M.; et al. Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice. Nutrients 2022, 14, 4659. https://doi.org/10.3390/nu14214659
Shen L-H, Fan L, Zhang Y, Shen Y, Su Z-T, Peng G-N, Deng J-L, Zhong Z-J, Wu X-F, Yu S-M, et al. Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice. Nutrients. 2022; 14(21):4659. https://doi.org/10.3390/nu14214659
Chicago/Turabian StyleShen, Liu-Hong, Lei Fan, Yue Zhang, Yu Shen, Zhe-Tong Su, Guang-Neng Peng, Jun-Liang Deng, Zhi-Jun Zhong, Xiao-Feng Wu, Shu-Min Yu, and et al. 2022. "Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice" Nutrients 14, no. 21: 4659. https://doi.org/10.3390/nu14214659
APA StyleShen, L.-H., Fan, L., Zhang, Y., Shen, Y., Su, Z.-T., Peng, G.-N., Deng, J.-L., Zhong, Z.-J., Wu, X.-F., Yu, S.-M., Cao, S.-Z., & Zong, X.-L. (2022). Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice. Nutrients, 14(21), 4659. https://doi.org/10.3390/nu14214659