Beyond Vision: Unveiling the Psychiatric Dimensions of Keratoconus
Abstract
1. Introduction
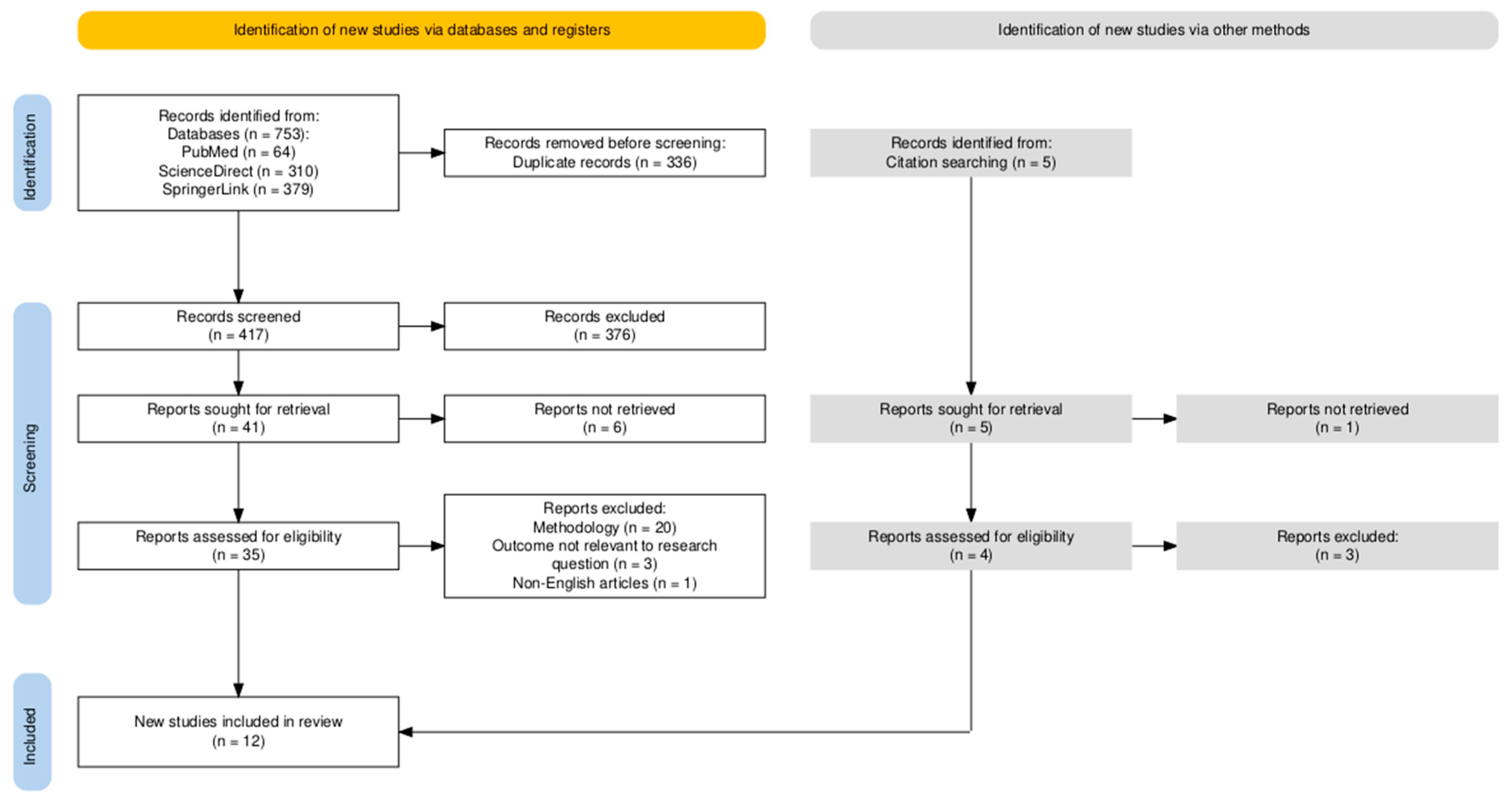
2. Materials and Methods
3. Results
3.1. Depression and Keratoconus
| Authors | S1 | S2 | S3 | S4 | C | E1 or O1 | E2 or O2 | E3 or O3 | Total | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Marx-Gross et al. [16] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Low |
| Moschos et al. [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | Low |
| Lin et al. [1] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | Low |
| Aslan et al. [18] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | Low |
| Bak-Nielsen et al. [19] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | Low |
| Woodward et al. [20] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | Low |
| Authors | 1. Inclusion | 2. Setting | 3. Exposure | 4. Criteria | 5. Confounders Id. | 6. Confounder Adj. | 7. Outcomes | 8. Analysis | 9. Sample | 10. Ethics | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yildiz et al. [21] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Moderate |
| Florek et al. [23] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Moderate-High |
| Al-Dairi et al. [22] | Yes | No | No | Unclear | Unclear | No | No | No | Yes | Yes | Low |
| Jonas et al. [24] | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | High |
| Alfardan et al. [25] | No | Yes | No | Unclear | Yes | No | Unclear | No | No | Yes | Low-Moderate |
| Authors | Publication Year | Country | Type of Study | Number of Patients/Controls | Depression Diagnostic Method | Main Result | Limitations of the Study |
|---|---|---|---|---|---|---|---|
| Lin et al. [1] | 2021 | Taiwan | case–control | 5055/20220 | Medical records | KC → ↓ depression | Reliance on medical records KC severity and visual parameters not reported |
| Bak-Nielsen et al. [19] | 2019 | Denmark | case–control | 2679/26790 | Medical records | KC diagnosis → ↑ depression risk | Reliance on medical records No KC severity data Long study period |
| Al-Dairi et al. [22] | 2020 | Saudi Arabia | cross-sectional | 330 | PHQ-9 | 40.6% of KC patients had depression | Sampling bias No clinical confirmation of depression Missing detailed clinical data No follow-up |
| Marx-Gross et al. [16] | 2023 | Germany | cohort study | 12423 | PHQ-9 | KC ↔ depression | No KC severity data Possible selection bias |
| Jonas et al. [24] | 2018 | China | cross-sectional | 3468 | ZDS | Major ocular diseases ↔ depression | Self-reported depression Cultural influences on reporting No follow-up |
| Woodward et al. [20] | 2016 | USA | case–control | 16053/16053 | Medical records | KC ↔ depression | Reliance on medical records No data on the uninsured Lack of detailed clinical data |
| Alfardan et al. [25] | 2023 | Saudi Arabia | Cross-sectional | 57 | Medical records | KC patients → high prevalence of psychiatric illness | Small sample size Reliance on medical records No control group |
| Yildiz et al. [21] | 2021 | Turkey | Cross-sectional | 94 | BDI | KC → high rates of depression | Small sample size No follow up No control group Age restricted to young adults |
| Florek et al. [23] | 2024 | Poland | Cross-sectional | 99/92 | HDRS, BDI | KC ↔ depression severity | Gender disparity Control group health self-reported Only symptom severity measured, not psychiatric disorder prevalence No follow-up |
| Moschos et al. [17] | 2018 | Greece | case–control | 56/47 | ZDI, PHQ-9 | KC ↑ depression levels ↓ Vision → ↑ depression KC → ↑ risk of depression | Small sample size No longitudinal follow-up Cannot assess effect of KC duration/severity |
| Aslan et al. [18] | 2021 | Turkey | case–control | 59/65 | BDI | KC → ↑ depression scores Vision and topographical parameters ↔ depression | Majority had mild KC Non-response rate unclear No adjustment for confounders |
3.2. Psychotic Disorders and Keratoconus
3.3. Personality Disorders and Keratoconus
3.4. Other Psychiatric Disorders and Keratoconus
3.5. Summary of Findings: Psychiatric Disorders in Keratoconus (See Table 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KC | Keratoconus |
| ADHD | Attention deficit hyperactivity disorder |
| TS | Tourette syndrome |
| ASD | Autism spectrum disorder |
| OCD | Obsessive–compulsive disorder |
| PHQ-9 | Patient Health Questionnaire-9 |
| ZDS | Zung Self-rating Depression Scale |
| BDI | Beck Depression Inventory |
| HDRS | Hamilton Rating Scale for Depression |
| MOCI | Maudsley Obsessive Compulsive Inventory |
| MR | Mendelian randomization |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor—alpha |
| MMPs | Matrix Metalloproteinases |
References
- Lin, K.-K.; Lee, J.-S.; Hou, C.-H.; Chen, W.-M.; Hsiao, C.-H.; Chen, Y.-W.; Yeh, C.-T.; See, L.-C. The Sociodemographic and Risk Factors for Keratoconus: Nationwide Matched Case-Control Study in Taiwan, 1998–2015. Am. J. Ophthalmol. 2021, 223, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Rodrigues, P.F.; Lamazales, L.L. Keratoconus Epidemiology: A Review. Saudi J. Ophthalmol. 2022, 36, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Soiberman, U.; Foster, J.W.; Jun, A.S.; Chakravarti, S. Pathophysiology of Keratoconus: What Do We Know Today. Open Ophthalmol. J. 2017, 11, 252–261. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An Updated Review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Asimellis, G.; Kaufman, E.J. Keratoconus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Unni, P.; Lee, H.J. Systemic Associations with Keratoconus. Life 2023, 13, 1363. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and Related Noninflammatory Corneal Thinning Disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Elder, M.J. Leber Congenital Amaurosis and Its Association With Keratoconus and Keratoglobus. J. Pediatr. Ophthalmol. Strabismus 1994, 31, 38–40. [Google Scholar] [CrossRef]
- Shinzawa, M.; Kato, N.; Kasai, K.; Konomi, K.; Chai, Y.; Shimazaki, J. Corneal Cross-Linking for Keratoconus Caused by Compulsive Eye Rubbing in Patients with Tourette Syndrome: Three Case Reports. Medicine 2019, 98, e15658. [Google Scholar] [CrossRef]
- Florek, S.; Pudlo, R.; Gościniewicz, P.; Mrukwa-Kominek, E. Mental Disorders in People with Keratoconus. Curr. Probl. Psychiatry 2023, 24, 33–39. [Google Scholar] [CrossRef]
- Safir, M.; Hecht, I.; Heller, D.; Pras, E.; Lifshitz, M.; Einan-Lifshitz, A. Psychiatric Comorbidities Associated with Keratoconus. JAMA Ophthalmol. 2023, 141, 1145. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghinia, R.; Arman, A.; Sobhi, N.; Mahmoudinezhad, G.; Molavi Vardanjani, H. Depression among Keratoconus Patients: A Systematic Review and Meta-Analysis. Front. Public Health 2024, 12, 1477411. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Cui, F.; Wu, X.; Zhang, C. Mendelian Randomization Analysis Does Not Reveal a Causal Influence between Keratoconus and Three Major Mental Disorders. Front. Psychiatry 2024, 15, 1370670. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.R.; Kulinich, A.G.; Scher, L.M.; Mannis, M.J. Vision Loss and Psychopathology. Pan-Am. J. Ophthalmol. 2021, 3, 7. [Google Scholar] [CrossRef]
- Demmin, D.L.; Silverstein, S.M. Visual Impairment and Mental Health: Unmet Needs and Treatment Options. OPTH 2020, 14, 4229–4251. [Google Scholar] [CrossRef]
- Marx-Gross, S.; Fieß, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K.-G. Much Higher Prevalence of Keratoconus than Announced Results of the Gutenberg Health Study (GHS). Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3241–3247. [Google Scholar] [CrossRef]
- Moschos, M.M.; Gouliopoulos, N.S.; Kalogeropoulos, C.; Androudi, S.; Kitsos, G.; Ladas, D.; Tsatsos, M.; Chatziralli, I. Psychological Aspects and Depression in Patients with Symptomatic Keratoconus. J. Ophthalmol. 2018, 2018, 7314308. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.G. Evaluation of Personality Features and Mental States of Keratoconus Patients. Beyoglu Eye J. 2021, 6, 272. [Google Scholar] [CrossRef]
- Bak-Nielsen, S.; Ramlau-Hansen, C.H.; Ivarsen, A.; Plana-Ripoll, O.; Hjortdal, J. A Nationwide Population-based Study of Social Demographic Factors, Associated Diseases and Mortality of Keratoconus Patients in Denmark from 1977 to 2015. Acta Ophthalmol. 2019, 97, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.A.; Blachley, T.S.; Stein, J.D. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus. Ophthalmology 2016, 123, 457–465.e2. [Google Scholar] [CrossRef]
- Yildiz, M.; Turhan, S.A.; Yargı, B.; Ergün, S.; Örnek, E.; Baz, F.; Toker, A.E. Psychiatric Morbidity of Patients with Keratoconus: A Cross-Sectional Study. J. Psychosom. Res. 2021, 143, 110384. [Google Scholar] [CrossRef]
- Al-Dairi, W.; Al Sowayigh, O.M.; Al Saeed, A.A.; Alsaad, A. Depression Among Keratoconus Patients in Saudi Arabia. Cureus 2020, 12, e11932. [Google Scholar] [CrossRef]
- Florek, S.; Gościniewicz, P.; Suszka, M.; Mrukwa-Kominek, E.; Pudlo, R. Psychological and Psychiatric Characteristics of People with Keratoconus. Reports 2024, 7, 67. [Google Scholar] [CrossRef]
- Jonas, J.B.; Wei, W.B.; Xu, L.; Rietschel, M.; Streit, F.; Wang, Y.X. Self-Rated Depression and Eye Diseases: The Beijing Eye Study. PLoS ONE 2018, 13, e0202132. [Google Scholar] [CrossRef]
- Alfardan, F.; Alsanad, M.H.; Altoub, H.A. Prevalence of Psychiatric Illness Among Keratoconus Patients. Cureus 2023, 15, e42141. [Google Scholar] [CrossRef] [PubMed]
- Schürhoff, F.; Leboyer, M.; Szöke, A. Comorbidity between Schizophrenia and Keratoconus. Psychiatry Res. 2017, 247, 315–316. [Google Scholar] [CrossRef]
- Aiello, F.; Gallo Afflitto, G.; Ceccarelli, F.; Garzione, F.; Pocobelli, G.; Pinci, C.; Di Lorenzo, G.; Siracusano, A.; Nucci, C. Keratoconus and Personality Traits: A Case–Control Study. Cornea 2024, 43, 237–244. [Google Scholar] [CrossRef]
- Stanojlovic, S.; Milovancevic, M.P.; Stankovic, B. Is There a Potential Link between Keratoconus and Autism Spectrum Disorders?: A Case Report and Literature Review. Medicine 2020, 99, e20247. [Google Scholar] [CrossRef]
- ALGarzaie, M.A.; Alsaqr, A.M. A Comparative Study of Corneal Topography in Children with Autism Spectrum Disorder: A Cross-Sectional Study. Vision 2021, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Palamar, M.; Dincer, G.; Teker, M.E.; Kayahan, B.; Gonul, A.S. Bilateral Keratoconus, Acute Hydrops and Unilateral Corneal Perforation Due to Tourette Syndrome. Saudi J. Ophthalmol. 2019, 33, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Hage, A.; Knoeri, J.; Leveziel, L.; Majoulet, A.; Blanc, J.-V.; Buffault, J.; Labbé, A.; Baudouin, C. EYERUBBICS: The Eye Rubbing Cycle Study. JCM 2023, 12, 1529. [Google Scholar] [CrossRef]
- Assayag, E.; Zadok, D.; Carmel, M.; Abulafia, A.; Weill, Y. Superior Keratoconus in Attention-Deficit Hyperactivity Disorder (ADHD): A Case Report and Literature Review. Cureus 2024, 16, e76013. [Google Scholar] [CrossRef]
- Panikkar, K.; Manayath, G.; Rajaraman, R.; Saravanan, V. Progressive Keratoconus, Retinal Detachment, and Intracorneal Silicone Oil with Obsessive-Compulsive Eye Rubbing. Oman J. Ophthalmol. 2016, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.R.; Xiang, X.; Stagg, B.C.; Ehrlich, J.R. Longitudinal Associations of Self-Reported Vision Impairment With Symptoms of Anxiety and Depression Among Older Adults in the United States. JAMA Ophthalmol. 2019, 137, 793. [Google Scholar] [CrossRef]
- Sabel, B.A.; Wang, J.; Cárdenas-Morales, L.; Faiq, M.; Heim, C. Mental Stress as Consequence and Cause of Vision Loss: The Dawn of Psychosomatic Ophthalmology for Preventive and Personalized Medicine. EPMA J. 2018, 9, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Iliuta, F.; Manea, M.; Budisteanu, M.; Andrei, E.; Linca, F.; Rad, F.; Cergan, R.; Ciobanu, A. Magnetic Resonance Imaging of Brain Anomalies in Adult and Pediatric Schizophrenia Patients: Experience of a Romanian Tertiary Hospital. Exp. Ther. Med. 2021, 22, 1098. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Rosen, R. Schizophrenia and the Eye. Schizophr. Res. Cogn. 2015, 2, 46–55. [Google Scholar] [CrossRef]
- Iliuta, F.; Manea, M.; Budisteanu, M.; Ciobanu, A.; Manea, M. Magnetic Resonance Imaging in Schizophrenia: Luxury or Necessity? (Review). Exp. Ther. Med. 2021, 22, 765. [Google Scholar] [CrossRef]
- Petrescu, C.; Petrescu, D.M.; Marian, G.; Focseneanu, B.E.; Iliuta, F.P.; Ciobanu, C.A.; Papacocea, S.; Ciobanu, A.M. Neurological Soft Signs in Schizophrenia, a Picture of the Knowledge in the Last Decade: A Scoping Review. Healthcare 2023, 11, 1471. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, D.; Ghita, R.V.; Iconaru, S.L.; Ciobanu, S.C.; Manea, M.; Badea, M.L.; Costescu, A.; Trusca, R.; Predoi, G.; et al. Multi-Level Evaluation of UV Action upon Vitamin D Enhanced, Silver Doped Hydroxyapatite Thin Films Deposited on Titanium Substrate. Coatings 2021, 11, 120. [Google Scholar] [CrossRef]
- Balmus, I.-M.; Alexa, A.I.; Ciuntu, R.-E.; Danielescu, C.; Stoica, B.; Cojocaru, S.I.; Ciobica, A.; Cantemir, A. Oxidative Stress Markers Dynamics in Keratoconus Patients’ Tears before and after Corneal Collagen Crosslinking Procedure. Exp. Eye Res. 2020, 190, 107897. [Google Scholar] [CrossRef]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An Inflammatory Disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef]
- Najmi, H. The Correlation between Keratoconus and Eye Rubbing: A Review. Int. J. Ophthalmol. 2019, 12, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.; Gil, J.Q.; Cruz, N.; Costa, C.; Rodrigues-Santos, P.; Sousa, L.M.; Almeida, J.S.; Fernandes, R.; Alves, N.; Rosa, A.; et al. Persistent Proinflammatory Cytokine Profile in the Tear Fluid of Stable Keratoconus: Rethinking Clinical Quiescence. Trans. Vis. Sci. Tech. 2025, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; D’Souza, S.; Khamar, P.; Ghosh, A.; Nuijts, R.M.M.A.; Sethu, S. Biochemical Markers and Alterations in Keratoconus. Asia-Pac. J. Ophthalmol. 2020, 9, 533–540. [Google Scholar] [CrossRef] [PubMed]
| Outcome | No. of Studies (Design) | Participants (KC/Control) | Main Findings | Certainty of Evidence (GRADE) | Comments |
|---|---|---|---|---|---|
| Depression | 11 (6 case–control/cohort, 5 cross-sectional) | 40373/63267 | Mixed results: 5 studies showed ↑ depressive symptoms; 4 found no difference; 2 showed ↓ or no association | ⊕⊕◯◯ Low | Heterogeneous methodologies; different tools used (PHQ-9, BDI, ZDS, HDRS); causal inference not possible; |
| ADHD | 1 (cross-sectional) | 1533 | ↑ prevalence of KC in males with ADHD ADHD severity ↔ KC severity | ⊕⊕⊕◯ Moderate | Confounders controlled; lack of data on eye rubbing limits interpretation; |
| Psychotic disorders | 1 (cross-sectional) + 3 case reports + 1 (genetic MR) | 60 | KC ↔ schizophrenia; rare co-occurrence; | ⊕◯◯◯ Very low | Evidence from limited case reports; not generalizable; |
| Personality disorders | 3 (cross-sectional) | 188/187 | ↑ obsessive-compulsive and neurotic traits ↑ risk of cluster C personality disorders | ⊕⊕◯◯ Low | May reflect chronic disease burden rather than KC itself; |
| TS | 2 (case reports + case series) | 4 | KC linked to compulsive eye rubbing | ⊕◯◯◯ Very low | Based on case reports; no comparative data; |
| ASD | 2 (1 case report, 1 cross-sectional) | 115 | No increased prevalence of KC-like features in ASD; isolated co-occurrence described; | ⊕◯◯◯ Very low | Sparse evidence; not statistically assessed; |
| OCD | 1 (case report) | 1 | Rapid KC progression associated with compulsive eye rubbing | ⊕◯◯◯ Very low | Anecdotal evidence; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuță, T.-G.; Manea, M.C.; Varlam, C.I.; Nuță, G.; Mareș, A.-M.; Iliuță, F.P. Beyond Vision: Unveiling the Psychiatric Dimensions of Keratoconus. Medicina 2025, 61, 1943. https://doi.org/10.3390/medicina61111943
Nuță T-G, Manea MC, Varlam CI, Nuță G, Mareș A-M, Iliuță FP. Beyond Vision: Unveiling the Psychiatric Dimensions of Keratoconus. Medicina. 2025; 61(11):1943. https://doi.org/10.3390/medicina61111943
Chicago/Turabian StyleNuță, Teodor-Georgian, Mihnea Costin Manea, Corina Ioana Varlam, Gabriela Nuță, Aliss-Mădălina Mareș, and Floris Petru Iliuță. 2025. "Beyond Vision: Unveiling the Psychiatric Dimensions of Keratoconus" Medicina 61, no. 11: 1943. https://doi.org/10.3390/medicina61111943
APA StyleNuță, T.-G., Manea, M. C., Varlam, C. I., Nuță, G., Mareș, A.-M., & Iliuță, F. P. (2025). Beyond Vision: Unveiling the Psychiatric Dimensions of Keratoconus. Medicina, 61(11), 1943. https://doi.org/10.3390/medicina61111943






