Abstract
Breast cancer is a significant global health challenge, with rising incidence rates and substantial morbidity and mortality worldwide. Conventional treatments, while effective, often lead to adverse effects and may not fully eradicate cancer cells, resulting in recurrence and progression of tumors. Addressing these challenges requires innovative treatment strategies. Nanotechnology, particularly polysaccharide-based nanoparticles (NPs), offers a promising approach due to their biocompatibility, tunable properties, and targeted drug delivery capabilities. Polysaccharide NPs, including starch, alginate, hyaluronic acid, and chitosan, possess inherent biocompatibility and can be tailored for specific applications. Furthermore, beyond their inherent biocompatibility, polysaccharide-based NPs shown substantial interest due to their natural abundance, ease of processing, and availability from renewable resources, solidifying their role as a sustainable choice for diverse biomedical applications. By functionalizing their surface with ligands, polysaccharide NPs can target breast cancer cells, enhance therapeutic efficacy while minimizing off-target effects. Moreover, these NPs can modulate biological processes relevant to cancer progression, such as angiogenesis and immune response. This review article provides a concise overview of the pathophysiology of breast cancer and the benefits of polysaccharides in drug delivery. Additionally, it emphasizes the significance of several polysaccharide-based NPs in breast cancer therapy, followed by a detailed discussion on the role of various polysaccharide-based NPs in breast cancer treatment.
1. Introduction
Breast cancer remains a considerable global health problem, with significant morbidity and mortality rates worldwide. The incidence of breast cancer continues to rise, making it the most commonly diagnosed cancer among women globally []. Globally, breast cancer is accountable for a considerable proportion of cancerous tumor cases and cancer-related mortalities among women. According to the latest prevalence statistics from the World Health Organization (WHO), breast cancer is the highest causality of cancer incidence and the second leading rationale of cancer mortality among females worldwide. In 2020, over 2.2 million new cases of breast cancer were diagnosed worldwide, resulting in an estimated 685,000 deaths []. In India, breast cancer cases shown a significant public health challenge, with increasing incidence rates observed in recent years [,]. Several factors contribute to the high prevalence rate of breast cancer in India, including changing lifestyle patterns, delayed childbearing, reduced breastfeeding duration, and increasing urbanization [,].
Many conventional treatments for breast cancer, i.e., chemotherapy, immunotherapy, and radiation therapy, can yield consequential adverse events, including fatigue, hair loss, nausea, and increased risk of infections or low immunogenicity. Additionally, tumor cells may acquire resistance to conventional therapies over time, resulting in treatment failure and disease recurrence []. This resistance can emerge due to congenital mutations, tumor heterogeneity, or adaptive transformations within the tumor microenvironment, making it challenging to achieve long-term remission []. Despite aggressive treatment regimens, conventional therapies may not completely eradicate the cancer cells, especially in cases of chronic or metastatic disease [,]. Therefore, the residual cancer cells can lead to disease relapse and progression, highlighting the need for more effective treatment strategies []. In some cases, conventional treatments may be saturated, further expose the patients to unnecessary risks and burdens without significant clinical benefit. This highlights the importance of personalized treatment approaches tailored to individual patient characteristics and tumor biology. Addressing these challenges requires a multifaceted approach, including the development of novel treatment modalities, personalized medicine approaches, supportive care interventions, and efforts to improve access to care and reduce treatment-related inconsistency [,,]. By overcoming these challenges, we can improve the effectiveness, safety, and quality of breast cancer treatment, ultimately enhancing patient outcomes and survivorship. Therefore, breast cancer remains a substantial global health challenge, necessitating the development of innovative treatment strategies.
Nanotechnology has emerged as a promising approach, offering targeted drug delivery and improved therapeutic efficacy, while simultaneously reducing systemic toxicity []. In this context, polysaccharide-based nanoparticles (NPs) developed using natural sources have attracted significant attention due to their excellent biocompatibility, structural versatility, and easily tunable properties. Unlike many synthetic polymer-based NPs, which may exhibit long-term toxicity, and lipid-based NPs, which often reported with limited stability, polysaccharide-based NPs offer a safer and more stable alternative, making them highly promising candidates for breast cancer therapy. Polysaccharide-based NPs, i.e., starch, alginate, hyaluronic acid (HA), dextran, cellulose, chitosan (CS), etc., possess inherent biocompatibility and can be functionalized for targeted drug delivery [,]. The mucoadhesive properties of polysaccharide-based NPs enable effective uptake at the tumor site, while their biodegradability ensures minimal toxicity [,,]. Polysaccharide-based NPs hold significant promise for breast cancer aid due to their promising physicochemical characteristics and versatile mechanical properties. By functionalizing their surfaces with ligands, polysaccharide-based NPs can specifically target receptors overexpressed on breast cancer cells, improving therapeutic efficacy while minimizing the off-target effects [,]. Polysaccharide NPs can be functionalized with proteins or ligands that selectively affix to receptors overexpressed on breast cancer cells, facilitating their uptake and accumulation within the tumor microenvironment []. In addition, these NPs may exert additional therapeutic effects through modulation of biological processes relevant to cancer progression, i.e., angiogenesis, apoptosis, and immune response [,]. Several polysaccharide NPs may inhibit angiogenesis of cancerous cells via targeting vascular endothelial growth factor (VEGF) receptors [] or enhance immune infiltration into the tumor microenvironment [], leading to improved antitumor or anticancer immune responses []; therefore, can be combined with other treatment modalities, i.e., radiation therapy, immunotherapy, or chemotherapy, to achieve synergistic therapeutic effects []. Thus, polysaccharide NPs can improve cancer therapy efficacy and reduce recurrence risk by enhancing drug delivery and overcoming resistance mechanisms.
To the best of our knowledge, only a few review articles have comprehensively addressed the role of polysaccharide-based NPs in breast cancer therapy []. A 2024 review primarily focused on the use of polysaccharide-based NPs for antisense oligonucleotide delivery []. Therefore, this review article aims to provide an updated overview of recent advances in various polysaccharide-based NPs designed for the delivery of diverse therapeutic agents, including chemotherapeutic, targeted, phytochemical, and immunotherapeutic agents. This comprehensive manuscript further examines both in vitro and in vivo findings that highlight their efficacy and translational potential. Collectively, polysaccharide-based NPs emerge as promising platforms for targeted breast cancer therapy, offering new avenues to enhance breast cancer treatment outcomes.
2. Pathophysiology of Breast Cancer
Breast cancer arises due to mutations in genes that are pivotal for controlling cell division, cell cycle, programmed cell death (apoptosis), and frequently implicated genes include TP53, PIK3CA, MYC, PTEN, CCND1, ERBB2, FGFR1, and GATA3 []. Beyond genetic alterations, epigenetic changes, such as DNA methylation and modifications to histone proteins, significantly contribute to the initiation and development of breast cancer [,]. These epigenetic changes are reversible, making them highly appealing for therapeutic exploration. The role of estrogen in breast cancer development is substantial, as it strongly influences breast tissue during hormonal transitions such as adolescence, pregnancy, and the menstrual cycle. Estrogen enhances cell growth in breast tissue, thereby increasing the probability of genetic mutations that can initiate cancer. Furthermore, estrogen is particularly associated with driving the growth of cancers that rely on estrogen signaling, especially ER-positive breast cancer []. A crucial molecular player in breast cancer is human epidermal growth factor receptor-2 (HER2). HER2, a member of the tyrosine kinase receptor family, activates several intracellular pathways upon stimulation. These include the RAS pathway and PI3K-AKT-MAPK pathway, which collectively promote cell proliferation, survival, and the spread of cancer to other parts of the body []. The immune system also interacts with tumor microenvironment in complex ways. Initially, the immune system recognizes and attacks tumor cells by targeting neoantigens produced by mutations []. However, chronic exposure to these antigens leads to the activation of immune checkpoint molecules such as CTLA-4 and PD-L1, which suppress immune responses. This suppression allows immunosuppressive cells to accumulate at the tumor site, fostering an environment for tumor survival [,,].
3. Importance of Nanotechnology in Breast Cancer Treatment
The growing field of nanotechnology presents a promising outlook for diagnosing, imaging, and treating cancer [,]. Breast cancer, one of the significant or prevalent malignancies impacting women’s health worldwide, has particularly benefited from the advancements in nanotechnology. Nanotechnology has revolutionized the prior or premature detection and diagnosis of breast cancer microenvironment through the development of highly sensitive and specific nanoscale imaging agents [,,,]. NPs functionalized with targeting ligands, i.e., antibodies or peptides, can specifically bind to cancer biomarkers, enabling precise detection using various imaging techniques, i.e., positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), etc. []. Additionally, nanosensors capable of detecting cancer-related biomolecules in biological fluids offer non-invasive diagnostic approaches with high accuracy and sensitivity []. Theranostic NPs enable progressive imaging and treatment of breast cancer via integrating diagnostic and therapeutic functionalities or payloads in a single platform. By incorporating imaging agents (e.g., fluorescent dyes, contrast agents) and therapeutic payloads (e.g., chemotherapeutic drugs, small interfering RNA), theranostic nanomaterials facilitate personalized medicine by providing real-time monitoring of treatment response and disease progression [,].
One of the significant challenges in cancer treatment is achieving targeted drug delivery to tumor sites while minimizing systemic toxicity. NPs serve as versatile carriers for anticancer therapeutics, allowing for targeted delivery to breast tumors [,]. Surface modification of NPs with ligands specific to overexpressed receptors on cancer cells enhances their accumulation at the tumor site through active targeting mechanisms [,,]. NPs sensitive to stimuli can be specifically designed to release the therapeutics due to the changes in pH, temperature, or enzymatic activity. This “smart” stimuli-drug delivery technique results in a higher drug concentration at the tumor site, effectively reducing off-target effects and enhancing the therapeutic effectiveness of the anticancer agents [,]. Using a NP-based technique may avoid problems associated with macromolecules, i.e., lack of permeation, cell toxicity, lack of specificity, and high doses []. On the other hand, concerns associated with multidrug resistance (MDR) and P-glycoprotein (P-gp) efflux may potentially undergo certain modifications. By utilizing nanotherapeutic strategies, medical professionals can target cancer cells with unprecedented precision and accuracy, resulting in improved patient outcomes and a brighter outlook for those affected by this devastating disease. These include photothermal therapy (PTT) and photodynamic therapy (PDT), which utilize light-absorbing NPs to generate localized hyperthermia or reactive oxygen species (ROS), respectively, for tumor ablation []. Nanovaccines and immunomodulatory NPs are also promising immunotherapeutic solutions for breast cancer, further encouraging the immune system to identify and combat cancerous cells, resulting in precise and effective treatment options [].
4. Polysaccharides—A Multifunctional Polymer for Innovative Nanoparticle Design
Polysaccharides constitute a vital class of hydrophilic polymers originating from natural sources, widely recognized for their intrinsic biocompatibility and functional versatility. Their extensive integration into water-based polymer systems and nanotechnology arises from their remarkable attributes, including biodegradability, non-toxicity, and compatibility with biological systems []. These characteristics are critical for the effective application of NPs, positioning polysaccharides as an optimal category of materials for NP development. Polysaccharide-based NPs exhibit significant advantages, such as enhanced drug loading capacity, rapid drug release profiles, precise targeting capabilities, high stability, and minimal toxicity under physiological conditions. These properties not only enhance their suitability for drug delivery but also underscore their relevance in environmentally sustainable and advanced technological innovations []. Furthermore, beyond their inherent biodegradability and biocompatibility, polysaccharides have garnered substantial interest due to their natural abundance, ease of processing, and availability from renewable resources, demonstrating their role as a sustainable choice for diverse biomedical and tissue engineering applications [,]. Furthermore, polysaccharides undergo chemical functionalization primarily through the activation of their inherent carboxyl and hydroxyl groups, which are distributed along their structural backbone. By leveraging the reactivity of these functional groups, it becomes possible to materialize the polysaccharide derivatives with precisely controlled characteristics, such as reduced hydrophobicity or improved solubility []. This targeted modification significantly enhances the versatility and applicability of polysaccharides, particularly in the development of NP-based drug delivery systems, including those specifically designed for cancer therapy [,,].
5. Polysaccharide-Based Nanoparticles for Breast Cancer Therapy
5.1. Chitosan
CS, also known as chitin, is a polymeric structure that consists of N-acetyl-D-glucosamine and D-glucosamine units of the polysaccharide category []. Polyionic polysaccharides include several desirable characteristics, i.e., biodegradability, biocompatibility, enhanced cell membrane permeability, and cellular absorption, which further contribute to various biomedical applications. CSNPs possess mucoadhesive properties that enable them to encapsulate or coat therapeutic substances on their surface, thereby controlling the therapeutic or drug delivery to the targeted site. The CS nanocarrier releases the drug when it becomes protonated at a pKa value of 6.4. Cancer cells exhibit a lower pH of 5, which causes the drug to adhere to the negatively charged mucosal surface through electrostatic contact. This results in an improved binding of the drug to the target cells, making it more effective for cancer treatment [,]. The anti-metastatic effectiveness of the CSNPs can be attributed to their capability to permeate epithelial cells, resulting in augmented drug permeability and subsequent disruption of tight junctions at the molecular level [,,,].
The premature detection and instantaneous treatment of breast cancer are strongly recommended using advancement of NPs for therapies and diagnosis of breast cancer. On this note, Ekinci et al. developed methotrexate (MTX) and Tc [99mTc] radionuclide-loaded CSNPs via an ionotropic gelation method for characterizing the potential efficacy and biodistribution of the NPs in breast cancer treatment. The investigation demonstrated that the MTX-CSNPs exhibited a mean hydrodynamic diameter ranging from 148 to 157 nm, a significant polydispersity index (PDI) value (from 0.415 to 0.451), zeta potential (from 15.7 to 18.7 mV), with an MTX % of encapsulation efficiency (%EE) ranging from 64% to 70%. The Tc [99mTc]-MTX-CSNPs exhibited a high labeling capacity (99%) and demonstrated stability up to 6 h at room temperature. The optimized NP exhibited a significant absorption into the 4T1 cancer cell line compared to R/H-[99mTc] Tc. The in vivo result obtained using BALB/c mice exhibited that the cancer cells shown a more significant absorption of Tc [99mTc]-MTX-CSNPs compared to Tc [99mTc] alone. Thus, this study further furnished additional evidence advocating the diagnostic and therapeutic potential treatment of breast cancer using CS-based NPs [].
Several drug delivery scientists have expressed significant attraction to nanotherapeutic delivery systems based on essential oils conjugated with polysaccharide-based polymeric systems, primarily due to their synergistic anti-inflammatory and pro-angiogenic properties for breast cancer. To harness the challenges of conventional therapeutics or delivery systems, Xu et al. assessed the anticancer potential of essential oil derived from Cinnamomum cassia (CEO) encapsulated in CSNPs (CS-CEO). The study determined that the spherical and uniform CS-CEO NPs exhibited a smaller particle size (215.40 ± 3.90 nm), zeta potential (51.70 ± 1.90 mV), higher %EE (83.37 ± 0.4%), drug loading capacity (26.42 ± 0.65%), and demonstrated high stability at 4 °C temperature. The in vitro anticancer efficacy of CS-CEO NPs was validated by the higher expression of Caspase-3 and AIF protein at a concentration of 52 μg/mL. The researchers additionally documented the suppression of tumor cell proliferation, apoptosis, and other related processes in 4T1 breast cancer cells. Therefore, this finding furnishes evidence for the CEO’s physical stability and effective encapsulation within CSNPs, which can be demonstrated as a significant phytomedicine for treating breast cancer, as documented in traditional medicine [].
The transportation of restoratives or drugs to cancer cells possess significant challenges, mainly due to the constraints related to physicochemical attributes (i.e., low %EE, poor stability, rapid degradation, and bio-distribution) of the delivery system. In order to address these constraints, Rajaei et al. developed 5-fluorouracil (5-FU) loaded CS/agarose/graphene oxide (CS/AG/GO)-based hydrogel system via a double emulsification technique (i.e., w/o/w) for the breast cancer treatment. The resultant nanocomposite exhibited several desirable properties after double emulsification, including an average nanosized particle size of 197 nm, PDI value (i.e., 0.34), high zeta potential (+23.5 mV), %EE (97%), and drug loading capacity (57%). The investigators additionally documented the continuous release of 5-FU at a pH of 5.4 over 48 h and demonstrated the efficient cytotoxicity of CS/AG/GO/5-FU against the MCF-7 cell line, resulting in a 23% reduction in cell viability. These findings further concluded that CS/AG/GO-based hydrogel acted as a pH-sensitive drug delivery system and further enhanced the in vitro delivery efficiency of 5-FU against breast cancer [].
In the field of cancer treatment and diagnosis, CSNPs have received significant consideration due to their diverse biological and physicochemical features, i.e., glycol-based CSNPs exhibited low immunogenicity, minimal or no toxicity in living organisms, and substantial biodegradability and biocompatibility. However, there are no significant investigations available on the dose-dependent study of these NPs. In this context, Chang et al. synthesized glycol CS conjugated with 5β-cholanic acid, which exhibited NPs with uniform size distributions (i.e., 265.36 to 288.3 nm) under aqueous conditions. In a dose and time-dependent rationale, the researchers observed a notable increase in cellular uptake in the 4T1 and H9C2 cardiomyocyte cell lines compared to macrophages and fibroblast cell lines. This absorption resulted in severe cell necrosis under conditions that were clinically relevant and highly concentrated. The in vivo intravenous administration of glycol-based CSNPs (90 mg/kg) to healthy mice exhibited significant biodistribution or accumulation in the major organs, i.e., heart, after 6 h and remained located for a sustained period of 72 h. This study found that the repeated administration of high doses of glycol-based CSNPs can lead to severe damage to the heart, including inflammation, tissue necrosis, fibrotic changes, and impaired organ function. The findings demonstrate that CSNPs should be utilized with caution in therapeutic environments, and further, the toxicological framework demonstrated the safe integration of CSNPs into breast cancer treatments [].
Several investigations reported that HA exhibited a high affinity for cell surface receptors, i.e., CD44 has emerged as a prevalent ligand to coat CSNPs to target breast cancer via facilitating the access of NPs into cells and enhancing the concentration of medicine within cancer cells via endocytosis [,]. Therefore, Meylina et al. strive to develop HA coated CSNPs to deliver Alpha Mangostin (AM) (AM-CS/HA) specifically to the MCF-7 cell line. The AM-CS/HA exhibited a spherical shape, average diameter (i.e., 304 nm), PDI value (i.e., 0.3), positive zeta potential (i.e., +24.43 mV), %EE (90%), and drug loading (8.5%). The AM-CS/HA exhibited a continuous release of AM at an acidic pH (5.5), surpassing the physiological pH (7.4) and beyond the release observed in an acidic pH of 5.5. The cytotoxicity of AM-CS/HA on MCF-7 cells demonstrated significantly higher (at 4.37 g/mL) compared to AM NPs without HA coating (AM-CS; 4.48 g/mL) and non-coated AM (5.27 g/mL). The AM-CS/HA system enhances the ability of AM to kill cancer cells and could be useful in treating breast cancer. The study suggested that the targeted delivery of AM via NPs has the potential to be a practical therapeutic approach for breast cancer treatment. However, additional research is necessary to assess the bioavailability, toxicity, and anticancer efficacy of AM-loaded CSNPs coated with HA using in vivo introspections [].
In a recent year, there are multiple investigations reported on CS-decorated or based nanoparticulate systems, i.e., 4-carboxy phenyl boronic acid (4-CPBA)/Palbociclib (PALB)/CS lipid NPs [], CS/Piperine (PIP)/metal–organic framework (MOF) [PIP@MIL-100(Fe)] [], CS/Bismuth oxide NPs/Curcumin (CUR)/5-aminolevulinic acid nanocomposite [], Folate-functionalized carboxymethyl CS (CMCS)/calcium phosphate (CF/CaP) NPs [], CS/ZnO NPs/Cisplatin nanoconjugates [], Mesoporous silica/CS/Polyethylene glycol-MUC1 aptamer [], 5-FU/CS-bimetallic (Au/Pd) NPs [], etc., have shown substantial potentiality due to favorable characteristics (as mentioned earlier), and binding affinity to the receptors in breast cancer therapy.
5.2. Alginate
Alginate, a copolymeric structure, is derived from the combination of L-guluronic acid (G) and D-mannuronic acid (M), further created MG blocks. Alginate is commonly obtained from marine algae and other plant species, including Laminaria digitata, Laminaria saccharina, and Macrocystis pyrifera, and retains a gelling capability that confers both mechanical strength and flexibility, enabling it to withstand moisture present at the drug delivery application site effectively []. Alginate NPs provide enhanced drug loading and target-based delivery capabilities, positively reasonable for application in cancer treatment. In the past year, Ahamady et al. materialized an optimized system that incorporated capsaicin-loaded alginate NPs within polycaprolactone-CS nanofiber mats. The alginate NPs further demonstrated significant capsaicin loading, particle size (19.42 nm), and higher %EE (98.7%). The study reported an electrospinning technique to fabricate polycaprolactone-CS nanofibers with varying ratios (100:0, 80:20, and 60:40), and further in vitro characterization on MCF-7 human breast cells provided additional evidence supporting the efficacy of the NPs in inhibiting the proliferation of cancer cells compared to human fibroblast cells. Therefore, this preliminary investigation further provides optimism for the regulation of capsaicin release for more than 120 to 500 h using alginate NPs, which could prove to be efficacious if further examined in vivo and clinical settings in the context of breast cancer treatment [].
Exemestane (EXE) is a popular and conventional oral medication utilized to treat breast cancer. However, its side effects and reduced bioavailability are causes for concern. As a practicable solution, Jayapal et al. suggested combining alginate NPs with EXE via gelation technique, which can potentially lessen side effects, regulate drug release, and enhance the interaction between the drug and its target receptors. The research findings yielded a particle size of 197 nm, a higher Zeta potential (−18.3 mV), an approximate %EE (i.e., 98%), and a drug release duration of up to 7 h. The study demonstrated that the cytotoxicity analysis on Dalton’s lymphoma ascites cells (DLA) indicated that the alginate NPs loaded with different concentrations (i.e., 10 to 200 µg/mL) of EXE exhibited the highest inhibitory effect on the cancerous cells. Therefore, it has been demonstrated that alginate NPs serve as effective nanocarriers for loading EXE anticancer medication, resulting in prolonged release in alkaline conditions (pH 7.4). Thus, this in vitro study further proves the efficacy and improved target-receptor binding capacity of alginate NPs-loaded therapeutics for breast cancer treatment [].
Mastectomy is the most effective treatment for breast cancer, as demonstrated clinically. However, it comes with significant challenges, including a high rate of tumor recurrence and the adverse effects of chemotherapy. Hence, it is imperative to develop a therapeutic approach that may efficiently facilitate the process of postoperative wound healing and impede the recurrence of local tumors. Therefore, Su et al. strived to construct a local drug delivery platform called CCNACA, which utilizes carbon dots-CUR nano-drug release (CCNPs) as a 3D printing scaffold. The platform was constructed utilizing CCNPs, sodium alginate, nanoclays, and caffeic acid grafted CS as raw materials. The primary objective of this platform was to enable the visualization of the % cumulative release rate. The in vitro drug release study conducted over 14 days showed that the CCNACA scaffolds exhibited a tumor inhibition rate of 73.77% ± 1.68% on breast cancer cells (MCF-7). Furthermore, the authors demonstrated that introducing CCNACA scaffolds into surgical incisions can effectively suppress remaining cancer cells after surgery, diminish inflammation, stimulate the growth of new blood vessels, and restore damaged tissues resulting from surgical procedures. Thus, the investigation further proposed a local therapeutic delivery system that has significant promise in the realms of wound healing and tumor recurrence prevention after breast cancer surgery [].
The current research focuses on advancing inorganic NPs-based biomedicine for anticancer therapy due to their cellular absorption significantly influencing the efficacy of inorganic NPs as anticancer agent. In this scenario, the cellular uptake of inorganic NPs is contingent upon various physical and chemical characteristics, i.e., dimensions, morphology, surface modification, passive diffusion, and endocytosis of the NPs. Recently, Karthikeyan et al. investigated inorganic SnO2 (tin dioxide) and sodium alginate (SA) into SnO2 (SASnO2) using an one-pot green technique, which further possesses superior antibacterial, antioxidant, and anticancer characteristics compared to SnO2 NPs. The study reported that the in vitro MCF-7 cells exhibited greater cellular uptake of SASnO2 NPs (19 nm) than SnO2 NPs (38 nm). Therefore, this contemporary investigation further concluded that the increased surface area of these SASnO2 NPs facilitates more significant interaction with biological membranes and internalization by cancer cells, leading to improved efficacy in breast cancer therapy [].
The utilization of magnetic drug targeting has surfaced as a viable approach for the efficient delivery of phytochemicals in the context of breast cancer. Therefore, Bulatao et al. investigated the cytotoxicity of lutein (LUT) loaded CS/alginate iron oxide NPs (LUT-CS/Alg-Fe3O4-NPs) against breast cancer cells, further optimized via Box–Behnken design considering various dependent variables. The study reported that the optimized LUT-CS/Alg-Fe3O4-NPs demonstrated biocompatibility and exhibited a notable increase in cytotoxicity towards MCF-7 cells when exposed to a permanent magnet. The produced NPs were confirmed to be superparamagnetic based on their remanent magnetization and minimal magnetic coercivity. Furthermore, the increase in magnetic activity was found to be four times greater than that of free LUT. Therefore, these findings imply that LUT-CS/Alg-Fe3O4-NPs possessed significant effectiveness for magnetically targeted delivery systems for effective therapy of breast cancer [].
The three-dimensional (3D) hydrogel structure is fascinating in terms of various physicochemical and mechanical properties. In this context, Ziaei et al. modulated a pH-sensitive in situ structure using oxidized alginate and gelatin-containing doxorubicin (DOX) loaded CS/gold NPs (CS/AuNPs)-based nanogels. The study resulted in satisfactory size distribution (209 nm), positive zeta potential (+19 mV), and higher %EE (72.6%) of DOX. The viscoelastic properties of hydrogels exceed the viscous properties of all hydrogels across the specified frequency range. Further analysis of the mechanical and textural properties demonstrated that β-GP and CS/AuNPs nanogel displayed exceptional mechanical properties. The results of the MTT analysis indicate that the hydrogels exhibited cytocompatibility with MCF-7 cells. The Live/Dead cell assay further revealed that the grown cells on hydrogels without DOX exhibited a high degree of viability when exposed to CS/AuNPs nanogels. The findings from the study indicated that both the hydrogel-encapsulated medication and unbound DOX at equivalent concentrations were able to induce significant apoptosis in the MCF-7 cell line. This suggests that the hydrogels developed in the study have a promising potential for localized breast cancer therapy [].
In past few years, several investigations reported, i.e., MTX/alginate NPs [], Iron oxide/sodium alginate/thymoquinone nanocomposites [], Chitosan/alginate-based platelet/DOX nanoconjugates [], Capsaicin/alginate NPs/polycaprolactone-chitosan nanofibers [], Cisplatin/DOX/niosomal alginate nanocarrier [], Alginate-coated gold nanorods (AuNRs) [], Gelatin/alginate/DOX-loaded niosomal (Nio-DOX@GT-AL) nanocomposite [], Alginate/chitosan/Molybdenum nanocomposite [], Turmeric/alginate NPs [], Metformin/CUR/chitosan/alginate NPs [], etc., have shown substantial efficacy against breast cancer cells. However, the bioavailability and pharmacokinetic parameters of multi-drug treatment in breast cancer have not been explored in vivo models.
5.3. Hyaluronic Acid
HA is a biological polysaccharide comprising N-acetyl-D-glucosamine and D-glucuronic acid and has been demonstrated to be biocompatible, biodegradable, non-immunogenic, non-toxic, and can target CD44 receptor overexpression in tumor cells. Due to these properties, HA possess potential effectiveness for biomedical and pharmaceutical applications. HA has improved drug retention time and delivery profiles when incorporated into developing nano-sized drug delivery systems (DDSs) [].
In a recent investigation, Omar et al. developed α1-acid glycoprotein (AGP)-conjugated HA (AGP-HA NPs) to modulate the responsiveness of breast cancer towards chemotherapy and inhibit the spread of breast tumors. The outcome of in vitro experiments conducted on MCF-7 cells demonstrated the significant efficacy of AGP-HA NPs in inhibiting of tumor migration by 79% up to 24 h. The study showed that AGP-HA NPs effectively regulated MDA-MB-231 cells and restored their sensitivity to the chemotherapeutic drug, i.e., DOX. Additionally, the results obtained using flow cytometry and confocal spectroscopy revealed that the AGP-HA NPs exhibited an increased capacity for DOX absorption and retention, facilitating its delivery to the cell nucleus within a 4 h of incubation period. Therefore, the investigation concluded that HA-based NPs shown a promising and efficacious therapeutic alternative for enhancing anticancer properties of chemotherapeutic molecules, and may potentially enhance the survival rate if investigated in vivo [].
Recently, the combination of chemotherapeutic agents has demonstrated greater effectiveness in treating multi-drug resistance cancer (MDRC). Thus, in order to target the MDRC treatment, Thummarati et al. designed dual pH-responsive NPs composed of gemcitabine (GEM) and CUR onto HA using a solvent diffusion method. The conjugates developed based on ester and hydrazone bonds, leading to the materialization of GeChH (random graft) and (GhC)hH (block graft). The study demonstrated that average particle size (221 nm), narrower PDI value (0.01), and release profile of GEM and CUR from the nanoconjugates exhibited faster drug release in the acidic microenvironment, whereas controlled at physiological pH (i.e., 7.4), suggesting pH-dependent behavior. Furthermore, the cytotoxicity study of GhChH NPs exhibited more significant cytotoxicity and a synergistic effect in various cell lines (i.e., Caco-2, HCT116, PANC-1, and A549) compared to GeChH NPs and GEM/CUR suspension. In addition, the % of uptake of the (GhC)hHFITC NPs demonstrated higher values or numbers compared to GeChHFITC NPs in A549 and HCT116 cell lines. By employing an HA blockade, the NP uptake percentage experienced a notable decrease, indicating the pivotal role of CD44 receptor targeting by HA in enhancing the specificity of the cancer cells. Thus, these newly developed NPs can potentially deliver both GEM and CUR in a pH-responsive manner, which could be advantageous for treating breast cancer [].
In recent times, a substantial body of research has emerged, demonstrating a strong correlation between breast cancer and vitamin D3 (VD3). Nevertheless, there have been limited investigations into the development of VD3-loaded delivery approaches targeting breast cancer. Therefore, Gao et al. characterized the conjugation of VD3 and HA (HA-VD) to effectively target breast cancer by facilitating the administration of DOX. The developed formulation further confirmed the % degree of substitution of 18.6%, whereas, at the molar concentration ratio of 1:2, the formulations possessed 0.0137 mg/mL of critical micelle concentration. The HA-VD micelles loaded with DOX (DOX/HA-VD) shown 6.2% of drug loading, with an %EE of 79.5%, and further demonstrated as viable or stable in blood for 48 h. The % cumulative release of DOX exhibited a reduction following encapsulation compared to the release rate seen in the absence of HA-VD. The decrease in IC50 of the DOX/HA-VD micelle showed a statistically significant difference compared to free DOX and blank micelles. VD3 and DOX had a synergistic effect on BC cells at a cell inhibition rate of 50%. Therefore, the utilization of HA-VD micelles has been shown to significantly enhance the therapeutic efficacy of DOX in targeting breast cancer [].
In another investigation, Zeng et al. highlighted the synergistic effects of dasatinib (DAS) and olaparib (OLA) on triple-negative breast cancer (TNBC) via pH-sensitive ester bonds to establish a connection between DAS and HA (HA-DAS). Subsequently, the researchers demonstrated that the incorporation of OLA and HA-DAS served as the carrier for synthesizing nanomicelles (HDO-NPs). The physicochemical characterization study indicated that HDO-NPs exhibited a spherical shape, monodispersed particle size, excellent stability, etc. Furthermore, cellular investigations demonstrated that the HDO-NPs were efficiently absorbed by attaching to the CD44 protein overexpression of MDA-MB-231 cells, leading to a higher drug concentration within the cells. The results of the in vivo investigation exhibited that the HDO-NPs exhibit efficient targeting of tumor tissues and remarkable therapeutic efficacy against tumors. Additionally, these NPs greatly extend the in vivo circulation period of pharmaceuticals and successfully enhance drug bioavailability, therefore exhibiting a promising potential for utilization in the therapeutic management of TNBC [].
Several substantial investigations on HA-based NPs in laboratory settings, i.e., HA/poly(methacrylic acid)/mesoporous silica nanocomposite [], Chemodynamic therapy (CDT) agent (HA-Fc-Mal)/HA NPs [], HA/copper sulfide NPs/diethyldithiocarbamate (DDTC)/losartan nanocomposite [], HA/manganese dioxide (HMnO2)/enzalutamide/MK-8776 nanoconjugates [], Dopamine-reduced GO nanomaterials/HA/DOX NPs [], HA modified chitosan/chebulinic acid NPs [], HA-coated berberine (BBR)/indocyanine NPs [], etc., have been documented that the HA-based NPs loaded several therapeutic compounds possess improved anticancer potentiality in breast cancer treatment.
5.4. Dextran
Several NPs based on dextran exhibit potential carriers or drug delivery systems for treating breast cancer owing to various favorable attributes, including biocompatibility, biodegradability, and a high surface area-to-volume ratio. These characteristics enable the improved loading of therapeutic agents, i.e., chemotherapeutic drugs, peptides, or nucleic acids. Dextran NPs can permeate through blood vessels and inadequate lymphatic drainage typical of solid tumors. This permits to accumulate in the tumor tissue through the improved permeability and retention capability consequence. Dextran NPs provide a flexible platform for surface modification, facilitating the integration of imaging agents for diagnostic applications or therapeutic agents for combination therapy. Furthermore, it is worth noting that dextran NPs often elicit limited immunological responses, hence decreasing the probability of unfavorable reactions or immune-mediated elimination following their administration.
The emergence of TNBC has revealed numerous therapeutic targets, indicating that a single target for the treatment may be unsuccessful, and has further emphasized the necessity to innovate substantial technologies for combination therapy. Paclitaxel (PTX), widely recognized as a groundbreaking development in the field of chemotherapy, encounters various challenges stemming from its limited solubility and permeability. The significance of interferons in cancer has been elucidated by ongoing scientific advancements. Therefore, Bakrania et al. developed DEAE-Dextran-loaded PTX NPs to induce interferon in TNBC treatment. The study exhibited significant synergistic cytotoxicity across different cell lines and further suppressed ROS by inducing β-interferon, countering ROS activation by PTX. The nanoformulation was additionally linked to FITC for internalization investigations. These tests revealed that the highest level of cellular uptake occurred 60 min after treatment with FITC, which illuminated the nuclear membrane. The in vivo xenograft model was developed to examine the mechanistic understanding of nuclear-targeted nanoformulation and demonstrated a synergistic release of β-interferon at the targeted site. Furthermore, the combinatorial nanoformulation induced various mechanisms by inhibiting VEGF and NOTCH1 and by overexpressing both β and β-interferon. Thus, the combination therapy shows potential as a versatile nanomaterial for delivering drugs into the nucleus of TNBC [].
Dextran NPs provide a flexible platform for surface modification, facilitating the integration of imaging agents for diagnostic applications or therapeutic agents for combination therapy. To utilize this advantage, Wang et al. developed carboxymethyl dextran (CMD) loaded PTX/docosahexaenoic acid (DHA) for the conjugation, which resulted in CMD-DHA-PTX-(conjugate S, and conjugate L). The study resulted in conjugates (i.e., Gly Gly and Lys-Gly Gly) exhibiting elevated PTX loading capacity, improved water solubility, and the capacity for self-assembly into NPs (88.7 nm to 94.7 nm), further exhibiting continuous release of PTX in blood and tumor cells. In addition, conjugate S demonstrated enhanced pharmacokinetic characteristics and more excellent tumor site distribution compared to the original PTX, conjugate L, and abraxane. The two conjugates demonstrated superior anti-tumor effectiveness compared to the leading PTX formulation and abraxane in MCF-7-induced nude mice. The administration of conjugate S therapy resulted in the complete eradication of xenograft tumors without inducing any reduction in body weight. This study suggested that the materialization of dextran-based dual-chemotherapeutic conjugates shows excellent potential and innovation in delivering anticancer molecules to various types of tumors [].
Similarly, Li et al. investigated the potential of cisplatin-loaded LHRH-modified dextran NPs (Dex-SA-CDDP-LHRH) to effectively suppress primary tumor development and metastasis. The study demonstrated that Dex-SA-CDDP-LHRH NPs effectively controlled the targeting ability of LHRH and selectively affixed to the LHRH receptors. The Dex-SA-CDDP-LHRH NPs exhibited superior cellular absorption and heightened cytotoxicity compared to the non-targeted Dex-SA-CDDP NPs. In addition, using non-targeted and targeted NPs led to a significant decrease in the overall toxicity of CDDP and an increase in the tolerated dose of CDDP (4 to 30 mg·kg−1). The treatment of Dex-SA-CDDP-LHRH led to a substantial augmentation in the accumulation of CDDP within both the primary tumor and the organs harboring metastases, resulting in a significant decrease in the nephrotoxic impacts of CDDP. Therefore, the CDDP/LHRH-decorated NPs exhibited a notable improvement in anticancer and anti-metastasis effectiveness compared to the non-targeted NPs, as evidenced by dose-dependent therapeutic effects. The findings of this study indicate that Dex-SA-CDDP-LHRH NPs exhibit significant promise in targeted chemotherapy for metastatic breast cancer [].
The presence of drug resistance in cancer treatment significantly hampers the efficacy of chemotherapeutic drugs, resulting in unfavorable clinical results. The majority of chemotherapeutic agents can stimulate autophagy, enhancing tumor resistance to these medicines and diminishing the efficacy of drug transport to tumor cells. In another investigation, Li et al. developed a nanocomposite composed of carboxymethyl β-dextran (CMD)/protamine sulfate (PS) to facilitate the transportation of docetaxel (DTX), chloroquine (CQ), and Atg5 siRNA within cancerous cells. The study demonstrated that the electrostatic interaction drives the CQ + DTX + Atg5 siRNA NPs, further resulting in the encapsulation of CQ and Atg5 siRNA in NPs, which can augment the responsiveness of tumor cells to DTX. The NPs demonstrated significant therapeutic efficacy in TNBC and excellent biosafety. Hence, this novel multifunctional nanoplatform was developed utilizing CMD and PS, which effectively boosts the sensitivity of chemotherapeutic drugs by inhibiting autophagy. This approach presents promising prospects for addressing the issue of conventional drug resistance and improving therapeutic efficacy in TNBC [].
Several investigations further reported, i.e., Fe3O4 NPs/dextran (Fe3O4@Dex)/glucosamine [], CMD/thienopyridine derivative [], Streptavidin (SA)-functionalized CMD/melamine NPs (MNPs) [], Dextran/iron oxide NPs (SDIO) [], SPIONs/CMD/triptorelin (SPION@CMD@triptorelin) nanoconjugate [], CUR/poly (lactic-co-glycolic) acid/chitosan/dextran/PEG nanoemulsion [], CMD/trimethyl chitosan (TMC) NPs/NIK/STAT3-specific siRNA/BV6 [], Indomethacin (IND)-grafted dextran/DOX nanomicelles [], etc., utilization of dextran in conjugate or grafting agent to improve the bioavailability and pharmacokinetics properties of several nanoconjugates for the treatment of breast cancer.
5.5. Cellulose
Cellulose, a polysaccharide that occurs naturally in plants, exhibits biocompatibility and high tolerability towards cellulose NPs inside biological systems. Cellulose NPs can decompose naturally, exhibit minimal immune response, and can be functionalized with specific ligands or antibodies that specifically attach to excessively present receptors on breast cancer cells. Cellulose NPs possess physicochemical features, including size, surface chemistry, and shape, which can be readily customized using a range of production and modification methodologies. The adaptability of NPs enables them to be tailored to individual needs for precise medication administration, imaging, or other therapeutic uses in breast cancer treatment.
In a recent year, Karimi et al. investigated gefitinib-loaded cellulose acetate butyrate NPs (Gnb-NPs), further loaded into CS/β-glycerophosphate thermo-responsive hydrogels via the solvent evaporation method for intratumoral delivery in breast cancer-induced animals. The study reported that significant spherical particle size (156.50 ± 2.40 nm), zeta potential (−4.90 ± 0.04 mV), %EE (99.77 ± 0.09%), swelling, gelling time, injectability, and release behavior of the formulated Gnb-NPs-Hydrogel were further evaluated for anticancer activity on the 4T1 cell line. The cytotoxic effect of Gnb-NPs-hydrogel was reported to be more pronounced compared to free Gnb and Gnb-hydrogel. Moreover, administering Gnb-NPs-Hydrogel through intratumor injection demonstrated the highest effectiveness against tumors in living organisms. Thus, the Gnb-NPs-hydrogel demonstrated a significant potential effect as a viable breast cancer therapy option [].
Generally, the chemotherapeutic agents utilized in the treatment of cancer exhibit limited selectivity towards both normal and cancerous cells, thereby exacerbating the associated cytotoxic effects. The key to resolving this problem lies in efficiently encapsulating anticancer drugs; therefore, Fawal et al. addressed the low solubility and selectivity of disulfiram (DS) via loading into cellulose acetate (CA)/poly (ethylene oxide) (PEO) matrices (DS@CA/PEO) via the electrospinning technique for targeting breast cancer. The study reported that fabrication of DS into the CA/PEO scaffold exhibited superior compatibility compared to the DS-free scaffold when tested on normal human cells (Wi-38). Simultaneously, the DS-free scaffold showed comparable anticancer activity against the Caco-2 and MDA-MB-231 cell lines. The greater affinity of DS@CA/PEO for cancer cells compared to normal cells was linked to the preservation of apoptotic activity and the ability of DS to inhibit aldehyde dehydrogenase. This effectiveness resulted in eliminating stem cells from colon and breast tumors, as demonstrated by flow cytometry. Therefore, this investigation examined the potential of the dual-responsive DS@CA/PEO nanofiber scaffold for specific anticancer properties for breast cancer treatment [].
Breast cancer treatment regimens have transitioned from single agents to combinatorial approaches, providing combined effectiveness and minimizing adverse effects. An extended platform for tissue-specific co-delivery can be provided by self-assembled nanogels that consist of natural polysaccharides and functional proteins. In a recent investigation, Attalah et al. developed lactoferrin (Lf)/CMC-based nanogels loaded with pemetrexed (PMT)/honokiol (HK). The produced Lf-CMC NGs were utilized to encapsulate an inclusion complex consisting of HK and hydroxypropyl-β-cyclodextrin, resulting in a %EE of 66.67%. The cross-linked Lf-CMC NPs demonstrated an average hydrodynamic diameter (193.4 nm) and a negative surface charge (−34.5 mV). The MDA-MB-231 cells effectively absorbed PMT/HK-loaded Lf-CMC NGs, which further exhibited enhanced cytotoxicity. This was evidenced by a low combination index value and a more extensive dose reduction index compared to the free drugs. A study on a mouse model of Ehrlich ascites tumor (EAT) demonstrated the substantial effectiveness of PMT/HK-Lf-CMC NPs in preventing tumor expansion. This was attributed to the improved caspase-3 expression and decreased VEGF-1 and Ki-67 protein expression levels in the cells [].
A recent study by Salahuddin et al. investigated the antibacterial and cytotoxic effects of methylene blue (MB) loaded extracted cellulose, explicitly targeting the MCF-7 cells. The microscopic images of cellulose obtained through acid hydrolysis exhibited a filamentous structure with a diameter ranging from 30 to 50 nm and a length spanning or fragmentation from 270 to 1290 nm. The drug release study reported 52% of MB release at pH 7.4 up to 1400 h, whereas the changes in the pH (i.e., 5.4 and 6.7) led to lesser or decreased % of release (45% and 42%, respectively). The cytotoxicity assay of MB/cellulose NPs against MCF-7 exhibited % of cell viability decreased from 52.4% to 6.9% due to an increase in the concentration of MB from 12.5 to 100 mg/mL. The antibacterial efficacy of cellulose-loaded MB was assessed against a wide range of bacteria, demonstrating its efficacy against all tested bacterial species [].
In addition, the recent years further reported several investigations on cellulose-based NPs, i.e., Lignin/MTX/iron oxide NPs [], Gefitinib/cellulose acetate butyrate NPs (Gnb-NPs) [], Cellulose nanocrystals (CNCs)/poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol) (PCLA) [], 5-FU/MgO/methyl cellulose NPs [], Silver NPs/Ethyl cellulose (AgNPs-EC) NPs [], SF/cellulose acetate/gold-silver NPs (CA/SF/Au-Ag) composite nanofiber [], Nano cellulose/CUR [], CUR/polyvinyl alcohol/cellulose nanocrystals (PVA/CNCs) [], etc., have been extensively investigated in various models for breast cancer therapy.
5.6. Starch
Starch-based nanomaterials are currently prominent candidates in the field of biomedical research, offering a broad spectrum of potential applications in the area of targeted drug delivery and inhibition of tumor growth. Starch-based NPs possess extensive adaptability, effectively encapsulating a wide range of therapeutic agents. Furthermore, the utilization of ligand-functionalized NPs in starch-based nano-drug delivery systems holds significant promise for cancer therapy. In this study, Mariadoss et al. developed a novel approach to treat TNBC by synthesizing aptamer-laden starch NPs (Apt-p-CA-AStNPs) loaded with p-coumaric acid (p-CA). The investigation utilized the FT-IR technique to validate the functional groups linked to p-CA and amino starch (AS) in p-CA-AStNPs, followed by gel electrophoresis. The results indicated Apt-p-CA-AStNPs NPs exhibited an average zeta potential (29.2 ± 1.35 mV), particle size (218.97 ± 3.07), PDI value (0.299 ± 0.050), and %EE and loading capacity of 80.30 ± 0.53%, and 10.35 ± 0.85%, respectively. The Apt-p-CA-AStNPs demonstrated a burst release within a 5 h period of the experiment under the pH of 5.4. The Apt-p-CA-AStNPs effectively suppressed the apoptosis-related proteins in the MDA-MB-231 cell line by regulating ROS, mitochondrial membrane potential, and DNA damage []. Conjugated bio-nanomaterials, including magnetic cores, have emerged as a promising platform for developing advanced biological composites for breast cancer therapy. To utilize the characteristics of such biomaterials loaded magnetic NPs, He et al. developed dual core–shell NP-loaded Ag NPs functionalized with CS-starch composite. The MTT assay of breast cancer cells treated with Fe3O4@CS-Starch/Ag nanocomposite showed cytotoxicity at IC50 concentration. In the Fe3O4@CS-Starch/Ag nanocomposite, the % viability of breast cancer cells exhibited a dose-dependent reduction, further exhibiting different IC50 values of 183, 194, 207, 279, and 252 µg/mL against multiple cell lines (i.e., Hs 578Bst, UACC-3133, Hs 319 T, UACC-732, and MDA-MB-453, respectively) [].
In general, cancer stem cells (CSCs) exhibited self-regeneration, differentiation, and tumor initiation, which are widely acknowledged as the primary cause of therapy resistance, metastasis, and regression. Concurrently eliminating CSCs and large cancer cells is essential for effective cancer treatment. Thus, Xu et al. utilized hydroxyethyl starch-polycaprolactone NPs (DEPH NPs) co-loaded with Dox/erastin for the elimination of CSCs and cancer cells via the regulation of ROS. This resulted in a highly synergistic impact in a combination of Dox and erastin simultaneously administered by DEPH NPs. Erastin can potentially reduce intracellular glutathione (GSH) levels, which might hinder the movement of intracellular Dox and enhance the production of ROS generated by Dox. The elevated amounts of ROS inhibited the self-renewal of CSCs via downregulation of the hedgehog pathway, facilitated the differentiation of CSCs, and made differentiated cancer cells susceptible to necrosis. In addition, DEPH NPs demonstrated notable efficacy in eradicating cancer cells, mainly CSCs, hence playing a crucial role in inhibiting tumor growth, tumor-initiating potential, and metastasis in TNBC. Therefore, this study proves that combining Dox and erastin is significantly effective in eradicating cancer cells and CSCs, showing potential as a treatment for solid tumors with CSCs [].
There has been significant interest in utilizing prodrug-based nanomedicines that exhibit superior drug loading capacity and tumor targeting at lower doses for cancer treatment, especially for solid tumors, i.e., TNBC. Nevertheless, the anticancer potential of prodrug nanomedicines is significantly hindered by the complex tumor mechanical microenvironment (TMME), which hinders drug delivery and promotes the proliferation of CSCs. In a recent year, Wang et al. developed carbamate disulfide-bridged DOX dimeric/IR780/hydroxyethyl starch-folic acid conjugates to function for theragnostic purposes to target TNBC and achieve drug release, resulting in high GSH concentration in CSCs. The study reported the increased sensitivity of chemotherapy, mediated by FDINs, which further plays an important role in regulating TMME, depleting extracellular matrix proteins. In addition, FDINs effectively eradicate CSCs by challenging the distinct niche of CSCs and depleting intracellular GSH within CSCs. Consequently, FDINs effectively inhibit the growth of tumors in subcutaneous and orthotopic 4T1-induced tumors. Thus, this investigation proffers new perspectives on the significant development of prodrug-based nanomedicines to establish enhanced therapeutic efficacy against TNBC [].
Epigenetic medicines, e.g., histone deacetylase inhibitors, frequently encounter diminished effectiveness due to their inadequate solubility in water, leading to restricted bioavailability and a lowered therapeutic index. A biocompatible starch NP-loaded CG-1521 is currently considered for potential preclinical development for breast tumors to address the inadequate therapeutic index. The optimized CG-NPs are further characterized for physicochemical properties, i.e., morphology, zeta potential, loading capacity, and release kinetics. Additionally, their cytotoxic and apoptotic behavior has been assessed in the MCF-7 cell line. This study further demonstrated that the encapsulation of CG-1521 leads to a notable decrease in the rate at which the drug is released while also resulting in a significant cytotoxic capacity of the NPs compared to an equivalent dose of free CG-1521. The application of CG-NPs induced cell cycle and substantial cell death in the MCF-7 cell line via apoptosis, further exhibiting a comparable influence on gene expression as that of a free drug. The results indicated that incorporating CG-1521/starch NPs can potentially enhance the distribution of histone deacetylase inhibitors for breast cancer treatment while maintaining the drug’s effective mechanism of action [].
Several investigations, i.e., Starch/polydopamine coated core–shell NPs [], Bionized nanoferrite NPs/anti-HER2 antibody (BH NPs) [], Tyramine-functionalized starch (Tyr-St)/tannic acid (TA)/phenolated-magnetic NPs (Fe3O4-PhOH NPs)/Dox [], Hydroxychloroquine (HCQ)/Fmoc/hydroxyethyl starch (HES)/PTX [], Polyethylenimine-β-cyclodextrin (roFPC)/Dox/hTERT siRNA [], etc., have been further developed and investigated at in vitro settings to understand the efficacy in breast cancer therapy.
5.7. Chondroitin Sulfate
The utilization of chondroitin sulfate NPs has shown great potential in treating breast cancer, owing to its numerous advantageous features such as precise delivery, encapsulation of anticancer drugs, enhanced drug absorption, and the ability to overcome drug resistance. These NPs are designed to be modified to transport various therapeutic molecules concurrently, hence enabling the occurrence of synergistic effects between medications or combination therapies. Therefore, to utilize these, Tan et al. developed albumin corona NP-mediated chondroitin sulfate-loaded DOX and investigated the efficacy against 4T1 breast cancer cells. The BC-DOX-NPs synergized with the gp60, CD44, and SPARC receptors on tumor cells, resulting in enhanced transcytosis and improved accumulation and uptake inside tumor tissues (Figure 1). This interaction was facilitated by the combined action of BSA and CS. The coexistence of BSA and CS facilitated the effective targeting of CD44 by BC-DOX-NPs, resulting in cytotoxicity in the 4T1 cell line compared to CS-DOX-NPs or free DOX. The administration of BC-DOX-NPs in orthotopic 4T1 tumor-bearing mice resulted in enhanced accumulation of chemotherapeutics at the tumor site compared to CS-DOX-NPs or free DOX. Thus, this investigation further demonstrated the suppression of tumor proliferation and reduced drug deposition in crucial organs in the case of multi-drug treatment or resistance in breast cancer [].
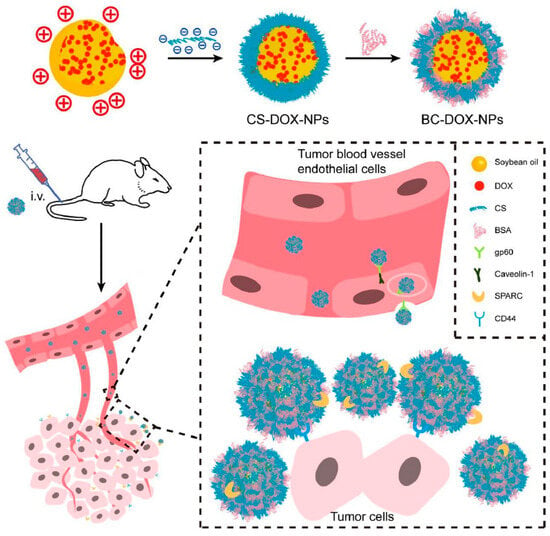
Figure 1.
Diagrammatic representation of developed intravenous (i.v) administration of albumin corona NPs-mediated CS/DOX and investigated the efficacy against 4T1 breast cancer cells. BC-DOX-NPs synergized with the gp60, CD44, etc., receptors on tumor cells, resulting in enhanced transcytosis and improved accumulation and uptake inside tumor tissues, reproduced from Ref. [], licensed under CC BY 4.0.
MDR poses a significant barrier to the efficacy of chemotherapeutics in the treatment of breast cancer. To address the MDR in cancer therapeutics, Shi et al. developed quercetin/chlorin e6/PTX-loaded redox-responsive chondroitin sulfate NPs in the treatment of multidrug-resistant (MDR) breast cancer and lung metastasis. The results of the cell line investigation demonstrated that the presence of NPs led to decreased P-gp expression in MCF-7 and ADR cells. Consequently, this downregulation led to an enhancement in the antitumor effectiveness of PTX against MCF-7 and ADR cells. Furthermore, applying near-infrared (NIR) laser irradiation can stimulate NPs to produce ROS within cells. This, in turn, can result in the disruption of the mitochondrial membrane and facilitate the escape of medicines via lysosomes. This novel nanoplatform demonstrated efficient in vivo MDR suppression and effectiveness in preventing metastasis by enhancing chemo-photodynamic treatment. Thus, this study indicated that the versatile nanoplatform exhibited promising potential for efficient breast cancer treatment [].
The utilization of polysaccharide-based biomaterials as a delivery system has been recently investigated widely due to their high payload and %EE. In a recent year, Setayash et al. grafted octadecylamine onto chondroitin sulfate (ChoS-ODA1) at different molar ratios (10, 20, and 30)-loaded CUR nanocomposites to treat breast cancer. The study reported that ChoS-ODA3 with 10% CUR was chosen for subsequent testing because it exhibited a higher %EE of 79.56% ± 5.56. The CUR-laden nanogels exhibited an in vitro release profile of over 80% over 70 h. Furthermore, the MTT study of the MCF-7 cell line revealed no significant cytotoxicity towards the nanogel (blank), whereas the CUR-loaded nanogels caused substantial cell death within 24 h. Additionally, the findings of this investigation demonstrated that the utilization of CUR-loaded ChoS nanogels effectively enhanced cellular absorption compared to the administration of free CUR. The nanogels that have been manufactured that incorporate CUR are anticipated to exhibit efficacy in future investigations about cancer therapy [].
To utilize the receptor-mediated capacity of ChoS-based NPs, Li et al. developed DTX-loaded redox-responsive ChoS to target tumors by attaching to the CD44 receptor, expressed on the surfaces of different tumor cells. Small-molecular DTX is susceptible to redox reactions synthesized via alterations of DTX-loaded cystamine-containing disulfide links (Cys-DTX) nanoconjugates. The study reported that the nanosized DTX delivery is anticipated to possess significant permeability and cytotoxicity of the Cys-DTX NPs, intended to facilitate the targeted transportation of the encapsulated redox-responsive Cys-DTX prodrug. Furthermore, the study observed that the Cys-DTX/ChoS-ss-DTX NPs exhibited a redox-responsive release of DTX via controlled manner. Additionally, these NPs demonstrate enhanced permeability in tissues, heightened cytotoxicity, and reduced adverse events compared to unbound DTX. The findings of this study indicate that the Cys-DTX/ChoS-ss-DTX NPs exhibit promising potential for future application in tumor treatment [].
Similarly to the objective of the previous investigation, Singhai et al. also developed α-tocopheryl succinate (α-TOS) and ChoS mediated multiwalled carbon-nanotubes (MWCNTs) (α-TOS–ChoS–MWCNTs)-loaded DOX to enable the exact targeting of overexpressed CD44 receptors on cells unique to TNBC. The study reported that α-TOS-ChoS-MWCNTs/Dx composite demonstrated enhanced cellular localization compared to formulations without ChoS, indicating a higher level of selectivity. Notably, the formulation with α-TOS-ChoS-MWCNTs/Dx demonstrated enhanced cellular localization compared to formulations without ChoS, indicating a higher level of selectivity. The Kiton Red 620 assay demonstrated a statistically significant reduction in the proliferation of MDA-MB-231 cells, as indicated by a GI50 value of 0.791 ± 0.015. The investigation on apoptosis utilizing the Annexin V/PI test demonstrated significant apoptosis in MDA-MB-231 cells (53.40 ± 3.32%) when exposed to α-TOS-ChoS-MWCNTs/Dx, as compared to alternative formulations. The results indicated that using ChoS, α-TOS, and Dx combined demonstrated efficacy and safety in treating TNBC [].
In recent years, several investigations, i.e., Berberine derivative (BD)/chondroitin sulfate (ChoS) [], PTX/quercetin (QC)/chondroitin sulfate (ChoS)/mesoporous silica NPs (MSNs) [], HA/chondroitin sulfate (ChoS)/DOX [], ChoS-deoxycholic acid (ChoS-DOCA) NPs [], ChoS-deoxycholic acid-polyethylene glycol-maleimide (ChoS-DOCA-PEG-MAL = CDPM) nanostructures [], Chondroitin sulfate/deoxycholic acid (DOCA) nanocomposite [], Chondroitin sulfate/polyethyleneimine (BPEI)/BPEI conjugated graphene (BPEI-GO)/Dox [], Celecoxib/honokiol/chondroitin sulfate/lactoferrin (LF) [], etc., have been assessed in various models for breast cancer therapy.
5.8. Pullulan
Another significant polysaccharide-based NP, i.e., pullan, has shown great potential in treating breast cancer, owing to similar characteristics of other polysaccharides. Therefore, to investigate the potential of pullulan-based NPs, Bonzi et al. developed novel biocomposite-based pullulan NPs loaded with alendronate (ALN) and PTX Pull-(GGPNle-β-PTX) with the specific purpose of specifically targeting bone metastases in breast cancer. The investigation results showed that Pull-(GGPNle-γ-PTX)-(PEG-ALN) exhibited a pronounced attraction towards hydroxyapatite, a material that closely resembles bone tissue. Under pathological conditions, the release of PTX from the bioconjugate was observed rapidly by the cleavage of Cathepsin K. The results of many tests conducted on human MDA-MB-231-BM, murine 4T1, murine K7M2, and human SAOS-2 osteosarcoma cells consistently demonstrated that the bioconjugate exhibited a significant anti-angiogenic activity compared to conjugate ALN. In addition, the nanoconjugate could impede the migration of tumor cells and exhibit significant anti-angiogenic properties. The findings exhibit remarkable promise for targeted therapy of bone neoplasms, including breast cancer, bone metastases, and osteosarcoma [].
Breast cancer regulates the expression of human epidermal growth factor receptor-2 (HER-2), which is further characterized by its high invasiveness, unfavorable clinical outcomes, and elevated likelihood of recurrence. Therefore, the study investigated by Xu et al. involved trastuzumab functionalized pullulan-DOX (Tz-P-DOX) NPs for targeting the overexpression of epidermal growth factors in breast cancer. The acquired NPs exhibited an average hydrodynamic diameter (6.7 ± 2.0 nm) and PDI of 0.218 ± 0.012. The study findings demonstrated a notable cellular uptake and cytotoxicity between Tz-P-Dox and non-targeted P-Dox when tested in HER-2-positive cell lines, including BT474 and MCF-7 cells. The outcome of this investigation indicated that NPs functionalized with trastuzumab exhibit significant promise as a viable therapeutic option for the treatment of HER-2-overexpressing breast cancer conditions [].
The primary challenge in integrating chemotherapy and gene therapy lies in developing a viable and biocompatible carrier for the concurrent delivery of therapeutic medication and genes. Therefore, Chen et al. investigated the potential of a novel amphiphilic bifunctional pullulan derivative (PDP) as a nano-carrier for the simultaneous delivery of chemotherapeutics and genes in the context of cancer therapy. The study resulted in favorable blood compatibility and minimal cytotoxicity when exposed to 160.8 nm of particle size and 28 mV of zeta potential of the nanocarrier. The %EE of the drug in PDP micelles was significantly greater (84.05%) compared to the loading capacity of 7.64% for DOX, exhibited a prolonged drug release profile, and demonstrated excellent DNA-binding ability. The utilization of flow cytometry revealed that MCF-7 cells effectively internalize PDP/DOX micelles, resulting in a greater level of cytotoxicity against the MCF-7 cell line compared to free DOX. In addition, it was shown that PDP micelles exhibited adequate transportation of the tumor suppressor gene p53 into MCF-7 cells. Furthermore, the introduction of exogenous p53 protein resulted in the induction of cell death in MCF-7 cells. Furthermore, it was shown that the co-delivery of DOX and gene p53 resulted in increased cytotoxicity, a higher rate of tumor cell apoptosis, and more efficient inhibition of cancer cell migration. The co-administration of DOX and p53 in mice with tumors demonstrated a higher level of effectiveness in combating tumors compared to the individual administration of DOX or p53 alone. These findings indicate that cationic PDP micelles have great potential for efficiently delivering functional genes and chemotherapeutic drugs, enhancing antitumor effectiveness, and reducing systemic toxicity [].
To minimize the cytotoxicity of chemotherapeutic agents, Asgari et al. fabricated an electrospun composite-based poly (epichlorohydrin) (PCH) grafted GO and further loaded with PTX and Cur, which are subsequently encapsulated into pullulan nanofibers through electrospinning. The compounds exhibited a prolonged release (93 h) from the nanofibers loaded with nanocarriers under pH 7.4. A synergistic cytotoxicity of PTX + Cur was confirmed via MTT assay and optical microscopic study. The nanofiber system, encapsulated with nanocarriers, is an innovative and adjustable drug delivery method designed for localized chemotherapeutic purposes [].
In addition, very limited investigations have been reported on pullulan-based NPs, i.e., Pullulan/poly(β-amino) ester (PBAE)/MTX [], Urocanic acid/pullulan/Adriamycin (ADR) [], Polyethylene sebacate (PES)-Gantrez® AN 119/DOX hydrochloride NPs/pullulan [], Pullulan (PUL)-Nalpha-Boc-L-histidine (bHis) conjugates/deoxycholic acid (DO) [], etc., for breast cancer treatment. Table 1 further summarizes several recent advancements in polysaccharide-based NPs and their potential outcome for the treatment of breast cancer.

Table 1.
Recent investigations on various polysaccharide-based NPs and their significant effectiveness for breast cancer treatment.
6. Patents
An increasing number of studies have shown that polysaccharide-based NPs hold great potential as effective carriers for delivering anticancer drugs specifically to breast cancer cells. Although CS-based NPs have not yet been used in clinical or commercial cancer treatments, the large number of related patents shows strong efforts to protect new ideas and move this research closer to real-world use. Conversely, while other polysaccharide-based NPs have shown strong preclinical efficacy, the number of patents filed for these systems remains relatively limited. This imbalance points to untapped opportunities for deeper exploration and innovation aimed at addressing current limitations and promoting the clinical advancement of polysaccharide nanocarriers in cancer therapeutics. Table 2 presents a selected compilation of published patents concerning the application of diverse polysaccharide-based NPs in the delivery of anticancer agents for breast cancer treatment.

Table 2.
Patents published on polysaccharide-based NPs for breast cancer therapy (Source: Espacenet.com).
7. Conclusions and Future Perspectives
Polysaccharide-based NPs have emerged as a promising platform for targeted breast cancer therapy, offering a combination of precision and biocompatibility that addresses critical challenges in current treatments. Their inherent biodegradability, hydrophilicity, and functional versatility allow for the creation of sophisticated drug delivery systems capable of enhancing therapeutic efficacy while minimizing systemic toxicity. By utilizing natural materials, such as starch, CS, HA, and alginate, these NPs provide a sustainable and patient-friendly approach to cancer management. One of the key advantages of polysaccharide-based NPs lies in their ability to be functionalized for specific targeting. By attaching ligands or proteins to their surfaces, these NPs can selectively bind to receptors overexpressed on breast cancer cells, ensuring precise drug delivery to the tumor microenvironment. This specificity not only improves the effectiveness of the treatment but also reduces the risk of off-target effects, a major limitation of conventional therapies. Polysaccharide-based NPs further distinguish themselves by their capacity to combat drug resistance, a pervasive challenge in advanced breast cancer cases. Their ability to modulate biological processes such as angiogenesis and immune responses enhances the anti-tumor effects while minimizing the likelihood of disease recurrence. In addition, targeting VEGF receptors with polysaccharide NPs inhibits angiogenesis, a critical process in tumor progression. The ability of polysaccharides to undergo chemical modification further expands their versatility, enabling precise control over characteristics such as solubility, hydrophobicity, and stability. This adaptability is crucial for tailoring NPs to meet the unique demands of breast cancer treatment, such as controlled drug release and enhanced bioavailability under physiological conditions.
Although polysaccharide-based NPs show significant promise for cancer therapy, several barriers must be addressed before clinical translation can be achieved. Despite hundreds of studies demonstrating innovations in their design and preclinical efficacy, clinical investigations remain limited. This gap suggests that excessive complexity and the use of unapproved materials often hinder regulatory approval and raise safety concerns. Therefore, prioritizing simpler, safer, and regulatory-compliant formulations may accelerate clinical advancement. Key priorities include establishing scalable manufacturing processes, ensuring formulation consistency, and meeting regulatory standards for safety and quality. While rodent models have confirmed the anticancer potential and biocompatibility of these systems, studies in larger animal models are essential to evaluate pharmacokinetics (ADME), immune interactions, and potential adverse effects. Such data will be critical to support Phase I clinical trials and eventual clinical adoption. Achieving this goal will require close collaboration between academia, industry, and healthcare institutions, fostering the translation of these nanotherapeutic platforms into mainstream cancer care and offering new hope for breast cancer treatment.
Author Contributions
S.M.: Writing—Original draft, Review and Editing, Artwork. B.H.J.G.: Conceptualization, Writing—Original draft, Review and Editing, Artwork. U.H.: Writing—Original draft, Review and Editing. S.N.: Writing—Review and Editing, Project administration. M.G.A.: Writing—Review and Editing, Project administration, Supervision. F.F.: Writing—Original draft, Review and Editing. K.P.: Writing—Review and Editing, Project administration, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP 2/78/46.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Sourav Mohanto and Benachakal Honnegowda Jaswanth Gowda would like to thank Yenepoya (Deemed to be University), Mangalore, India, for providing the Senior Research Fellowship (SRF) during the drafting of this manuscript. Umme Hani would like to thank King Khalid University, Saudi Arabia, for their support. Soumya Narayana and Mohammed Gulzar Ahmed would like to thank Yenepoya (Deemed to be University), Mangalore, India, for their support. Farhat Fatima would like to thank Prince Sattam Bin Abdulaziz University, Saudi Arabia, for their support. Karthika Paul would like to thank JSS College of Pharmacy, Mysuru, India, for their support. The graphical abstract was created using BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
NPs: Nanoparticles; VEGF: Vascular endothelial growth factor; ROS: Reactive oxygen species; SF: Silk fibroin; MDR: Multidrug resistance; P-gp: P-glycoprotein; MTX: Methotrexate; %EE: % encapsulation efficiency; CSNPs: Chitosan nanoparticles; ChoS: Chondroitin sulfate; 5-FU: 5-Fluorouracil; HA: Hyaluronic acid; DOX or Dox: Doxorubicin; CUR or Cur: Curcumin; GO: Graphene oxide; MDRC: Multi-drug resistance cancer; OLA: Olaparib; PTX: Paclitaxel; CMD: Carboxymethyl dextran; DTX: Docetaxel; CSCs: Cancer stem cells; GSH: Glutathione; TNBC: Triple-negative breast cancer.
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 23 July 2023).
- Indian Council of Medical Research. Indian Council of Medical Research National Cancer Registry Programme; Indian Council of Medical Research: Bangalore, India, 2020. [Google Scholar]
- Sathishkumar, K.; Sankarapillai, J.; Mathew, A.; Nair, R.A.; Gangane, N.; Khuraijam, S.; Barmon, D.; Pandya, S.; Majumdar, G.; Deshmane, V.; et al. Breast Cancer Survival in India across 11 Geographic Areas under the National Cancer Registry Programme. Cancer 2024, 130, 1816–1825. [Google Scholar] [CrossRef]
- Thakur, P.; Seam, R.K.; Gupta, M.K.; Gupta, M.; Sharma, M.; Fotedar, V. Breast Cancer Risk Factor Evaluation in a Western Himalayan State: A Case-Control Study and Comparison with the Western World. South Asian J. Cancer 2017, 6, 106–109. [Google Scholar] [CrossRef]
- Barrios, C.H. Global Challenges in Breast Cancer Detection and Treatment. Breast 2022, 62 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Hegde, G.V.; de la Cruz, C.; Eastham-Anderson, J.; Zheng, Y.; Sweet-Cordero, E.A.; Jackson, E.L. Residual Tumor Cells That Drive Disease Relapse after Chemotherapy Do Not Have Enhanced Tumor Initiating Capacity. PLoS ONE 2012, 7, e45647. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Gonçalves, A.; Bertucci, F. Personalized Medicine: Present and Future of Breast Cancer Management. Crit. Rev. Oncol. Hematol. 2014, 91, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Shah, H.; Yalamarty, S.S.K.; Ataide, J.A.; Torchilin, V.P. Recent Advances with Precision Medicine Treatment for Breast Cancer Including Triple-Negative Sub-Type. Cancers 2023, 15, 2204. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mitra, O.; Tripathi, G.; Adur, I.; Mohanto, S.; Nama, M.; Samanta, S.; Gowda, B.J.; Subramaniyan, V.; Sundararajan, V.; et al. Nanomaterials-Assisted Photothermal Therapy for Breast Cancer: State-of-the Advances and Future Perspectives. Photodiagnosis Photodyn. Ther. 2024, 45, 103959. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Ahmad, S.; Baboota, S.; Ali, J. 10–Polysaccharide-Based Amorphous Solid Dispersions (ASDs) for Improving Solubility and Bioavailability of Drugs. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 271–317. ISBN 978-0-08-102553-6. [Google Scholar]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Silant’ev, V.E.; Shmelev, M.E.; Belousov, A.S.; Patlay, A.A.; Shatilov, R.A.; Farniev, V.M.; Kumeiko, V. V How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers 2023, 15, 2516. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of Oral Bioavailability of Natural Compounds and Probiotics by Mucoadhesive Tailored Biopolymer-Based Nanoparticles: A Review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Moghaddam, F.D.; Heidari, G.; Zare, E.N.; Djatoubai, E.; Paiva-Santos, A.C.; Bertani, F.R.; Wu, A. Carbohydrate Polymer-Based Nanocomposites for Breast Cancer Treatment. Carbohydr. Polym. 2023, 304, 120510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Zhang, J.-G.; Yang, X.; Du, W.-L.; Yu, Z.-L.; Lv, Z.-Y.; Mou, X.-Z. Carbohydrates Based Stimulus Responsive Nanocarriers for Cancer-Targeted Chemotherapy: A Review of Current Practices. Expert Opin. Drug Deliv. 2022, 19, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Immunosuppressive Effects of Vascular Endothelial Growth Factor. Oncol. Lett. 2022, 24, 369. [Google Scholar] [CrossRef] [PubMed]
- Hyun, G.H.; Jeong, D.-H.; Yang, Y.Y.; Cho, I.H.; Ha, Y.-J.; Xing, X.; Abbott, D.W.; Hsieh, Y.S.Y.; Kang, Y.P.; Cha, J.-H.; et al. Multivalent Carbohydrate Nanocomposites for Tumor Microenvironment Remodeling to Enhance Antitumor Immunity. ACS Nano 2023, 17, 11567–11582. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Cai, J.; Liao, W.; Wen, J.; Ye, F.; Nie, Q.; Chen, W.; Zhao, C. Algae-Derived Polysaccharides and Polysaccharide-Based Nanoparticles: A Natural Frontier in Breast Cancer Therapy. Int. J. Biol. Macromol. 2025, 297, 139936. [Google Scholar] [CrossRef] [PubMed]
- Shayegh, F.; Türk, Z.; Armani, A.; Zarghami, N. New Insights into Polysaccharide-Based Nanostructured Delivery Systems in Breast Cancer: Possible Application of Antisense Oligonucleotides in Breast Cancer Therapy. Int. J. Biol. Macromol. 2024, 272, 132890. [Google Scholar] [CrossRef]
- Bazzazan, M.A.; Fattollazadeh, P.; Shahbaz, S.K.; Rezaei, N. Polymeric Nanoparticles as a Promising Platform for Treating Triple-Negative Breast Cancer: Current Status and Future Perspectives. Int. J. Pharm. 2024, 664, 124639. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Emerging Role of Histone Deacetylase Inhibitors as Anti-Breast-Cancer Agents. Drug Discov. Today 2019, 24, 685–702. [Google Scholar] [CrossRef]
- Williams, C.; Lin, C.Y. Oestrogen Receptors in Breast Cancer: Basic Mechanisms and Clinical Implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, A.; Veer, L.J.V.; Bissell, M.J. An “Elite Hacker”. Cell Adh. Migr. 2012, 6, 236–435. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive Cells in Cancer: Mechanisms and Potential Therapeutic Targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mitra, O.; P, S.; Bhattacharjee, A.; Mohanto, S.; Gowda, B.H.J.; Kar, S.; Ramaiah, S.; Anbarasu, A.; Ahmed, M.G. Exploring the Emerging Trends in the Synthesis and Theranostic Paradigms of Cerium Oxide Nanoparticles (CeONPs): A Comprehensive Review. Mater. Today Chem. 2024, 35, 101894. [Google Scholar] [CrossRef]
- Hani, U.; Gowda, B.H.J.; Haider, N.; Ramesh, K.; Paul, K.; Ashique, S.; Ahmed, M.G.; Narayana, S.; Mohanto, S.; Kesharwani, P. Nanoparticle-Based Approaches for Treatment of Hematological Malignancies: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 233. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The Use of Nanoparticulates to Treat Breast Cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.L. Nanomedicine Applications in the Treatment of Breast Cancer: Current State of the Art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Nur Khatijah, M.Z.; Al-Sowayan, B.S. Nanotechnology: A Revolutionary Approach to Prevent Breast Cancer Recurrence. Asian J. Surg. 2023, 46, 13–17. [Google Scholar] [CrossRef]
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.; Ullah, O.; Balant, S.; Rahman, M.H. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int. J. Mol. Sci. 2022, 23, 2856. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and Their Applications in Early Diagnosis of Cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Choi, K.Y.; Liu, G.; Lee, S.; Chen, X. Theranostic Nanoplatforms for Simultaneous Cancer Imaging and Therapy: Current Approaches and Future Perspectives. Nanoscale 2012, 4, 330–342. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic Nanomedicine for Cancer Detection and Treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast Cancer Vaccines: New Insights into Immunomodulatory and Nano-Therapeutic Approaches. J. Control. Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Ahmad, A.; Gulraiz, Y.; Ilyas, S.; Bashir, S. Polysaccharide Based Nano Materials: Health Implications. Food Hydrocoll. Health 2022, 2, 100075. [Google Scholar] [CrossRef]
- Visan, A.I.; Cristescu, R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics 2023, 15, 2227. [Google Scholar] [CrossRef] [PubMed]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Luisa Martínez-López, A.; Ribeiro, B.; Pinto, R.J.B.; Stana Kleinschek, K.; et al. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626–647. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, W.; Lin, X.; Feng, Y. Polysaccharide-Based Nanomaterials for Ocular Drug Delivery: A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 601246. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-Based Nanomedicines for Cancer Immunotherapy: A Review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.K.; Shankar, R. Polysaccharides Based Nanomaterials for Targeted Anti-Cancer Drug Delivery. J. Drug Target. 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Nandgude, T.; Pagar, R. Plausible Role of Chitosan in Drug and Gene Delivery against Resistant Breast Cancer Cells. Carbohydr. Res. 2021, 506, 108357. [Google Scholar] [CrossRef]
- de Oliveira Pedro, R.; Hoffmann, S.; Pereira, S.; Goycoolea, F.M.; Schmitt, C.C.; Neumann, M.G. Self-Assembled Amphiphilic Chitosan Nanoparticles for Quercetin Delivery to Breast Cancer Cells. Eur. J. Pharm. Biopharm. 2018, 131, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Yu, G.; Shen, L.; Fan, J.; Xu, L.; Zhang, H.; Zhao, N.; Zeng, Z.; Hu, T.; et al. Aptamer Internalization via Endocytosis Inducing S-Phase Arrest and Priming Maver-1 Lymphoma Cells for Cytarabine Chemotherapy. Theranostics 2017, 7, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, X.; Hunziker, P. Carbohydrate-Based Amphiphilic Nano Delivery Systems for Cancer Therapy. Nanoscale 2016, 8, 16091–16156. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of Chitosan Modified Nanocarriers in Breast Cancer. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent Development of Aptamer Conjugated Chitosan Nanoparticles as Cancer Therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Ekinci, M.; Koksal-Karayildirim, C.; Ilem-Ozdemir, D. Radiolabeled Methotrexate Loaded Chitosan Nanoparticles as Imaging Probe for Breast Cancer: Biodistribution in Tumor-Bearing Mice. J. Drug Deliv. Sci. Technol. 2023, 80, 104146. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Dong, W.; Zhao, G.; Lu, Y.; Huang, X.; Liang, X. Cinnamon Cassia Oil Chitosan Nanoparticles: Physicochemical Properties and Anti-Breast Cancer Activity. Int. J. Biol. Macromol. 2023, 224, 1065–1078. [Google Scholar] [CrossRef]
- Rajaei, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Díez-Pascual, A.M. Chitosan/Agarose/Graphene Oxide Nanohydrogel as Drug Delivery System of 5-Fluorouracil in Breast Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104307. [Google Scholar] [CrossRef]
- Chang, H.; Yhee, J.Y.; Jeon, S.; Shim, M.K.; Yoon, H.Y.; Lee, S.; Kim, K. In Vivo Toxicity Evaluation of Tumor Targeted Glycol Chitosan Nanoparticles in Healthy Mice: Repeated High-Dose of Glycol Chitosan Nanoparticles Potentially Induce Cardiotoxicity. J. Nanobiotechnol. 2023, 21, 82. [Google Scholar] [CrossRef]
- Al-Othman, N.; Alhendi, A.; Ihbaisha, M.; Barahmeh, M.; Alqaraleh, M.; Al-Momany, B.Z. Role of CD44 in Breast Cancer. Breast Dis. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Louderbough, J.M.V.; Schroeder, J.A. Understanding the Dual Nature of CD44 in Breast Cancer Progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Elamin, K.M.; Wathoni, N. Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers 2023, 15, 1025. [Google Scholar] [CrossRef]
- Pooja, Y.S.; Rajana, N.; Yadav, R.; Naraharisetti, L.T.; Godugu, C.; Mehra, N.K. Design, Development, and Evaluation of CDK-4/6 Inhibitor Loaded 4-Carboxy Phenyl Boronic Acid Conjugated PH-Sensitive Chitosan Lecithin Nanoparticles in the Management of Breast Cancer. Int. J. Biol. Macromol. 2024, 258, 128821. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Ocaña, A.; Alonso-Moreno, C.; Galvão Frem, R.C.; Chorilli, M. Chitosan-Coated MIL-100(Fe) Nanoparticles for Enhanced Piperine Release in Breast Cancer Treatment. J. Mol. Struct. 2024, 1305, 137801. [Google Scholar] [CrossRef]
- Dastgir, M.; Ayyami, Y.; Pourfarshid, A.; Ghorbani, M.; Mortezazadeh, T. Evaluation of the Theranostic Characteristics of Chitosan-Decorated Bismuth Oxide Nanoparticles Conjugated Curcumin and 5-Aminolevulinic Acid as a Biocompatible Targeted Nanoplatform in CT and Breast Cancer Radiation Therapy. J. Drug Deliv. Sci. Technol. 2024, 92, 105279. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Shen, Y. Folate-Modified Carboxymethyl Chitosan-Based Drug Delivery System for Breast Cancer Specific Combination Therapy via Regulating Mitochondrial Calcium Concentration. Carbohydr. Polym. 2024, 323, 121434. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.Y.; Elshoky, H.A.; El-Sayed, N.M.; Fahmy, H.M.; Ali, M.A. Ameliorative Effect of Zinc Oxide-Chitosan Conjugates on the Anticancer Activity of Cisplatin: Approach for Breast Cancer Treatment. Int. J. Biol. Macromol. 2024, 257, 128597. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Dabiri, A.; Mashayekhi, F.; Rahimmanesh, I.; Bidram, E.; Karbasi, S.; Rafienia, M.; Javanmard, S.H.; Ertas, Y.N.; Zarrabi, A.; et al. Smart Co-Delivery of Plasmid DNA and Doxorubicin Using MCM-Chitosan-PEG Polymerization Functionalized with MUC-1 Aptamer against Breast Cancer. Biomed. Pharmacother. 2024, 173, 116465. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Zhang, X.; Jeong, M.S.; Jang, E.-S.; Wang, M.-H. Multifunctional Chitosan-Bimetallic Nanocarrier Deliver 5-Fluorouracil for Enhanced Treatment of Pancreatic and Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2024, 259, 129165. [Google Scholar] [CrossRef]
- Mohanto, S.; Chakraborty, P.; Pandian, C.S.; Manna, S.; Dutta, J. A Comprehensive Review On Alginate As Wound Dressing Biomaterial. Curr. Appl. Polym. Sci. 2020, 4, 3–14. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-Loaded Alginate Nanoparticles Embedded Polycaprolactone-Chitosan Nanofibers as a Controlled Drug Delivery Nanoplatform for Anticancer Activity. J. Colloid Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef]
- Jayapal, J.J.; Dhanaraj, S. Exemestane Loaded Alginate Nanoparticles for Cancer Treatment: Formulation and in Vitro Evaluation. Int. J. Biol. Macromol. 2017, 105, 416–421. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Hu, X.; Lu, Y.; Zhang, J.; Jin, W.; Liu, W.; Shu, Y.; Cheng, Y.Y.; Li, W.; et al. Caffeic Acid-Grafted Chitosan/Sodium Alginate/Nanoclay-Based Multifunctional 3D-Printed Hybrid Scaffolds for Local Drug Release Therapy after Breast Cancer Surgery. Carbohydr. Polym. 2024, 324, 121441. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Varaprasad, K.; Kim, S.; Kumar Jangid, A.; Lee, W.; Syedahamed Haja Hameed, A.; Kim, K. Size-Dependent Cellular Uptakeof Sodium Alginate Passivated Tin Dioxide Nanoparticles in Triple-Negative Breast Cancercells. J. Ind. Eng. Chem. 2023, 123, 476–487. [Google Scholar] [CrossRef]
- Bulatao, B.P.; Nalinratana, N.; Jantaratana, P.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Lutein-Loaded Chitosan/Alginate-Coated Fe3O4 Nanoparticles as Effective Targeted Carriers for Breast Cancer Treatment. Int. J. Biol. Macromol. 2023, 242, 124673. [Google Scholar] [CrossRef]
- Alioghli Ziaei, A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In Situ Forming Alginate/Gelatin Hybrid Hydrogels Containing Doxorubicin Loaded Chitosan/AuNPs Nanogels for the Local Therapy of Breast Cancer. Int. J. Biol. Macromol. 2023, 246, 125640. [Google Scholar] [CrossRef] [PubMed]
- Taran, Z.; Yektaniroumand Digehsaraei, S.; Salouti, M.; Amini, B.; Mahmazi, S.; Kalantari, M. Methotrexate Loaded in Alginate Beads for Controlled Drug Release against Breast Cancer. Gene 2023, 851, 146941. [Google Scholar] [CrossRef]
- Alzahrani, B.; Elderdery, A.Y.; Alsrhani, A.; Alzerwi, N.A.N.; Althobiti, M.M.; Elkhalifa, A.M.E.; Rayzah, M.; Idrees, B.; Kumar, S.S.; Mok, P.L. Sodium Alginate Encapsulated Iron Oxide Decorated with Thymoquinone Nanocomposite Induces Apoptosis in Human Breast Cancer Cells via PI3K-Akt-MTOR Pathway. Int. J. Biol. Macromol. 2023, 244, 125054. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalil, I.A.; Mahmoud, M.Y.; Bakr, A.F.; Ghoniem, M.G.; Al-Farraj, E.S.; El-Sherbiny, I.M. Layer-by-Layer Development of Chitosan/Alginate-Based Platelet-Mimicking Nanocapsules for Augmenting Doxorubicin Cytotoxicity against Breast Cancer. Int. J. Biol. Macromol. 2023, 225, 503–517. [Google Scholar] [CrossRef]
- Sharafshadeh, M.S.; Tafvizi, F.; Khodarahmi, P.; Ehtesham, S. Preparation and Physicochemical Properties of Cisplatin and Doxorubicin Encapsulated by Niosome Alginate Nanocarrier for Cancer Therapy. Int. J. Biol. Macromol. 2023, 235, 123686. [Google Scholar] [CrossRef]
- Loke, Y.L.; Beishenaliev, A.; Wang, P.-W.; Lin, C.-Y.; Chang, C.-Y.; Foo, Y.Y.; Faruqu, F.N.; Leo, B.F.; Misran, M.; Chung, L.Y.; et al. ROS-Generating Alginate-Coated Gold Nanorods as Biocompatible Nanosonosensitisers for Effective Sonodynamic Therapy of Cancer. Ultrason. Sonochem. 2023, 96, 106437. [Google Scholar] [CrossRef]
- Zaer, M.; Moeinzadeh, A.; Abolhassani, H.; Rostami, N.; Tavakkoli Yaraki, M.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Bashiri, Z.; Moeinabadi-Bidgoli, K.; Moradbeygi, F.; et al. Doxorubicin-Loaded Niosomes Functionalized with Gelatine and Alginate as PH-Responsive Drug Delivery System: A 3D Printing Approach. Int. J. Biol. Macromol. 2023, 253, 126808. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jeong Lee, S.; Hur, W.; Gupta, P.K.; Eun Son, S.; Been Lee, H.; Yeon Park, J.; Nyeon Kim, S.; Hun Seong, G. Preparation and Photothermal Effect of Chitosan-Alginate-Molybdenum Diselenide Nanocomposite Scaffolds for Cancer Therapy. J. Photochem. Photobiol. A Chem. 2023, 442, 114759. [Google Scholar] [CrossRef]
- Kreua-ongarjnukool, N.; Soomherun, N.; Niyomthai, S.T.; Soonklang, C. Turmeric-Loaded Alginate Particulate-Based Burst Release Delivery System Containing a Gas-Generating Agent. J. Drug Deliv. Sci. Technol. 2023, 87, 104850. [Google Scholar] [CrossRef]
- Dasuni Wasana, P.W.; Hasriadi; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P.; Rojsitthisak, P. Metformin and Curcumin Co-Encapsulated Chitosan/Alginate Nanoparticles as Effective Oral Carriers against Pain-like Behaviors in Mice. Int. J. Pharm. 2023, 640, 123037. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, S.; Wang, C.; Sun, T.; Yang, L. Hyaluronic acid-based nano drug delivery systems for breast cancer treatment: Recent advances. Front. Bioeng. Biotechnol. 2022, 10, 990145. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Fardous, R.; Alhindi, Y.M.; Aodah, A.H.; Alyami, M.; Alsuabeyl, M.S.; Alghamdi, W.M.; Alhasan, A.H.; Almalik, A. α(1)-Acid Glycoprotein-Decorated Hyaluronic Acid Nanoparticles for Suppressing Metastasis and Overcoming Drug Resistance Breast Cancer. Biomedicines 2022, 10, 414. [Google Scholar] [CrossRef]
- Thummarati, P.; Suksiriworapong, J.; Sakchaisri, K.; Nawroth, T.; Langguth, P.; Roongsawang, B.; Junyaprasert, V.B. Comparative Study of Dual Delivery of Gemcitabine and Curcumin Using CD44 Targeting Hyaluronic Acid Nanoparticles for Cancer Therapy. J. Drug Deliv. Sci. Technol. 2022, 77, 103883. [Google Scholar] [CrossRef]
- Gao, N.; Fu, Y.; Gong, H.; Liu, H.; Li, W. Hyaluronic Acid and Cholecalciferol Conjugate Based Nanomicelles: Synthesis, Characterization, and Cytotoxicity against MCF-7 Breast Cancer Cells. Carbohydr. Res. 2022, 522, 108706. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.; Zhang, Y.; Xu, X.; Yuan, X.; Li, J. PH-Responsive Hyaluronic Acid Nanoparticles for Enhanced Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2022, 17, 1437–1457. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Synthesis of Quercetin-Loaded Hyaluronic Acid-Conjugated PH/Redox Dual-Stimuli Responsive Poly(Methacrylic Acid)/Mesoporous Organosilica Nanoparticles for Breast Cancer Targeted Therapy. Int. J. Biol. Macromol. 2024, 263, 130168. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Q.; Wang, Z.; Liu, Y.; Yang, S.; Zhao, X.; Peng, J. A Chemo/Chemodynamic Nanoparticle Based on Hyaluronic Acid Induces Ferroptosis and Apoptosis for Triple-Negative Breast Cancer Therapy. Carbohydr. Polym. 2024, 329, 121795. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Lei, L.; Tong, F.; Zhang, H.; Zhang, Y.; Yang, W.; Tang, Y.; Lin, R.; Xia, X.; et al. Combination Losartan with Hyaluronic Acid Modified Diethyldithiocarbamate Loaded Hollow Copper Sulfide Nanoparticles for the Treatment of Breast Cancer and Metastasis. Chin. Chem. Lett. 2024, 35, 108765. [Google Scholar] [CrossRef]
- Gao, F.; Wu, Y.; Wang, R.; Yao, Y.; Liu, Y.; Fan, L.; Xu, J.; Zhang, J.; Han, X.; Guan, X. Precise Nano-System-Based Drug Delivery and Synergistic Therapy against Androgen Receptor-Positive Triple-Negative Breast Cancer. Acta Pharm. Sin. B 2024, 14, 2685–2697. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; Melo, B.L.; Mendonça, A.G.; Correia, I.J.; de Melo-Diogo, D. Hyaluronic Acid-Functionalized Graphene-Based Nanohybrids for Targeted Breast Cancer Chemo-Photothermal Therapy. Int. J. Pharm. 2024, 651, 123763. [Google Scholar] [CrossRef]
- Shah, H.S.; Zaib, S.; Usman, F.; Sarfraz, M.; Faiz, R.; Rehman, S.A.; Khan, A.A.; Alanazi, A.M.; Khan, R.; Nasrullah, U.; et al. Synthesis, Characterization, Pharmacological and Computational Evaluation of Hyaluronic Acid Modified Chebulinic Acid Encapsulated Chitosan Nanocomposite for Cancer Therapy. Int. J. Biol. Macromol. 2024, 263, 130160. [Google Scholar] [CrossRef]
- Sun, J.; Ye, T.; Chen, X.; Li, B.; Wei, Y.; Zheng, H.; Piao, J.-G.; Li, F. A Self-Assembly Active Nanomodulator Based on Berberine for Photothermal Immunotherapy of Breast Cancer via Dual Regulation of Immune Suppression. Int. J. Pharm. 2024, 653, 123898. [Google Scholar] [CrossRef]
- Bakrania, A.K.; Variya, B.C.; Rathod, L.V.; Patel, S.S. DEAE-Dextran Coated Paclitaxel Nanoparticles Act as Multifunctional Nano System for Intranuclear Delivery to Triple Negative Breast Cancer through VEGF and NOTCH1 Inhibition. Eur. J. Pharm. Biopharm. 2018, 122, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Lv, H.; Huang, X.; Dong, P.; Wang, Q.; Yang, H.; Wang, S.; Li, X.; Hu, J.; et al. Complete Regression of Xenografted Breast Tumors by Dextran-Based Dual Drug Conjugates Containing Paclitaxel and Docosahexaenoic Acid. Eur. J. Med. Chem. 2022, 240, 114567. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Zhang, Y.; Lv, S.; Li, Q.; Chen, X. Targeted Delivery of Cisplatin by LHRH-Peptide Conjugated Dextran Nanoparticles Suppresses Breast Cancer Growth and Metastasis. Acta Biomater. 2015, 18, 132–143. [Google Scholar] [CrossRef]
- Li, Y.; Jia, F.; Gao, Y.; Wang, X.; Cui, X.; Pan, Z.; Wang, W.; Li, M.; Lu, J.; Wu, Y. Self-Assembled Nanocomposites of Carboxymethyl β-Dextran/Protamine Sulfate for Enhanced Chemotherapeutic Drug Sensitivity of Triple-Negative Breast Cancer by Autophagy Inhibition via a Ternary Collaborative Strategy. Int. J. Biol. Macromol. 2023, 233, 123663. [Google Scholar] [CrossRef]
- Lewińska, A.; Radoń, A.; Gil, K.; Błoniarz, D.; Ciuraszkiewicz, A.; Kubacki, J.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Krogul-Sobczak, A.; et al. Carbon-Coated Iron Oxide Nanoparticles Promote Reductive Stress-Mediated Cytotoxic Autophagy in Drug-Induced Senescent Breast Cancer Cells. ACS Appl. Mater. Interfaces 2024, 16, 15457–15478. [Google Scholar] [CrossRef]
- Lopes, F.A.C.; Fernandes, A.V.F.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Rodrigues, A.R.O.; et al. Magnetoliposomes Containing Multicore Nanoparticles and a New Antitumor Thienopyridine Compound with Potential Application in Chemo/Thermotherapy. Biomedicines 2022, 10, 1547. [Google Scholar] [CrossRef]
- Kurihara, Y.; Yokota, H.; Takahashi, M. Water-Dispersible Carboxymethyl Dextran-Coated Melamine Nanoparticles for Biosensing Applications. ACS Omega 2022, 7, 41641–41650. [Google Scholar] [CrossRef]
- Zhou, J.; Meli, V.S.; Yu-Tin Chen, E.; Kapre, R.; Nagalla, R.; Xiao, W.; Borowsky, A.D.; Lam, K.S.; Liu, W.F.; Louie, A.Y. Magnetic Resonance Imaging of Tumor-Associated-Macrophages (TAMs) with a Nanoparticle Contrast Agent. RSC Adv. 2022, 12, 7742–7756. [Google Scholar] [CrossRef] [PubMed]
- Yousefvand, M.; Mohammadi, Z.; Ghorbani, F.; Irajirad, R.; Abedi, H.; Seyedi, S.; Papi, A.; Montazerabadi, A. Investigation of Specific Targeting of Triptorelin-Conjugated Dextran-Coated Magnetite Nanoparticles as a Targeted Probe in GnRH(+) Cancer Cells in MRI. Contrast Media Mol. Imaging 2021, 2021, 5534848. [Google Scholar] [CrossRef] [PubMed]
- Sampath, M.; Pichaimani, A.; Kumpati, P.; Sengottuvelan, B. The Remarkable Role of Emulsifier and Chitosan, Dextran and PEG as Capping Agents in the Enhanced Delivery of Curcumin by Nanoparticles in Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 162, 748–761. [Google Scholar] [CrossRef]
- Nikkhoo, A.; Rostami, N.; Farhadi, S.; Esmaily, M.; Moghadaszadeh Ardebili, S.; Atyabi, F.; Baghaei, M.; Haghnavaz, N.; Yousefi, M.; Aliparasti, M.R.; et al. Codelivery of STAT3 SiRNA and BV6 by Carboxymethyl Dextran Trimethyl Chitosan Nanoparticles Suppresses Cancer Cell Progression. Int. J. Pharm. 2020, 581, 119236. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Zheng, Y.; Yan, G.; Wang, X.; Wang, J.; Tang, R. Indomethacin-Grafted and PH-Sensitive Dextran Micelles for Overcoming Inflammation-Mediated Multidrug Resistance in Breast Cancer. Carbohydr. Polym. 2020, 237, 116139. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Taymouri, S.; Minaiyan, M.; Mirian, M. Evaluation of Thermosensitive Chitosan Hydrogel Containing Gefitinib Loaded Cellulose Acetate Butyrate Nanoparticles in a Subcutaneous Breast Cancer Model. Int. J. Pharm. 2022, 624, 122036. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Abu-Serie, M.M.; El-Gendi, H.; El-Fakharany, E.M. Fabrication, Characterization and in Vitro Evaluation of Disulfiram-Loaded Cellulose Acetate/Poly(Ethylene Oxide) Nanofiber Scaffold for Breast and Colon Cancer Cell Lines Treatment. Int. J. Biol. Macromol. 2022, 204, 555–564. [Google Scholar] [CrossRef]
- Atallah, M.A.; Sallam, M.A.; Abdelmoneem, M.A.; Teleb, M.; Elkhodairy, K.A.; Bekhit, A.A.; Khafaga, A.F.; Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green Self-Assembled Lactoferrin Carboxymethyl Cellulose Nanogels for Synergistic Chemo/Herbal Breast Cancer Therapy. Colloids Surf. B Biointerfaces 2022, 217, 112657. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, N.; Akelah, A.; Elnagar, M.; Abdelwahab, M.A. Antibacterial and Cytotoxicity of Methylene Blue Loaded-Cellulose Nanocarrier on Breast Cancer Cell Line. Carbohydr. Polym. Technol. Appl. 2021, 2, 100138. [Google Scholar] [CrossRef]
- Pathania, K.; Sah, S.P.; Salunke, D.B.; Jain, M.; Yadav, A.K.; Yadav, V.G.; Pawar, S. V Green Synthesis of Lignin-Based Nanoparticles as a Bio-Carrier for Targeted Delivery in Cancer Therapy. Int. J. Biol. Macromol. 2023, 229, 684–695. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef]
- Jaisankar, E.; Azarudeen, R.S.; Thirumarimurugan, M. A Study on the Effect of Nanoscale MgO and Hydrogen Bonding in Nanofiber Mats for the Controlled Drug Release along with In Vitro Breast Cancer Cell Line and Antimicrobial Studies. ACS Appl. Bio. Mater. 2022, 5, 4327–4341. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M.; Tolba, N.S. Silver Nanoparticle-Coated Ethyl Cellulose Inhibits Tumor Necrosis Factor-α of Breast Cancer Cells. Drug Des. Devel. Ther. 2021, 15, 2035–2046. [Google Scholar] [CrossRef]
- Arumugam, M.; Murugesan, B.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospinning Cellulose Acetate/Silk Fibroin/Au-Ag Hybrid Composite Nanofiber for Enhanced Biocidal Activity against MCF-7 Breast Cancer Cell. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112019. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Manjusha, V.; Chithra Sekhar, V. A New Biodegradable Nano Cellulose-Based Drug Delivery System for PH-Controlled Delivery of Curcumin. Int. J. Biol. Macromol. 2021, 183, 2044–2054. [Google Scholar] [CrossRef]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced Anti-Cancer Activity by Localized Delivery of Curcumin Form PVA/CNCs Hydrogel Membranes: Preparation and in Vitro Bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart Drug Delivery of P-Coumaric Acid Loaded Aptamer Conjugated Starch Nanoparticles for Effective Triple-Negative Breast Cancer Therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef]
- He, C.; Guo, Y.; Karmakar, B.; El-kott, A.; Ahmed, A.E.; Khames, A. Decorated Silver Nanoparticles on Biodegradable Magnetic Chitosan/Starch Composite: Investigation of Its Cytotoxicity, Antioxidant and Anti-Human Breast Cancer Properties. J. Environ. Chem. Eng. 2021, 9, 106393. [Google Scholar] [CrossRef]
- Xu, C.; Li, S.; Chen, J.; Wang, H.; Li, Z.; Deng, Q.; Li, J.; Wang, X.; Xiong, Y.; Zhang, Z.; et al. Doxorubicin and Erastin Co-Loaded Hydroxyethyl Starch-Polycaprolactone Nanoparticles for Synergistic Cancer Therapy. J. Control. Release 2023, 356, 256–271. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Wang, H.; Li, Z.; Chen, J.; Zhang, Z.; Zeng, H.; Yu, X.; Yang, X.; Yang, X.; et al. Hydroxyethyl Starch-Folic Acid Conjugates Stabilized Theranostic Nanoparticles for Cancer Therapy. J. Control. Release 2023, 353, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Alp, E.; Damkaci, F.; Guven, E.; Tenniswood, M. Starch Nanoparticles for Delivery of the Histone Deacetylase Inhibitor CG-1521 in Breast Cancer Treatment. Int. J. Nanomed. 2019, 14, 1335–1346. [Google Scholar] [CrossRef]
- Steeves, M.; Combita, D.; Whelan, W.; Ahmed, M. Chemotherapeutics Loaded Poly(Dopamine) Core-Shell Nanoparticles for Breast Cancer Treatment. J. Pharmacol. Exp. Ther. 2024, 390, 78–87. [Google Scholar] [CrossRef]
- Healy, S.; Henderson, E.T.; Ke, S.; Kelly, J.; Simons, B.W.; Hu, C.; Ivkov, R.; Korangath, P. Spatial Analysis of Nanoparticle Distribution in Human Breast Xenografts Reveals Nanoparticles Targeted to Cancer Cells Localized with Tumor-Associated Stromal Cells. Nanotheranostics 2023, 7, 393–411. [Google Scholar] [CrossRef]
- Eskandani, M.; Derakhshankhah, H.; Zare, S.; Jahanban-Esfahlan, R.; Jaymand, M. Enzymatically Crosslinked Magnetic Starch-Grafted Poly(Tannic Acid) Hydrogel for “Smart” Cancer Treatment: An in Vitro Chemo/Hyperthermia Therapy Study. Int. J. Biol. Macromol. 2023, 253, 127214. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, C.; Zhang, F.; Zhou, Z.; Sun, M. “Attractive/Adhesion Force” Dual-Regulatory Nanogels Capable of CXCR4 Antagonism and Autophagy Inhibition for the Treatment of Metastatic Breast Cancer. J. Control. Release 2022, 341, 892–903. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-Responsive Drug/Gene Delivery System Based on Polyethylenimine Cyclodextrin Nanoparticles for Potential Cancer Therapy. Carbohydr. Polym. 2022, 276, 118747. [Google Scholar] [CrossRef]
- Tan, T.; Yang, Q.; Chen, D.; Zhao, J.; Xiang, L.; Feng, J.; Song, X.; Fu, Y.; Gong, T. Chondroitin Sulfate-Mediated Albumin Corona Nanoparticles for the Treatment of Breast Cancer. Asian J. Pharm. Sci. 2021, 16, 508–518. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Liu, M.; Wang, R.; Qiu, N.; Liu, Y.; Yang, H.; Ji, J.; Zhai, G. Chondroitin Sulfate-Based Nanoparticles for Enhanced Chemo-Photodynamic Therapy Overcoming Multidrug Resistance and Lung Metastasis of Breast Cancer. Carbohydr. Polym. 2021, 254, 117459. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-Assembled Formation of Chondroitin Sulfate-Based Micellar Nanogel for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ji, J.; Li, L.; Zhai, G. Redox-Responsive Nanoparticles Based on Chondroitin Sulfate and Docetaxel Prodrug for Tumor Targeted Delivery of Docetaxel. Carbohydr. Polym. 2021, 255, 117393. [Google Scholar] [CrossRef]
- Singhai, N.J.; Maheshwari, R.; Jain, N.K.; Ramteke, S. Chondroitin Sulphate and α-Tocopheryl Succinate Tethered Multiwalled Carbon Nanotubes for Dual-Action Therapy of Triple-Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 102080. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhao, J.; Rong, H.; Chen, Y.; Xiong, M.; Ye, X.; Yu, S.; Hu, H. Coordinated Regulation of BACH1 and Mitochondrial Metabolism through Tumor-Targeted Self-Assembled Nanoparticles for Effective Triple Negative Breast Cancer Combination Therapy. Acta Pharm. Sin. B 2022, 12, 3934–3951. [Google Scholar] [CrossRef]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and Quercetin Co-Loaded Functional Mesoporous Silica Nanoparticles Overcoming Multidrug Resistance in Breast Cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef] [PubMed]
- Oommen, O.P.; Duehrkop, C.; Nilsson, B.; Hilborn, J.; Varghese, O.P. Multifunctional Hyaluronic Acid and Chondroitin Sulfate Nanoparticles: Impact of Glycosaminoglycan Presentation on Receptor Mediated Cellular Uptake and Immune Activation. ACS Appl. Mater. Interfaces 2016, 8, 20614–20624. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Bile Acid-Conjugated Chondroitin Sulfate A-Based Nanoparticles for Tumor-Targeted Anticancer Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, H.S.; Kang, N.-W.; Lee, S.Y.; Kim, D.H.; Kim, S.; Yoon, I.-S.; Cho, H.-J.; Kim, D.-D. Blood Component Ridable and CD44 Receptor Targetable Nanoparticles Based on a Maleimide-Functionalized Chondroitin Sulfate Derivative. Carbohydr. Polym. 2020, 230, 115568. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhai, G. Self-Assembled Nanoparticles Based on Chondroitin Sulfate-Deoxycholic Acid Conjugates for Docetaxel Delivery: Effect of Degree of Substitution of Deoxycholic Acid. Colloids Surf. B Biointerfaces 2016, 146, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef] [PubMed]
- AbdElhamid, A.S.; Zayed, D.G.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zein-El-Dein, E.A.; El-Gizawy, S.A.; Elzoghby, A.O. Lactoferrin-Tagged Quantum Dots-Based Theranostic Nanocapsules for Combined COX-2 Inhibitor/Herbal Therapy of Breast Cancer. Nanomedicine 2018, 13, 2637–2656. [Google Scholar] [CrossRef]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Eldar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel Pullulan Bioconjugate for Selective Breast Cancer Bone Metastases Treatment. Bioconjug. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef]
- Xu, R.; Sui, J.; Zhao, M.; Yang, Y.; Tong, L.; Liu, Y.; Sun, Y.; Fan, Y.; Liang, J.; Zhang, X. Targeted Inhibition of HER-2 Positive Breast Cancer Cells by Trastuzumab Functionalized Pullulan-Doxorubicin Nanoparticles. Polym. Test. 2022, 113, 107669. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible Cationic Pullulan-g-Desoxycholic Acid-g-PEI Micelles Used to Co-Deliver Drug and Gene for Cancer Therapy. Mater. Sci. Eng. C 2017, 70, 418–429. [Google Scholar] [CrossRef]
- Asgari, S.; Pourjavadi, A.; Setayeshmehr, M.; Boisen, A.; Ajalloueian, F. Encapsulation of Drug-Loaded Graphene Oxide-Based Nanocarrier into Electrospun Pullulan Nanofibers for Potential Local Chemotherapy of Breast Cancer. Macromol. Chem. Phys. 2021, 222, 2100096. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, C.; Zhou, P.; Liu, Y.; An, T.; Sun, D.; Zhang, N.; Wang, Y. Core-Shell Nanoparticles Based on Pullulan and Poly(β-Amino) Ester for Hepatoma-Targeted Codelivery of Gene and Chemotherapy Agent. ACS Appl. Mater. Interfaces 2014, 6, 18712–18720. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Y.; Wang, Y.; Wu, J.; Yang, X.; Li, R.; Wang, Y.; Zhang, N. PH-Sensitive Pullulan-Based Nanoparticle Carrier for Adriamycin to Overcome Drug-Resistance of Cancer Cells. Carbohydr. Polym. 2014, 111, 908–917. [Google Scholar] [CrossRef]
- Pranatharthiharan, S.; Patel, M.D.; Malshe, V.C.; Devarajan, P. V Polyethylene Sebacate Doxorubicin Nanoparticles: Role of Carbohydrate Anchoring on in Vitro and in Vivo Anticancer Efficacy. Drug Deliv. 2016, 23, 2980–2989. [Google Scholar] [CrossRef]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-Organized Nanogels Responding to Tumor Extracellular PH: PH-Dependent Drug Release and in Vitro Cytotoxicity against MCF-7 Cells. Bioconjug. Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef]
- De, A.; Wadhwani, A.; Sauraj; Roychowdhury, P.; Kang, J.H.; Ko, Y.T.; Kuppusamy, G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Mehata, A.K.; Bharti, S.; Singh, P.; Viswanadh, M.K.; Kumari, L.; Agrawal, P.; Singh, S.; Koch, B.; Muthu, M.S. Trastuzumab Decorated TPGS-g-Chitosan Nanoparticles for Targeted Breast Cancer Therapy. Colloids Surf. B Biointerfaces 2019, 173, 366–377. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K.; Gärtner, F.; Gonçalves, R.M.; De Wever, O.; Oliveira, M.J. Chitosan/γ-PGA Nanoparticles-Based Immunotherapy as Adjuvant to Radiotherapy in Breast Cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Masson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Oliveira, C.; Gonçalves, C.S.; Martins, E.P.; Neves, N.M.; Reis, R.L.; Costa, B.M.; Silva, T.H.; Martins, A. Fucoidan/Chitosan Nanoparticles Functionalized with Anti-ErbB-2 Target Breast Cancer Cells and Impair Tumor Growth in Vivo. Int. J. Pharm. 2021, 600, 120548. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic Alginate/Chitosan Nanoparticles for Targeted Delivery of Curcumin into Human Breast Cancer Cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Q.; Zhou, Z.; He, N.; Li, S.; Zhao, C. Co-Delivery of Doxorubicin and Hydroxychloroquine via Chitosan/Alginate Nanoparticles for Blocking Autophagy and Enhancing Chemotherapy in Breast Cancer Therapy. Front. Pharmacol. 2023, 14, 1176232. [Google Scholar] [CrossRef]
- Rosch, J.G.; Winter, H.; DuRoss, A.N.; Sahay, G.; Sun, C. Inverse-Micelle Synthesis of Doxorubicin-Loaded Alginate/Chitosan Nanoparticles and in Vitro Assessment of Breast Cancer Cytotoxicity. Colloid Interface Sci. Commun. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Osanloo, M.; Pishamad, S.; Ghanbariasad, A.; Zarenezhad, E.; Alipanah, M.; Alipanah, H. Comparison Effects of Ferula Gummosa Essential Oil and Beta-Pinene Alginate Nanoparticles on Human Melanoma and Breast Cancer Cells Proliferation and Apoptotic Index in Short Term Normobaric Hyperoxic Model. BMC Complement. Med. Ther. 2023, 23, 428. [Google Scholar] [CrossRef]
- Espinosa-Cano, E.; Huerta-Madroñal, M.; Cámara-Sánchez, P.; Seras-Franzoso, J.; Schwartz, S.J.; Abasolo, I.; San Román, J.; Aguilar, M.R. Hyaluronic Acid (HA)-Coated Naproxen-Nanoparticles Selectively Target Breast Cancer Stem Cells through COX-Independent Pathways. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112024. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Liang, X.; Yin, Y.; Zuo, T.; Chen, D.; Shen, Q. Hyaluronic Acid-Modified Cationic Nanoparticles Overcome Enzyme CYP1B1-Mediated Breast Cancer Multidrug Resistance. Nanomedicine 2019, 14, 447–464. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Costa, E.C.; Correia, I.J. Hyaluronic Acid Functionalized Nanoparticles Loaded with IR780 and DOX for Cancer Chemo-Photothermal Therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Photodynamic Therapy/Chemotherapy against Breast Cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jia, W.; Dong, P.; Liu, J.; Wang, S.; Li, X.; Hu, J.; Zhao, L.; Shi, Y. Improved Antitumor Efficacy of a Dextran-Based Docetaxel-Coupled Conjugate against Triple-Negative Breast Cancer. Curr. Drug Deliv. 2024, 21, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Liu, J.; Lv, H.; Wu, J.; Zhang, N.; Wang, S.; Li, X.; Hu, J.; Wang, A.; Li, D.J.; et al. The Enhanced Antitumor Activity of the Polymeric Conjugate Covalently Coupled with Docetaxel and Docosahexaenoic Acid. Biomater. Sci. 2022, 10, 3454–3465. [Google Scholar] [CrossRef]
- Askar, M.A.; El Shawi, O.E.; Abou Zaid, O.A.R.; Mansour, N.A.; Hanafy, A.M. Breast Cancer Suppression by Curcumin-Naringenin-Magnetic-Nano-Particles: In Vitro and in Vivo Studies. Tumor Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Sajid, M.I.; Parang, K.; Tiwari, R.K. Synthesis, Characterization, and Cytotoxicity Evaluation of Dextran-Myristoyl-ECGKRK Peptide Conjugate. Int. J. Biol. Macromol. 2021, 191, 1204–1211. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S. A Convergent Fabrication of PH and Redox Dual-Responsive Hybrids of Mesoporous Silica Nanoparticles for the Treatment of Breast Cancer. J. Biomater. Sci. Polym. Ed. 2023, 34, 147–165. [Google Scholar] [CrossRef]
- Allahyari, M.; Motavalizadeh-Kakhky, A.R.; Mehrzad, J.; Zhiani, R.; Chamani, J. Cellulose Nanocrystals Derived from Chicory Plant: An Un-Competitive Inhibitor of Aromatase in Breast Cancer Cells via PI3K/AKT/MTOP Signalling Pathway. J. Biomol. Struct. Dyn. 2023, 42, 5575–5589. [Google Scholar] [CrossRef]
- Jutakridsada, P.; Suwannaruang, T.; Kasemsiri, P.; Weerapreeyakul, N.; Knijnenburg, J.T.N.; Theerakulpisut, S.; Kamwilaisak, K.; Chindaprasirt, P. Controllability, Antiproliferative Activity, Ag(+) Release, and Flow Behavior of Silver Nanoparticles Deposited onto Cellulose Nanocrystals. Int. J. Biol. Macromol. 2023, 225, 899–910. [Google Scholar] [CrossRef]
- Khella, K.F.; Abd El Maksoud, A.I.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef]
- Omrani, Z.; Pourmadadi, M.; Yazdian, F.; Rashedi, H. Preparation and Characterization of PH-Sensitive Chitosan/Starch/MoS(2) Nanocomposite for Control Release of Curcumin Macromolecules Drug Delivery; Application in the Breast Cancer Treatment. Int. J. Biol. Macromol. 2023, 250, 125897. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Tajiki, A.; Abdouss, M. A Green Approach for Preparation of Polyacrylic Acid/Starch Incorporated with Titanium Dioxide Nanocomposite as a Biocompatible Platform for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2023, 242, 124785. [Google Scholar] [CrossRef] [PubMed]
- Azimijou, N.; Karimi-Soflou, R.; Karkhaneh, A. CD44 Targeted-Chondroitin Sulfate Nanoparticles: Fine-Tuning Hydrophobic Groups to Enhance in Vitro PH-Responsiveness and in Vivo Efficacy for Advanced Breast Cancer Treatment. Biomater. Adv. 2024, 158, 213776. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, J.; Jiang, R.; Yuan, Q.; Ding, Y.; Ren, J.; Jiang, D.; Wang, Y.; Wang, L.; Chen, P.; et al. Versatile Chondroitin Sulfate-Based Nanoplatform for Chemo-Photodynamic Therapy against Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2024, 265, 130709. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Ling, Y.; Xiao, X.; Gong, J.; Jin, H.; Xu, J.; Chen, P.; Xie, X.; Zhang, L. Peptide-Modified Bioresponsive Chondroitin Sulfate Micelles for Targeted Doxorubicin Delivery in Triple-Negative Breast Cancer. Colloids Surf. B Biointerfaces 2023, 227, 113381. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Xie, X.; Zhu, W.; Lei, Z.; Lv, L.; Yu, H.; Xu, J.; Ren, J. Multifunctional Nanoparticles Codelivering Doxorubicin and Amorphous Calcium Carbonate Preloaded with Indocyanine Green for Enhanced Chemo-Photothermal Cancer Therapy. Int. J. Nanomed. 2023, 18, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Xu, F.; Yao, T.; Zhu, J.; Yu, H.; Wang, G.; Li, J. TPGS and Chondroitin Sulfate Dual-Modified Lipid-Albumin Nanosystem for Targeted Delivery of Chemotherapeutic Agent against Multidrug-Resistant Cancer. Int. J. Biol. Macromol. 2021, 183, 1270–1282. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Y.; Wen, S.; Wen, Y.; Wang, R.; Zhang, Q.; Qin, G.; Yi, H.; Wu, M.; Lu, L.; et al. Synergistically Enhanced Inhibitory Effects of Pullulan Nanoparticle-Mediated Co-Delivery of Lovastatin and Doxorubicin to Triple-Negative Breast Cancer Cells. Nanoscale Res. Lett. 2019, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, X.; Qin, J.; Chen, H.; Shen, Z.; Lv, F.; Nan, W.; Wang, Y.; Li, Q.; Zhang, Q. Synthesis, Characterization and In Vivo Efficacy of Biotin-Conjugated Pullulan Acetate Nanoparticles as a Novel Anticancer Drug Carrier. J. Biomed. Nanotechnol. 2017, 13, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/079907316/publication/US2022047559A1?q=pn%3DUS2022047559A1 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/068180514/publication/CN110339372A?q=pn%3DCN110339372A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/062593328/publication/CN108186607A?q=pn%3DCN108186607A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/063206357/publication/CN108434123A?q=pn%3DCN108434123A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/072666554/publication/US12377117B2?q=pn%3DUS12377117B2 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/089532444/publication/CN117414346A?q=pn%3DCN117414346A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/062240944/publication/WO2018098705A1?q=pn%3DWO2018098705A1 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/067793550/publication/CN110201181A?q=pn%3DCN110201181A (accessed on 20 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).