Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics
Abstract
1. Introduction
2. Results and Discussion
2.1. Absorptional Characteristics of Mango Leaf Samples from Different Varieties
2.2. Foaming and Emulsification Characteristics of Mango Leaf Samples
2.3. Bioactive Profiles of the Different Mango Leaf Varieties
2.4. Pearson Correlation Analysis Between Functional and Bioactive Properties
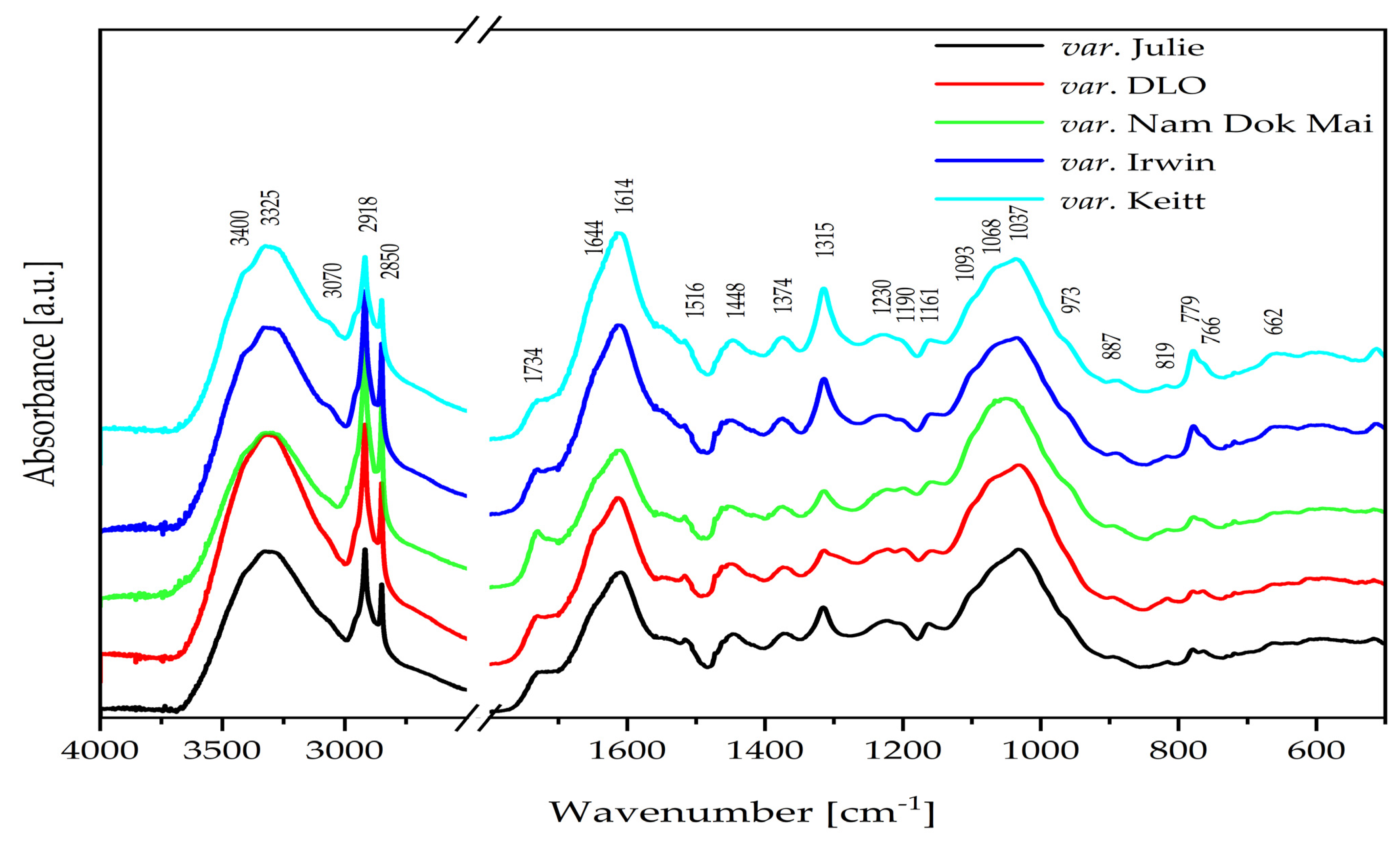
2.5. Mid-Infrared (IR) Spectra of Mango Leaves
3. Materials and Methods
3.1. Materials
3.2. Absorptional Characteristics of Mango Leaf Samples from Different Varieties
3.3. Foaming and Emulsification Characteristics of Mango Leaf Samples
3.4. Bioactive Profiles of the Different Mango Leaf Varieties
3.4.1. Extraction Procedure
3.4.2. Reducing Sugar Content
3.4.3. DPPH Radical Scavenging Activity
3.4.4. ABTS Radical Cation Decolorization Assay
3.4.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Mid-Infrared (IR) Spectra of Mango Leaves
3.6. Statistical Analysis
4. Conclusions
Study Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, V.M.; Rathod, V.K. Extraction of mangiferin from Mangifera indica leaves using three phase partitioning coupled with ultrasound. Ind. Crops Prod. 2014, 52, 292–297. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Rathod, V.K. A novel method to augment extraction of mangiferin by application of microwave on three phase partitioning. Biotechnol. Rep. 2014, 6, 8–12. [Google Scholar] [CrossRef]
- Saleem, M.; Tanvir, M.; Akhtar, M.F.; Iqbal, M.; Saleem, A. Antidiabetic potential of Mangifera indica L. cv. Anwar Ratol leaves: Medicinal application of food wastes. Medicina 2019, 55, 353. [Google Scholar] [CrossRef]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera indica (mango): A promising medicinal plant for breast cancer therapy and understanding its potential mechanisms of action. Breast Cancer Targets Ther. 2021, 13, 471–503. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.T.; Richi, A.E.; Riju, S.A.; Jalal, T.; Orchi, R.J.; Singh, S.; Bhagat, P.; Abdel-Wahab, Y.H.A.; Ansari, P. Evaluation of antidiabetic potential of Mangifera indica leaf in streptozotocin-induced type 2 diabetic rats: Focus on glycemic control and cholesterol regulation. Endocrines 2024, 5, 137–152. [Google Scholar] [CrossRef]
- Kaur, J.; Kaushik, D.; Kumar, M.; Babagil, G.E.; Amarowicz, R.; Proestos, C.; Oz, E.; Oz, F.; Esatbeyoglu, T.; Bordiga, M. Comprehensive analysis and characterization of Mangifera indica L. leaf powder. Food Sci. Nutr. 2025, 13, e70083. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sreenivasulu, A.; Veerendra, G.T.N.; Phani Manoj, A.V.; Haripavan, N. Synthesis and characterization of mango leaves biosorbents for removal of iron and phosphorous from contaminated water. Appl. Surf. Sci. Adv. 2022, 11, 100292. [Google Scholar] [CrossRef]
- Thiruketheeswaranathan, S. Comparative adsorption study of mango (Mangifera indica) leaves and neem (Azadirachta indica) leaves as an adsorbent for dye removal. J. Sci. 2022, 13, 49. [Google Scholar] [CrossRef]
- Rossouw, G.C.; Orr, R.; Bennett, D.; Bally, I.S.E. The roles of non-structural carbohydrates in fruiting: A review focusing on mango (Mangifera indica). Funct. Plant Biol. 2024, 51, FP23195. [Google Scholar] [CrossRef]
- Khaled, B.M.; Das, A.K.; Alam, S.M.S.; Saqib, N.; Rana, M.S.; Sweet, S.R.; Naznin, T.; Hossain, M.P.; Sardar, S.; Hossain, Z.; et al. Effect of different drying techniques on the physicochemical and nutritional properties of Moringa oleifera leaves powder and their application in bakery product. Appl. Food Res. 2024, 4, 100599. [Google Scholar] [CrossRef]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, É.; Dufossé, L.; Guérard, F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Pirca-Palomino, M.; Malange, Y.I.; Ramos-Escudero, F.; Muñoz, A.M.; Cancino-Chávez, K. Antioxidant properties, texture and sensory quality of sliced bread enriched with leaf powder from mango (Mangifera indica). Pol. J. Food Nutr. Sci. 2024, 74, 313–322. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Litz, R.E. Introduction: Botany and importance. In The Mango: Botany, Production and Uses; CABI: Wallingford, UK, 2009; pp. 1–18. [Google Scholar]
- Ueda, M.; Sasaki, K.; Utsunomiya, N.; Inaba, K.; Shimabayashi, Y. Changes in physical and chemical properties during maturation of mango fruit (Mangifera indica L. ‘Irwin’) cultured in a plastic greenhouse. Food Sci. Technol. Res. 2000, 6, 299–305. [Google Scholar] [CrossRef]
- Feuillard, T.; Abillon, J.-M. Estimation of hourly insolation in Guadeloupe. Sol. Wind Technol. 1990, 7, 541–543. [Google Scholar] [CrossRef]
- Bertin, A.; Frangi, J.P. Contribution to the study of the wind and solar radiation over Guadeloupe. Energy Convers. Manag. 2013, 75, 593–602. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C.; Hagerman, A.E.; Davies, N.W.; Beadle, C.L. Phenolic acclimation to ultraviolet-A irradiation in Eucalyptus nitens seedlings raised across a nutrient environment gradient. Photosynthetica 2007, 45, 36–42. [Google Scholar] [CrossRef]
- Younis, M.E.B.; Hasaneen, M.N.A.; Abdel-Aziz, H.M. An enhancing effect of visible light and UV radiation on phenolic compounds and various antioxidants in broad bean seedlings. Plant Signal. Behav. 2010, 5, 1197–1203. [Google Scholar] [CrossRef]
- Santos, I.; Fidalgo, F.; Almeida, J.M.; Salema, R. Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci. 2004, 167, 925–935. [Google Scholar] [CrossRef]
- Robin, G. Julie Mango in the Eastern Caribbean: A Comprehensive Manual; Caribbean Agricultural Research and Development Institute: St. Augustine, Trinidad and Tobago, 2001. [Google Scholar]
- Guamán-Balcázar, M.C.; Montes, A.; Pereyra, C.; de la Ossa, E.M. Precipitation of mango leaves antioxidants by supercritical antisolvent process. J. Supercrit. Fluids 2017, 128, 218–226. [Google Scholar] [CrossRef]
- Sadia, H.; Jeba, F.; Kamal, A.; Salam, A. Air pollution tolerance index of Mangifera indica plant species growing in the greater Dhaka region, Bangladesh. J. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Schieber, A. Bioactive compounds in mango (Mangifera indica L.). In Bioactive Foods in Promoting Health; Elsevier BV: Oxford, UK, 2010; pp. 507–523. [Google Scholar]
- Parvez, G.M.M. Pharmacological activities of mango (Mangifera indica): A review. J. Pharmacogn. Phytochem. 2016, 5, 1–7. [Google Scholar]
- Bohne, A.-V.; Schwenkert, S.; Grimm, B.; Nickelsen, J. Roles of tetratricopeptide repeat proteins in biogenesis of the photosynthetic apparatus. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 324, pp. 187–227. [Google Scholar] [CrossRef]
- Ralison, S.; Tounkara, F.; Karangwa, E.; Yong, S.; Guowei, L. In vitro antioxidant activities of protein hydrolysate from germinated black soybean (Glycine max L.). Adv. J. Food Sci. Technol. 2013, 5, 453–459. [Google Scholar] [CrossRef]
- Fafiolu, A.; Odugunawa, O.; Bambose, A.; Oso, A.; Isah, O.; Olantanji, J.; Jegede, A. Feeding value of mango leaf (Mangifera indica) for growing rabbits. J. Anim. Vet. Adv. 2006, 5, 800–804. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Chichester, UK, 2004; ISBN 978-0-47009-307-8. [Google Scholar]
- Feng, Y.; Jin, C.; Lv, S.; Zhang, H.; Ren, F.; Wang, J. Molecular mechanisms and applications of polyphenol-protein complexes with antioxidant properties: A review. Antioxidants 2023, 12, 1577. [Google Scholar] [CrossRef]
- Işık, M.; Dikici, E.; Altın, S.; Alp, C.; Kırboğa, K.K.; Köksal, E.; Beydemir, Ş. Phenolic content, antioxidant capacity, and therapeutic potential of mango (Mangifera indica L.) leaves. Food Sci. Nutr. 2025, 13, e70263. [Google Scholar] [CrossRef]
- Sferrazzo, G.; Palmeri, R.; Restuccia, C.; Parafati, L.; Siracusa, L.; Spampinato, M.; Carota, G.; Distefano, A.; Di Rosa, M.; Tomasello, B.; et al. Mangifera indica L. leaves as a potential food source of phenolic compounds with biological activity. Antioxidants 2022, 11, 1313. [Google Scholar] [CrossRef]
- Saxena, P.; Sharma, D.; Gautam, P.; Niranjan, A.; Rastogi, S. HPLC-DAD quantification of mangiferin, antioxidant potential and essential oil composition of the leaves of five varieties of Mangifera indica L. of North India. Nat. Prod. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Zając, A.; Dymińska, L.; Lorenc, J.; Ptak, M.; Hanuza, J. Depth profiling of horse fat tissue using mid-infrared and confocal Raman microscope. Spectrosc. Lett. 2018, 51, 81–88. [Google Scholar] [CrossRef]
- Das, S.P.; Ravindran, R.; Deka, D.; Jawed, M.; Das, D.; Goyal, A. Bioethanol production from leafy biomass of mango (Mangifera indica) involving naturally isolated and recombinant enzymes. Prep. Biochem. Biotechnol. 2013, 43, 717–734. [Google Scholar] [CrossRef]
- Villanueva, M.; De Lamo, B.; Harasym, J.; Ronda, F. Microwave radiation and protein addition modulate hydration, pasting and gel rheological characteristics of rice and potato starches. Carbohydr. Polym. 2018, 201, 374–381. [Google Scholar] [CrossRef]
- Harasym, J.; Satta, E.; Kaim, U. Ultrasound treatment of buckwheat grains impacts important functional properties of resulting flour. Molecules 2020, 25, 3012. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia esculenta), Rice (Oryza sativa) Flour, Pigeonpea (Cajanus cajan) Flour and Their Blends. LWT Food Sci. Technol. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Kiiru, S.; Ng’ang’a, J.; Konyole, S.; Roos, N.; Hetzer, B.; Marel, A.; Kinyuru, J. Physicochemical Characterisation and Impact of Gryllus bimaculatus Addition on Gluten-Free Flour Blends. Int. J. Food Sci. Technol. 2024, 59, 4620–4634. [Google Scholar] [CrossRef]
- Olędzki, R.; Lutosławski, K.; Nowicka, P.; Wojdyło, A.; Harasym, J. Non-Commercial Grapevines Hybrids Fruits as a Novel Food of High Antioxidant Activity. Foods 2022, 11, 2216. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. Quantitative Analysis of Reducing Sugars by 3,5-Dinitrosalicylic Acid (DNSA Method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Humana: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
| Sample | WHC [g H2O/g DM] | WAC [g H2O/g DM] | OAC [g oil/g DM] | HLI | WAI [g H2O/g DM] | WSI [g H2O/100 g DM] | SP [g H2O/g DM] |
|---|---|---|---|---|---|---|---|
| Julie | 5.08 ± 0.10 b | 3.53 ± 0.15 ab | 2.70 ± 0.17 b | 1.307 ± 0.030 a | 5.14 ± 0.18 b | 13.89 ± 0.95 a | 4.72 ± 0.18 b |
| DLO | 5.19 ± 0.33 b | 3.59 ± 0.08 b | 2.69 ± 0.04 b | 1.336 ± 0.040 ab | 3.68 ± 0.34 a | 28.69 ± 7.72 c | 2.82 ± 0.56 a |
| Nam Dok Mai | 5.53 ± 0.14 c | 3.48 ± 0.12 ab | 2.21 ± 0.08 a | 1.576 ± 0.025 c | 5.17 ± 0.29 b | 19.41 ± 2.70 b | 4.58 ± 0.33 b |
| Irwin | 5.19 ± 1.33 b | 3.76 ± 0.38 b | 2.77 ± 0.13 b | 1.354 ± 0.080 b | 4.72 ± 0.07 b | 15.68 ± 1.26 ab | 4.10 ± 0.10 b |
| Keïtt | 4.64 ± 0.05 a | 3.73 ± 0.12 b | 2.49 ± 0.08 ab | 1.501 ± 0.088 bc | 3.60 ± 0.19 a | 30.83 ± 6.47 c | 2.67 ± 0.39 a |
| Sample | FC [mL] | FS [%] | EA [%] | ES [%] |
|---|---|---|---|---|
| Julie | 65.29 ± 9.76 a | 121.9 ± 16.6 b | 1.33 ± 0.37 ab | 3.32 ± 1.31 b |
| DLO | 59.79 ± 2.25 a | 112.8 ± 4.2 a | 1.33 ± 0.37 ab | 2.22 ± 1.49 a |
| Nam Dok Mai | 82.69 ± 7.79 b | 153.1 ± 14.4 c | 1.66 ± 0.78 b | 2.10 ± 1.40 a |
| Irwin | 64.56 ± 1.55 a | 125.3 ± 1.3 b | 0.55 ± 0.00 a | 3.28 ± 0.00 b |
| Keïtt | 67.29 ± 2.32 a | 129.4 ± 0.9 b | 3.83 ± 0.78 c | 2.47 ± 0.39 a |
| Sample | Reducing Sugars | DPPH ETOH | DPPH H2O | ABTS ETOH | ABTS H2O | FRAP ETOH | FRAP H2O |
|---|---|---|---|---|---|---|---|
| [GE mg/g DM] | [TE mg/g DM] | [FeSO4 mg/g DM] | |||||
| Julie | 20.61 ± 0.58 a | 1.95 ± 0.25 a | 2.25 ± 0.35 a | 3.45 ± 0.45 a | 1.65 ± 0.18 a | 1.65 ± 0.15 a | 1.95 ± 0.12 a |
| DLO | 24.67 ± 1.57 bc | 1.85 ± 0.35 a | 2.45 ± 0.42 ab | 3.85 ± 0.65 b | 1.85 ± 0.28 ab | 1.78 ± 0.18 a | 2.05 ± 0.18 ab |
| Nam Dok Mai | 25.92 ± 0.37 c | 2.15 ± 0.45 a | 2.89 ± 0.58 b | 4.12 ± 0.78 b | 2.05 ± 0.15 b | 1.95 ± 0.12 a | 2.18 ± 0.25 b |
| Irwin | 28.04 ± 2.46 d | 2.65 ± 0.55 b | 3.15 ± 0.65 c | 4.35 ± 0.85 c | 2.15 ± 0.35 c | 2.05 ± 0.22 b | 2.35 ± 0.28 c |
| Keïtt | 27.36 ± 0.77 cd | 2.45 ± 0.35 b | 2.95 ± 0.48 bc | 4.15 ± 0.68 b | 1.95 ± 0.25 b | 1.85 ± 0.18 a | 2.25 ± 0.22 bc |
| WHC | WAC | OAC | HLI | WAI | WSI | SP | FC | FS | EA | ES | DPPH_H2O | ABTS_H2O | FRAP_H2O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHC | 1.000 | −0.629 | −0.471 | 0.486 | 0.648 | −0.515 | 0.626 | 0.378 | 0.537 | −0.714 | −0.204 | 0.495 | 0.528 | 0.527 |
| WAC | 1.000 | 0.213 | −0.137 | −0.271 | 0.447 | −0.291 | −0.043 | −0.191 | 0.204 | 0.343 | 0.612 | 0.367 | 0.717 | |
| OAC | 1.000 | −0.942 | 0.348 | 0.120 | 0.368 | −0.916 | −0.898 | −0.425 | 0.703 | 0.425 | 0.155 | 0.332 | ||
| HLI | 1.000 | −0.449 | −0.253 | −0.465 | 0.847 | 0.877 | 0.545 | −0.661 | −0.384 | −0.094 | −0.281 | |||
| WAI | 1.000 | −0.922 | 0.998 | 0.195 | 0.360 | −0.556 | 0.399 | 0.527 | 0.588 | 0.580 | ||||
| WSI | 1.000 | −0.938 | −0.357 | −0.487 | 0.706 | −0.694 | −0.419 | −0.484 | −0.442 | |||||
| SP | 1.000 | 0.229 | 0.393 | −0.573 | 0.443 | 0.548 | 0.608 | 0.598 | ||||||
| FC | 1.000 | 0.990 | 0.137 | −0.413 | −0.217 | 0.003 | −0.128 | |||||||
| FS | 1.000 | 0.173 | −0.381 | −0.165 | 0.055 | −0.072 | ||||||||
| EA | 1.000 | −0.409 | 0.140 | −0.086 | 0.117 | |||||||||
| ES | 1.000 | −0.075 | −0.188 | −0.001 | ||||||||||
| DPPH_H2O | 1.000 | 0.949 | 0.989 | |||||||||||
| ABTS_H2O | 1.000 | 0.930 | ||||||||||||
| FRAP_H2O | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, G.; Marchaux, I.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Zając, A.; Paroń, O.; Aurore, G.; Harasym, J. Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics. Molecules 2025, 30, 3381. https://doi.org/10.3390/molecules30163381
Lawrence G, Marchaux I, Pejcz E, Wojciechowicz-Budzisz A, Olędzki R, Zając A, Paroń O, Aurore G, Harasym J. Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics. Molecules. 2025; 30(16):3381. https://doi.org/10.3390/molecules30163381
Chicago/Turabian StyleLawrence, Génica, Ingrid Marchaux, Ewa Pejcz, Agata Wojciechowicz-Budzisz, Remigiusz Olędzki, Adam Zając, Oliwia Paroń, Guylène Aurore, and Joanna Harasym. 2025. "Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics" Molecules 30, no. 16: 3381. https://doi.org/10.3390/molecules30163381
APA StyleLawrence, G., Marchaux, I., Pejcz, E., Wojciechowicz-Budzisz, A., Olędzki, R., Zając, A., Paroń, O., Aurore, G., & Harasym, J. (2025). Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics. Molecules, 30(16), 3381. https://doi.org/10.3390/molecules30163381







