Abstract
Billions of tons of agro-industrial residues are produced worldwide. This is associated with the risk of pollution as well as management and economic problems. Simultaneously, non-edible portions of many crops are rich in bioactive compounds with valuable properties. For this reason, developing various methods for utilizing agro-industrial residues as a source of high-value by-products is very important. The main objective of the paper is a review of the newest studies on biologically active compounds included in non-edible parts of crops with the highest amount of waste generated annually in the world. The review also provides the newest data on the chemical and biological properties, as well as the potential application of phytochemicals from such waste. The review shows that, in 2020, there were above 6 billion tonnes of residues only from the most popular crops. The greatest amount is generated during sugar, oil, and flour production. All described residues contain valuable phytochemicals that exhibit antioxidant, antimicrobial and very often anti-cancer activity. Many studies show interesting applications, mainly in pharmaceuticals and food production, but also in agriculture and wastewater remediation, as well as metal and steel industries.
1. Introduction
The agricultural industry generates billions of tonnes of waste from the tillage and processing of various crops. The crops with the largest amounts of produced residues are rice, maize, soybean, sugarcane, potato, tomato, and cucumber, as well as some fruits, mainly bananas, oranges, grapes, and apples [1,2]. It has been estimated that European food processing companies generate annually approximately 100 Mt of waste and by-products, mostly during the production of drinks (26%), dairy and ice cream (21.3%), and fruits and vegetables (14.8%) [3].
In Table 1, the amounts of particular wastes generated worldwide are presented. Many of them are rich in biologically active compounds and have the potential to become important raw materials for obtaining valuable phytochemicals. Vegetable and fruit processing by-products are promising sources of valuable phytochemicals having antioxidant, antimicrobial, anti-inflammatory, anti-cancer, and cardiovascular protection activities [4]. The applications of these agro-industrial residues and their bioactive compounds in functional food and cosmetics production were presented in many studies [5,6,7]. Moreover, due to the potential health risk of some synthetic antioxidants such as BHA, the identification and isolation of natural antioxidants from waste has become increasingly attractive. Important criteria to decide if a product or by-product can be of interest to recover phytochemicals are the absolute concentration and preconcentration factor, as well as the total amount of product or by-product per batch [8].

Table 1.
Amount of residues from some crops produced in the world in 2020.
As interest in waste processing has been growing in recent years, many scientific papers have been published on new compounds in agro-industrial waste, new properties of valuable phytochemicals contained in crop residues and their applications. It seems necessary to summarize and collect the latest knowledge on this subject. In this work, an overview of the recent knowledge on the phytochemicals in some of the most popular food by-products, with the highest amount generated in the world, as well as on their properties and potential applications, have been presented in more detail (Figure 1).
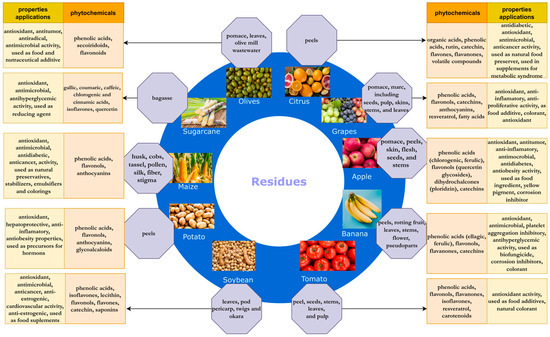
Figure 1.
Agricultural residues and the properties and applications of their phytochemicals.
2. Phytochemicals from Crop Residues
2.1. Sugarcane Bagasse
Large amounts of waste are generated during the processing of sugarcane. In fact, one metric ton of sugarcane generates 280 kg of bagasse. Sugarcane bagasse is one of the most abundant agro-food by-products and is a very promising raw material available at low cost for recovering bioactive substances [18,19]. Sugarcane bagasse consists mainly of cellulose (35–50%), hemicellulose (26–41%), lignin (11–25%), but also some amount of plant secondary metabolites (PSM), mainly anthocyanins and mineral substances [20,21,22,23,24,25].
Phenolic compounds are a very important group of natural substances identified in sugarcane waste. Nonetheless, steam explosion and ultrasound-assisted extraction (UAE) pretreatment was applied for the production of valuable phenolic compounds from the lignin included in this residue. Chromatographic analysis revealed that sugarcane bagasse is a good feedstock for the generation of phenolic acids. The concentration of total phenolics with the Folin-Ciocalteau method was between 2.8 and 3.2 g/L. Zhao et al. [26] have identified many phenolics, mainly flavonoids and phenolic acids, in sugarcane bagasse extract (Table 2). The total polyphenol content was detected as higher than 4 mg/g of dry bagasse, with total flavonoid content of 470 mg quercetin/g of polyphenol. The most abundant phenolic acids identified in the sugarcane bagasse extract were gallic acid (4.36 mg/g extract), ferulic acid (1.87 mg/g extract) and coumaric acid (1.66 mg/g extract). Spectroscopic analysis showed that a predominant amount of p-coumaric acid is ester-linked to the cell wall components, mainly to lignin. On the other hand, about half of the ferulic acid is esterified to the cell wall hemicelluloses. The purified sugarcane bagasse hydrolysate consisted mainly of p-coumaric acid. Besides, the purified products showed the same antioxidant activity, reducing power and free radical scavenging capacity as the standard p-coumaric acid. Al Arni et al. [27] stated that the major natural products contained in the lignin fraction were p-coumaric acid, ferulic acid, syringic acid, and vanillin.

Table 2.
Phytochemicals derived from sugarcane bagasse.
Gallic, coumaric, caffeic, chlorogenic, and cinnamic acids were the main phenolic compounds extracted from raw and alkaline pretreated sugarcane bagasse and identified by high-performance liquid chromatography (HPLC) [28]. The aromatic phenolic compounds (p-coumaric acid, ferulic acid, p-hydroxybenzaldehyde, vanillin, and vanillic acid) were reported in sugarcane bagasse pith. Five phenolic compounds (tricin 4-O-guaiacylglyceryl ether-7-O-glucopyranoside, genistin, p-coumaric acid, quercetin, and genistein) in 30% hydroalcoholic fraction of sugarcane bagasse were identified using ultra-high performance liquid chromatography/high-resolution time of flight mass spectrometry (UHPLC-HR-TOF-MS); (Table 2). The total phenolic content was 170.68 mg gallic acid/g dry extract [19].
Phenolic compounds derived from sugarcane bagasse exhibited many biological activities, which were used in various applications. The most important biological activities and the newest and most interesting applications have been summarized in Table 3.

Table 3.
Biological activities and potential applications of phytochemicals obtained from sugarcane bagasse.
2.2. Maize Residues
Maize (corn Zea mays L.) bran, husk, cobs, tassel, pollen, silk, and fiber are residues of corn production. They contain substantial amounts of phytochemicals, such as phenolic compounds, carotenoid pigments and phytosterols [39] (Table 4).

Table 4.
Phytochemicals identified in corn waste.
Corn bran is produced as a plentiful by-product during the corn dry milling process. Similar to other cereal grains, phenolics in corn bran exist in free insoluble bound and soluble-conjugated forms. Corn bran is a rich source of ferulic acid compared to other cereals, fruits and vegetables. Guo et al. [39] isolated four forms of ferulic acid and its derivates from corn bran. On the other hand, it has been reported that the hexane-derived extract from corn bran contains high levels of ferulate-phytosterol esters, similar in composition and function to oryzanol.
Another corn waste is a husk. It is the outer leafy covering of an ear of Zea mays L. The main constituents of the maize husk extracts determined in various phytochemical studies are phenolic compounds, e.g., flavonoids [41,50]. Saponins, glycosides, and alkaloids are present mainly in the aqueous and methanolic extracts, while phenols and tannins are numerous in methanolic ones [51]. Moreover, corn husk has high contents of anthocyanins [48,52]. Simla et al. [53] reported that anthocyanins concentration in corn husks ranges from 0.003 to 4.9 mg/g. The major anthocyanins of corn husk were identified as malonylation products of cyanidin, pelargonidin, and peonidin derivatives [54].
Important by-products of the corn industry are cobs. For every 100 kg of corn grain, approximately 18 kg of corn cobs are produced. Corn cob is one of the food waste-material having a phytochemical component that has a healthy benefit [55]. They contain cyanidin-3-glucoside and cyanidin-3-(6″malonylglucoside) as main anthocyanins, as well as pelargonidin-3-glucoside, peonidin-3-glucoside and their malonyl counterparts [48].
Corn tassel is a by-product from hybrid corn seed production and an excellent source of phytochemicals (the flavonol glycosides of quercetin, isorhamnetin and kaempferol) with beneficial properties [56]. In Thailand, purple waxy corn is considered a special corn type because it is rich in phenolics, anthocyanins, and carotenoids in the tassel [57]. Besides, corn tassels could be considered a great source of valuable products such as volatile oils.
Corn pollen is another corn waste. Significant amounts of phytochemicals, including carotenoids, steroids, terpenes and flavonoids, are present in maize pollen [52]. Bujang et al. (2021) showed that maize pollen contains a high total phenolic content and total flavonoid content of 783.02 mg gallic acid equivalent (GAE)/100 g and 1706.83 mg quercetin equivalent (QE)/100 g, respectively. The flavonoid pattern of maize pollen is characterized by an accumulation of the predominant flavonols, quercetin and traces of isorhamnetin diglycosides and rutin. According to Žilić et al. [58], the quercetin values in maize pollen were 324.16 μg/g and 81.61 to 466.82 μg/g, respectively.
Corn silk, another by-product from corn processing, contains a wide range of bioactive compounds in the form of volatile oils, steroids, saponins, anthocyanins [59], and other natural antioxidants, such as flavonoids [52] and phenolic compounds [41,58,59]. In the corn silk powder, the high phenolic content (94.10 ± 0.26 mg GAE/g) and flavonoid content (163.93 ± 0.83 mg QE/100 g) are responsible for its high antioxidant activity [60]. About 29 flavonoids have been isolated from corn silk. Most of them are C-glycoside compounds and have the same parent nucleus as luteolin [44]. Ren et al. [61] successfully isolated and separated compounds such as 2″-O-α-l-rhamnosyl-6-C-3″-deoxyglucosyl-3′-methoxyluteolin, ax-5′-methane-3′-methoxymaysin, ax-4″-OH-3′-methoxymaysin, 6,4′-dihydroxy-3′-methoxyflavone-7-O-glucoside, and 7,4′-dihydroxy-3′-methoxyflavone-2″-O-α-l-rhamnosyl-6-C fucoside from corn silk. Moreover, among flavonoids, Haslina and Eva [43] determined in corn silk: apigmaysin, maysin, isoorientin-2″-O-α-l-rhamnoside, 3-methoxymaysine, and ax-4-OH maysin.
This richness of biologically active compounds results in advantageous properties and applications. The most important properties and the newest studies on the application are listed in Table 5.

Table 5.
Biological activity and potential applications of phytochemicals obtained from corn wastes.
2.3. Potato Waste
Approximately 40–50% of potatoes are not suitable for human consumption. Industrial processing of potatoes (mashed and canned potatoes, chips, fries and ready meals) creates huge amounts of peel as waste [66,67]. Potato peel is a non-edible residue generated in considerable amounts by food processing plants. Depending on the peeling process, e.g., abrasion, lye or steam peeling, the amount of waste can range between 15 and 40% of the number of processed potatoes [68]. Industrial processing produces between 70 to 140 thousand tons of peels worldwide annually, which are available to be used in other applications [69].
Potato peels differ greatly from other agricultural by-products because they are revalorized as a source of functional and bioactive compounds, including phenolic compounds, glycoalkaloids, vitamins and minerals [70] (Table 6).

Table 6.
Phytochemicals identified in potato waste.
Potato peel is a good source of phenolic compounds because almost 50% of potato phenolics are located in the peel and adjoining tissues [74,83]. The results obtained by Wu et al. [77] showed that the potato peels contained a higher amount of phenolics than the flesh. Moreover, the polyphenols in potato peel are ten times higher than those in the pulp. Potato peel extract contains 70.82 mg of catechin equivalent (CE)/100 g of phenolic and had a high level of phenolic compounds (2.91 mg GAE/g dry weight) that was found to be greater than carrot (1.52 mg GAE/g dry weight), wheat bran (1.0 mg GAE/g dry weight), and onion (2.5 mg GAE/g dry weight) [67]. The results of Javed et al. [72] showed that the total phenolic content in potato peel ranged from 1.02 to 2.92 g/100 g and total flavonoids ranged from 0.51 to 0.96 g/100 g. Phenolic acids are the most abundant phenolic compounds in potato peel. They include derivatives of hydroxycinnamic and hydroxybenzoic acids (Table 6). Kumari et al. [84], using UHPLC-MS/MS, showed that chlorogenic and caffeic acids are important components of the free-form phenolics in potato peel. The results show that phenolic acids in potato peals are not only present in their free form but also occur in bound form. Javed et al. [72] showed that the extract of potato peel contains chlorogenic acid (753.0–821.3 mg/100 g), caffeic acid (278.0–296.0 mg/100 g), protocatechuic acid (216.0–256.0 mg/100 g), p-hydroxybenzoic acid (82.0–87.0 mg/100 g), gallic acid (58.6–63.0 mg/100 g), vanillic acid (43.0–48.0 mg/100 g), and p-coumaric acid (41.8–45.6 mg/100 g). Silva–Beltran et al. [78] showed that flavonoids such as rutin and quercetin were present in potato peel at low concentrations of 5.01 and 11.22 mg/100 g dry weight, respectively.
Many studies have noted that potato peels are excellent untapped source of steroidal alkaloids, e.g., glycoalkaloids (α-solanine and α-chaconine) and aglycone alkaloids (solanidine and demissidine; Table 6) [80,81,85]. α-solanine, α-chaconine, and the glycosides of solanidine constitute about 95% of the total potato peel glycoalkaloid content [86]. Higher amounts of these compounds were found in potato peel, unlike potato flesh [87]. There are various cultural, genetic and storage factors that influence the concentration of glycoalkaloids in potato peel [88]. Concerning cultivars, it was shown that the variety with blue flesh showed the highest concentration (5.68 mg/100 g fresh weight), followed by the red-leaved (5.26 mg/100 g fresh weight), while yellow or cream flesh. In the study of Singh et al. [89] of potato peel, glycoalkaloids were detected as 1.05 mg/100 g. The results of Rytel et al. [88] showed that the glycoalkaloid content of potato peel depends on the potato cultivar and ranges from 181 mg/kg to 3526 mg/kg of fresh potato tubers.
Besides, the peel of pigmented potatoes is an excellent source of anthocyanins, e.g., pelargonidin-3-(p-coumaryoly rutinoside)-5-glucoside and petunidin-3-(p-coumaroyl rutinoside)-5-glucoside. It has been proven that their content depends on the cultivar [90]. Ji et al. [80] showed that anthocyanidin levels were higher in the peel than in the tuber. The most important beneficial properties and potential applications of phytochemicals identified in potato waste are listed in Table 7.

Table 7.
Biological activity and potential applications of phytochemicals obtained from potato wastes.
2.4. Soybean Residues
Soybean waste has the potential as a sustainable source of phytochemicals and functional foods. It includes both leaves, pod pericarp, and twigs, as well as the residues after seeds processing, so-called okara. Okara is the residue of soybean milling after extraction of the aqueous fraction used for producing tofu and soy drink and presents high nutritional value [109]. The results of the last studies showed that an okara contains enough bioactive compounds that make it useful to obtain value-added products for use in food production, oil extraction, nutraceutical, pharmaceutical, and cosmetic formulations. Moreover, it was stated that okara isoflavones have good antioxidant activity. Although some nutrients like protein decrease in okara during soymilk processing, it still has many other phytochemicals and nutrients, making it their least expensive and most excellent source. Since it has good antimicrobial activity, it can be used in pharmaceutical industries, thus opening up new frontiers for drug exploration [109]. Various food enriched with okara, such as biscuits and cookies, have been mentioned in the literature [110,111]. Guimarăes et al. [112] reported that food products enriched with okara contained 0.411 mg/100 mL of β-carotene and 0.15 μm/g isoflavones.
One of the main phytochemicals in soybean waste are isoflavones: daidzein, genistein, glycitein, and their glycosides (e.g., acetyl-, malonyl-, and β-glycosides) [113]. Isoflavones are compounds belonging to the flavonoid group. In addition to the well-established antioxidant effect, isoflavones exhibit estrogenic activity because of their similar structure to estrogen [113,114]. The beneficial effects of isoflavones are the prevention of hormone-dependent cancer, coronary heart disease, osteoporosis, and menopausal symptoms [114]. Kumar et al. [115] proved that daidzein expressed anticancer activity against human breast cancer cells MCF-7. The extract from soybean waste material showed total phenolic content (TPC) in the range of 27.4–167 mg GAE/g, total flavonoids from 10.4 to 63.8 mg QE/g and antioxidant activity (AOA) from 26.5% to 84.7% [114]. Moreover, their values were highest in the leaves, followed by pod pericarp and twigs. As was stated by Šibul et al. [113], soybean roots are also a good source of daidzein and genistein, as well as other phenolic compounds. The concentrations of isoflavones in roots were higher than in herbs, 1584.5 and 93.48 μg/g of dry extract, respectively. The newest study on soybean pods stated that its ethanolic extract and fractions exhibited anticancer potential against human colorectal carcinoma (HTC-116) and prostate cancer (PC-3) [116]. Moreover, it was the first analysis of this material using ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS), resulting in the identification of 50 polyphenols belonging to phenolic acids, flavonoids and other groups. The authors stated that soybean pods might be useful material as an active food additive or a component in dietary supplements and preparations with anti-radical and anti-cancer properties.
Soybean by-products are a good source of lecithin. Lecithin is a natural emulsifier that stabilizes fat and improves the texture of many food products, such as salad dressings, desserts, margarine, chocolate, and baking and cooking goods [117]. Moreover, it also has health benefits such as lowering cholesterol and low-density lipoprotein level in the human blood, improving digestion, cognitive and immune function, as well as aiding in the prevention of gall bladder and liver diseases.
Saponins are another important group of phytochemicals derived from soybean waste [113]. Soyasaponins have been linked to anti-obesity, antioxidative stress, and anti-inflammatory properties, as well as preventive effects on hepatic triacylglycerol accumulation [118]. One of the latest applications of saponins derived from soybean by-products was as eco-friendly agents for washing pesticide residues in the vegetable and fruit industries [119].
Compounds identified and quantified in soybean waste are specified in Table 8. The newest studies on the applications and properties of soybean waste are presented in Table 9.

Table 8.
Phytochemicals identified and quantified in soybean waste.

Table 9.
Biological activity and potential applications of phytochemicals obtained from soybean residues.
2.5. Tomato Residues
During the industrial processing of tomatoes, a considerable amount of waste is generated. Tomato waste consists mainly of peel, seeds, stems, leaves, fibrous parts and pulp residues [124]. The wet tomato pomace constitutes the major part of this waste, which consists of 33% seed, 27% peel and 40% pulp, while the dried pomace contains 44% seed and 56% pulp and peel [125]. When tomatoes are processed into products like ketchup, juice or sauces, 3–7% of their weight becomes waste. The management of tomato by-products is considered an important problem faced by tomato processing companies due to their disposal into the environment [126,127].
Although tomato waste has no commercial value, it is a rich source of nutrients, colorants and highly biologically active compounds such as polyphenols, carotenes, sterols, tocopherols, terpenes, and others (Table 10) [128,129,130,131,132]. The number of these compounds depends on tomato variety, part of the tomato residues (seed, peels, and pulp), time and extraction method, used solvent, as well as fractions gained after the isolation procedure, e.g., alkaline-hydrolyzable, acid-hydrolyzable, and bound phenolics [133]. They reported a total phenolics average of 1229.5 mg GAE/kg, of which flavonoids accounted for 415.3 mg QE/kg. The most abundant phenolic acids quantified in dried tomato waste were ellagic (143.4 mg/kg) and chlorogenic (76.3 mg/kg) acids. Other phenolic acids determined in lower concentrations were gallic, salicylic, coumaric, vanillic and syringic [133]. The levels of vanillic (26.9 mg/kg) and gallic (17.1 mg/kg) was lower than those found by Elbadrawy and Sello [134] in tomato peel (33.1 and 38.5 mg/kg, respectively). Ćetković et al. [135] identified phenolic acids (chlorogenic, p-coumaric, ferulic, caffeic and rosmarinic acid), flavonols (quercetin and rutin and its derivatives), and flavanone (naringenin derivatives) as the major phenolic compounds in extracts of tomato waste. The results obtained by Aires et al. [136] showed that the major polyphenol found in tomato wastes were kaempferol-3-O-rutinoside and caffeic acid. Several papers [135,136,137,138] reported the amounts of caffeic, chlorogenic, p-coumaric acids, kaempferol and quercetin, among other phenolic compounds found in tomato by-products. In the tomato’s wastes, Di Donato et al. [139] identified two main flavonoid compunds e.g., kaempferol rutinoside and quercetin rutinoside. Rutin and chlorogenic acid were the most abundant individual phenolics found by García–Valverde et al. [140] in all studied tomato varieties.

Table 10.
Phytochemicals identified in tomato wastes.
Traditionally, the bioactivity of tomatoes and their products has been attributed to carotenoids (β-carotene and lycopene). The results of Nour et al. [133] confirmed that dried tomato wastes contain considerable amounts of lycopene (510.6 mg/kg) and β-carotene (95.6 mg/kg) and exhibited good antioxidant properties. The results obtained by Fărcaş et al. [145] confirmed lycopene as the main carotenoid of tomato waste in a concentration between 42.18 and 70.03 mg/100 g DW (dry weight). Simultaneously, peels contain around 5 times more lycopene compared to tomato pulp [146,147]. The lycopene content in peel was 734 μg/g DW, but significant amounts of β-carotene, cis-β-carotene and lutein were also determined. The study by Górecka et al. [148] showed that tomato waste could be considered a promising source of lycopene for the production of functional foods.
Peels, as one of the main residues of tomato, are a richer source of nutrients and biologically active compounds than the pulp [137,149]. Despite of high concentration of carotenoids, peels also contain a considerable amount of polyphenols. The results obtained by Hsieh et al. [97] showed that the main flavonoids detected in fresh tomato peel were quercetin, myricetin, apigenin, catechin, puerarin, fisetin, hesperidin, naringin, rutin and their levels were reported as 4.2, 2.9, 1.9, 0.9, 0.8, 0.5, 0.3, 0.2, and 0.2 mg/100 g, respectively. It has been proven that tomato peel extracts contain high amounts of kaemferol-3-O-rutinoside (from 8.5 to 142.5 mg/kg) [127], quercetin derivatives, p-coumaric acid and chlorogenic acid derivative [150,151]. The main phenolic acids identified in tomato peel are protocatechuic, vanillic, gallic, catechin and caffeic acid. Their corresponding concentrations were 5.52, 3.85, 3.31, 2.98, and 0.50 mg/100 g, respectively [134]. The results of Lucera et al. [152] showed that tomato peels contain 4.90 mg/g DW of total phenolic and 2.21 mg/g DW of total flavonoids. The total polyphenolic content in tomato peels and seeds was higher than in the pulp. On the other hand, tomato peel has a very small amount of anthocyanin [153].
Tomato seeds are considered a potential natural source of antioxidants due to their rich phytochemical profile. Many publications indicate that tomato seeds contain, e.g., carotenoids, proteins, polyphenols, phytosterols, minerals and vitamin E [154]. According to Eller et al. [155], the total content of phenolic compounds in the tomato seed extract was 20.66 mg/100 g. Quercetin-3-O-sophoroside, isorhamnetin-3-O-sophoroside, and kaempferol-3-O-sophoroside were present in the highest concentrations of the total phenolic compounds. Quercetin derivatives contributed approximately 37% of the total flavonoid content. Pellicanò et al. [156] found naringenin (84.04 mg/kg DW) as the most abundant flavonoid identified, followed by caffeic acid (26.60 mg/kg DW). Apart from phenolics, carotenoids are the next class of bioactive compounds present in tomato seeds. Qualitatively, the carotenoid composition (β-carotene and lycopene isoforms: lycopene all trans, lycopene cis 1, lycopene cis 2, lycopene cis 3) in tomato seeds is similar to that of the carotenoids in tomato fruit [157].
Tomato waste has attracted great interest due to its biological activity and potential applications of phytochemicals (Table 11).

Table 11.
Biological activity and potential applications of phytochemicals obtained from tomato wastes.
2.6. Banana Residues
Banana (Musa spp., Musaceae family) is one of the main fruit crops cultivated for its edible fruits in tropical and subtropical regions. The main by-product of bananas is its peels, which represent approx. 30% of the whole fruit [164]. Moreover, banana waste also includes small-sized, damaged, or rotting fruit, leaves, stems, and pseudoparts. Banana peels are sometimes used as feedstock for livestock, goats, monkeys, poultry, rabbits, fish, zebras, and many other species. They are rich in vitamin B6, manganese, vitamin C, fiber, potassium, biotin, and copper [165], but also in phytochemicals with high antioxidant capacity such as phenolics (flavonols, hydroxycinnamic acids, gallocatechin), anthocyanin (delphinidin, cyanidin), carotenoids (β-carotenoids, α-carotenoids, and xanthophylls), catecholamines, sterols and triterpenes (Table 12). Banana peels are natural antacids and are helpful in acid reflux, heartburn, and diarrhea [165].

Table 12.
Phytochemicals identified in banana wastes and their concentration.
Previous studies reported that the banana peel is rich in chemical compounds as antioxidant and antimicrobial activities [167,168,169,171]. Moreover, ethanoic extract from banana peel exhibited the strongest antihyperglycemic activity in comparison with the extract from pulp, seed, and flower [172]. Phytochemicals derived from banana peel were tested as a biofungicide against Fusarium culmorum and Rhizoctonia solani and as a bactericide against Agrobacterium tumefaciens for the natural preservation of wood during handling or in service. Encapsulation is successfully investigated as the method for stabilizing the banana peel extract and its bioactive compounds during storage [173].
Other phytochemical components present in the banana peel extracts, such as ethanediol and butanediol, were determined as highly reducing agents to synthesize silver nanoparticles, which are significant to the medical and chemical industries [173].
The harvesting of the fruits in the plantation requires the decapitation of the whole; therefore, the valuable banana by-products, in addition to peels, are the pseudostem, leaves, inflorescence, and fruit stalk, but also rhizome, which can also be used as a raw material for the acquisition of phytochemicals [174]. Kandasamy et al. [170] isolated three compounds from the pseudostem and rhizome of bananas, including chlorogenic acids, cycloeucalenol acetate, and 4-epicyclomusalenone. Crude extract and isolated compounds are characterized by strong antibacterial, antifungal, antiplatelet aggregation, and anticancer activities.
Using the inflorescence of bananas, anthocyanins can be obtained as good biocolorants with attractive colors, moderate stability in food systems, water solubility, and benefits for health [175]. Cyanidin-3-rutinoside, as the main compound, could be exploited as a cheap source of natural food colorant.
The newest application and explored properties of biologically active compounds from banana residues are presented in Table 13.

Table 13.
Biological activity and potential applications of phytochemicals obtained from banana residues.
2.7. Apple Residues
Poland is the main producer of apples in the world, with an annual production of over 4 million tons [177]. About 25% of apple biomass was wasted during crop and processing. Apple pomace as a waste from apple juice and cider processing consists mainly of apple skin/flesh, seeds, and stems [178]. Until recently, apple waste was used as livestock feed, bioenergy feedstock, as well as for food supplementation and pectin extraction, but still, it is far from being used at its full potential, particularly considering its application in the pharmaceuticals and cosmetics industry [179,180]. Nonetheless, apple pomace has the potential to become a source of valuable biomaterials for agriculture. It contains numerous phytochemicals in the form of pectin and dietary fibers, but also polyphenols, triterpenoids, and volatiles. Interestingly, apple pomace is a richer source of antioxidants than fresh fruits itself because it has a significantly lower content of water; moreover, many valuable bioactive compounds are found mainly in the peels and seeds [180].
Polyphenols are the main valuable constituents of apple pomace. Waldbauer et al. [181] reported that the total phenolic content in apple pomace is in the range of 262–856 mg of total phenols/100 g. This content differs between studies due to the use of different solvents, extraction conditions, and apple varieties [182,183].
Four major phenolic groups are hydroxycinnamic acids, dihydrochalcone derivatives (phloretin and its glycosides), flavan-3-ols (catechin and procyanidins), and flavonols (quercetin and its glycosides) [184,185].
Although the phytochemical composition of apple pomace has been studied for a long time, new compounds with beneficial properties are still being isolated and identified. Ramirez-Ambrosi et al. [186] identified 52 phenolic compounds using a newly developed, rapid, selective, and sensitive strategy of ultrahigh-performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry (UHPLC-DAD–ESI-Q-ToF-MS) with automatic and simultaneous acquisition of exact mass at high and low collision energy. Among new compounds, two dihydrochalcones (two isomers of phloretin-pentosyl-hexosides) and three flavonols (isorhamnetin-3-O-rutinoside, isorhamnetin-3-O-pentosides and isorhamnetin-3-O-arabinofuranoside) have been tentatively identified for the first time in apple pomace.
One of the compounds newly identified in the last few years in apple pomace is monoterpene–pinnatifidanoside D [185]. This compound has been isolated for the first time from Crataegus pinnatifida and exhibited small antiplatelet aggregation activity.
Mohammed and Mustafa [187] and Khalil and Mustafa [188] isolated and structurally elucidated novel furanocoumarins from apple seeds. Isolated compounds exhibited promising antimicrobial activity against Pseudomonas aeruginosa, Klebsiella pneumonia, Haemophilus influenzae, Escherichia coli, Candida albicans, and Aspergillus niger.
The main compounds determined in apple by-products with ranges of their concentrations are listed in Table 14.

Table 14.
Total phenolic content (TPC), total flavonoid content (TFC), and main phytochemicals identified and quantified in apple pomace.
Many have been written about the application of apple pomace itself. However, the present work concerns the properties and application of bioactive compounds derived from apple pomace. The newest studies reported valuable activities and interesting applications of phytochemicals from apple pomace are listed in Table 15. Preclinical studies have found apple pomace extracts and isolated compounds improved lipid metabolism, antioxidant status, and gastrointestinal function and had a positive effect on metabolic disorders (e.g., hyperglycemia, insulin resistance, etc.) [193]. As was reported by Gołębiewska et al. [194], despite medicine and cosmetics, apple pomace phytochemicals found recent applications in building and construction industries as green corrosion inhibitors and wood protectors [194].

Table 15.
Biological activity and potential applications of phytochemicals obtained from apple residues.
Phenolic content is related to the antioxidant properties of apple pomace, and procyanidins are considered the major contributors to the antioxidant capacity of apples. Despite high concentrations in apples, catechins and procyanidins are very often absent in the extract from apple pomace. The exposure of polyphenols to polyphenoloxidase during apple processing caused, in addition to native apple phytochemicals, their oxidation products also represent a significant part of the overall polyphenolic fraction. Moreover, the polyphenols can interact non-covalently with polysaccharides; thus, they become non-extractable. Fernandes et al. [178] reported that such complexes represented up to 40% of the available polyphenols from apple pomace, potentially relevant for agro-food waste valuation. Moreover, it has been revealed that the use of appropriate extraction procedures, such as microwave-superheated water extraction (MWE) of the hot water/acetone, as well as additional hydrolysis, made it possible to recover these valuable compounds from apple pomace. This knowledge will allow for designing more diversified solutions for agro-food waste valuation [178]. The strong antioxidant in apple pomace is quercetin, which has protective effects against breast and colon cancer, as well as heart and liver diseases [203].
Apple is a unique plant in the Rosaceae family due to the high content of phloridzin, a major phenolic compound in commercial varieties of apples [203]. Phloridzin has anti-diabetic potential and could be applied as a natural sweetening agent [200]. Phloridzin from apple waste was also tested as the substrate for the production of food dye through its enzymatic oxidation. The yellow product, so-called phloridzin oxidation products (POP), turned out to be a good alternative to tartrazine and other potentially toxic food yellow pigments [200,201].
Interesting phytochemicals of apple pomace are triterpenoids, particularly ursolic acid. It has attracted attention because of its therapeutic potential associated with several functional properties such as antibacterial, antiprotozoal, anti-inflammatory, and antitumor [196]. Woźniak et al. [190] optimized the method of its extraction using supercritical carbon dioxide. The data obtained allowed the prediction of the extraction curve for the process conducted on a larger scale.
As has been mentioned previously, apple pomace contains some amount of seeds. Walia et al. [192] proved that also apple seed oil could be a promising raw material for the production of natural antioxidants and anticancer agents. The authors tested the fatty acid composition and physicochemical and antioxidant properties of oil extracted from apple seeds separated from industrial pomace. The dominant fatty acids were oleic acid (46.50%) and linoleic acid (43.81%).
The major constituent in apple seed is also amygdalin, which may be metabolized to toxic hydrogen cyanide [203,204]. However, in the literature, there are also several reports of the positive pharmacological activity of amygdalin. Luo et al. [205] showed its anti-fibrotic properties in the case of liver fibrosis. Song and Xu [206] proved that amygdalin exhibits analgesic effects in mice, probably by inhibiting prostaglandins E2 and nitric oxide synthesis. Despite so many above reports, there is still a need for human and animal studies to confirm the protection against the disease’s effects of apple pomace.
2.8. Winery Waste
The major winery by-products are grape pomace and marc, including seeds, pulp, skins, stems, and leaves. Bioactive phytochemicals present in residues from wine-making are mainly represented by polyphenols belonging to various groups of compounds, such as phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids), flavonoids (flavanols or flavan-3-ols, anthocyanins, proanthocyanidins, flavones, and flavonols), and stilbenes and anthocyanins. The relative concentrations of the different phenolic compounds are influenced by genotype (red or white grapes), a distinct fraction of residues, as well as agro-climatic conditions [207]. The presence of polyphenolic compounds in grape residues supports the potential of the investigation and valorization of this agro-industrial waste. The compounds identified in grapes by-products with their concentrations are listed in Table 16.

Table 16.
Phytochemicals identified and quantified in grape residues.
The residues derived from the grape processing contain phytochemicals of interest for the production of preservatives, dyes, enriched foods, medicines, and products aimed at personal care, pharmaceutical, and cosmetic industries. The presence of bioactive compounds with antioxidant, antimicrobial, anti-inflammatory, anti-tumor, and protective activity of the cardiovascular system provides possibilities for many applications [221]. The potential beneficial role of phytochemicals of grape pomace in the prevention of disorders associated with oxidative stress and inflammation, such as endothelial dysfunction, hypertension, hyperglycemia, diabetes, and obesity, is due to the mechanisms concerned especially modulation of antioxidant/prooxidant activity, improvement of nitric oxide bioavailability, reduction of pro-inflammatory cytokines and modulation of antioxidant/inflammatory signal pathways [222].
It has been proven that the antioxidant properties of polyphenols in grape pomace help to prevent radical oxidation of the polyunsaturated fatty acids of low-density lipoproteins (LDL) and hence, are conducive to the prevention of cardiovascular diseases [223]. The compounds derived from grape pomace were also tested for their anti-inflammatory and anti-carcinogenic effect [224]. Álvarez et al. [225] studied the impact of procyanidins from grape pomace as inhibitors of human endothelial NADPH oxidase and stated the decrease in the production of reactive oxygen species. A rich source of procyanidins is grape seeds. They are widely consumed in some countries in the form of powder as a dietary supplement because of several related health benefits associated with procyanidins. They present antitumor-promoting activity, inhibit growth and induce apoptosis in human prostate cancer cells, as well as significantly reducing atherosclerosis in the aorta.
Seeds contain a very broad spectrum of procyanidins, with the dominant compounds being the dimers, trimers, and tetramers of catechin or epicatechin. Higher polymers are also present but at much lower abundance. Besides, every polymer can also be found as a gallic acid ester.
Very important is the anti-microbial activity of bioactive compounds included in grapes wastes. Mendoza et al. [226] demonstrated the antifungal properties of extracts from winery by-products against Botrytis cinerea, the causal agent of gray mold, considered the most important pathogen responsible for postharvest decay of fresh fruit and vegetables. Moreover, a few reports are available in the literature about the effective action of polyphenol-rich extracts from vinification by-products against various pathogenic bacteria and insects, e.g., Listeria monocytogenes, Leptinotarsa decemlineata, and Spodoptera littoralis [1]. The potential health benefits of plant phenolics cause much interest and consideration in a lot of agri-food applications for phenolics extracted from grape wastes [16]. There are a lot of studies on the application of phytochemicals from grape pomace in the meat industry [221].
To facilitate the industrial application of wine waste polyphenols, encapsulation was recently developed to improve the stability of valuable compounds in different conditions of light and temperature [227,228].
The examples of the newest potential applications and valuable properties of phytochemicals derived from winery waste are listed in Table 17.

Table 17.
Biological activity and potential applications of phytochemicals obtained from grape residues.
2.9. Citrus Residues
Citrus fruits from the family Rutaceae include oranges, lemons, limes, grapefruits, mandarins, and tangerines. They are well known for their nutritional value, as they are good sources of dietary fiber, pectin, vitamin C, vitamin B group, carotenoids, flavonoids, and limonoids (Table 18). It is estimated that approximately 140 chemical components have been isolated and identified from citrus peels, and flavonoids are the main group of phytochemicals with biological activity [245]. Afsharnezhad et al. [165] evaluated the antioxidant potential of extract from various fruit peels and stated that the maximum DPPH radical scavenging activity, total phenols, and total anthocyanins were observed in orange peels.

Table 18.
Phytochemicals identified and quantified in citrus residues.
Citrus peels are widely used by-products for the production of essential oils, which have great commercial importance due to their aroma, antifungal and antimicrobial properties. Citrus essential oil is employed in the food industry, perfumes, cosmetics, domestic household products, and pharmaceuticals [257]. The main ingredient is limonene, accounting for more than 94% of citrus essential oil [258]. It is used as an insect-killing agent in pesticides and a good biodegradable and non-toxic solvent [257]. Furthermore, limonene has shown regulatory effects on neurotransmitters and stimulant-induced changes in dopamine neurotransmission [258].
The citrus waste contained high amounts of organic and phenolic acids, as well as flavonoids. Among flavonoids, the main compounds are flavanones and flavones (such as naringenin, hesperetin, and apigenin glycosides) as well as polymethoxylated flavones (PMFs), not found in other fruit species [259,260]. Okino Delgado and Feuri [258] indicated that polymethoxylated flavones, at a dosage of 250 mg/kg, exhibit an anti-inflammatory effect comparable to ibuprofen. The most widely studied PMFs are tangeretin and nobiletin. They are exclusively derived from citrus peels. Lv et al. [261] stated that nobiletin and its derivatives showed anti-cancer activity. Generally, anticancer activity increases with the increasing number of methoxy groups because PMFs have then higher hydrophobicity for approaching and penetrating cancer cells [244]. Moreover, PMFs exhibit a broad spectrum of other biological activities such as anti-obesity, anti-atherosclerosis, antiviral and antioxidant properties [262,263].
Among flavanones, citrus peel is rich in eriocitrin, hesperidin, diosmin, neohesperidin, didymin, and naringin. Chiechio et al. [264] used red orange and lemon extract rich in flavanones for in vivo assays on male CD1 mice fed with a high-fat diet. The results showed that an 8-week treatment with the extract was able to induce a significant reduction in glucose, cholesterol, and triglyceride levels in the blood, with positive effects on the regulation of hyperglycemia and lipid metabolism. Barbosa et al. [265] tested flavanones obtained from citrus pomace by enzyme-assisted and conventional hydroalcoholic extraction as an agent against Salmonella enterica subsp. enterica. Tested extracts decreased the expression of genes associated with cell invasion. Moreover, the results suggest that extracts and flavanones inhibit Salmonella Typhimurium adhesion by interacting with fimbriae and flagella structures and downregulating fimbrial and virulence genes.
Citrus peels also contained some flavonols, such as rutin, isorhamnetin 3-O-rutinoside, quercetin-O-glucoside, and myricetin, as well as phenolic acids, but at a much lower concentration. It has been proven that Citrus reticulata waste extract, mainly including rutin, was the most effective against gram-negative bacteria and the three pathogenesis fungi: Bacillus subtilis, Candida albicans and Aspergillus flavus [266].
Citrus seeds are also a good source of valuable components, particularly oil rich in carotenoids (19.01 mg/kg), phenolic compounds (4.43 g/kg), tocopherols (135.65 mg/kg) and phytosterols (1304.2 mg/kg) [251]. This oil was characterized by high antioxidant activity ranging from 56.0% to 70.2%.
A summary of the main phytochemical constituents, together with their concentrations in citrus residues, as well as their newest applications and properties, is presented in Table 18 and Table 19, respectively.

Table 19.
Biological activity and potential applications of phytochemicals obtained from citrus residues.
2.10. Olive Waste
The cultivation of olive trees is a widespread practice in the Mediterranean region, accounting for about 98% of the world’s olive cultivation. A large number of phenolic compounds occur in both olive oil and olive waste that includes both leaves and the residues of oil production [275,276]. Their chemical characterization was reported by Dermeche et al. [277]. The main groups of phenolic compounds in olive mill wastes are phenolic acids, secoiridoids, and flavonoids, and the most abundant polyphenols are oleuropein, hydroxytyrosol, verbascoside, apigenin-7-glucoside, and luteolin-7-glucoside [278] (Table 20). Olive mill wastewater obtained during oil production is a complex mixture of vegetation waters and processing waste of the olive fruit; it is characterized by a dark color, strong odor, a mildly acidic pH, and a very high inorganic and organic load [279]. The organic fraction consists essentially of sugars, tannins, polyphenols, polyalcohols, proteins, organic acids, pectins and lipids [277]. About 30 million m3 of olive mill wastewater are produced annually in the world as a by-product of the olive oil extraction process; because of the high polyphenolic content (0.5–24 g/L), this by-product is difficult to biodegrade and a relevant environmental and economic issue [280].

Table 20.
Phytochemicals identified and quantified in olive waste.
Polyphenols also occur in the leaves [287]. These compounds confer bioactive properties on olive leaf extracts, such as antioxidant, antimicrobial, and antitumor activity; the capacity to reduce the risk of coronary heart disease was also reported [288]. Olive leaves can be collected as a by-product during oil processing (about 10% of the total weight of the olives) but can also be a residue of olive tree pruning. Some authors estimated that about 25 kg of by-products (twigs and leaves) could be obtained annually by pruning per tree [289]. To date, this by-product is often used as animal feed, even if this natural resource rich in antioxidant phenolic compounds should be valorized [290].
The qualitative and quantitative content of phenolic compounds is often heterogeneous in olive by-products; however, several studies reported the bioactive properties of these phenolic compounds, promising potential as antioxidant, anti-inflammatory, and antimicrobial agents. The antioxidant activities of olive mill wastewater and olive pomace have been demonstrated by different antioxidant assays as DPPH radical-scavenging activity, superoxide anion scavenging, LDL oxidation, and the protection of catalase against hypochlorous acid [281,291,292]. An overview of the pharmacology of olive oil and its active ingredients has been reported by Visioli et al. [293]. Recently, a novel stable ophthalmic hydrogel containing a polyphenolic fraction obtained from olive mill wastewater was formulated [294]. Among olive polyphenols, hydroxytyrosol is one of the main phenolic compounds; it can occur in its free form or as secoiridoids (oleuropein and its aglycone). For its polarity, it is more abundant in olive mill wastewater and pomace rather than in olive oil. Anticancer, antioxidant, and anti-inflammatory properties have been reported for hydroxytyrosol [295,296]. In vitro antioxidant and skin regenerative properties have been reported by Benincasa et al. [297].
Moreover, the polyphenol fraction obtained from olive mill wastewater showed activities against bacteria, fungi, plants, animals, and human cells; antibacterial activities against several bacterial species (Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa) have been reported by Obied et al. [298]. Fungicidal activities have also been reported [299]. Moreover, the effects of phenolic compounds from olive waste on Aspergillus flavus growth and aflatoxin B1 production were investigated [300,301]. The olive mill wastewater polyphenols did not inhibit the Aspergillus flavus fungal growth rate but significantly reduced the aflatoxin B1 production (ranging from 88 to 100%) at 15% concentration [302].
Finally, cytoprotection of brain cells by olive mill wastewater has been studied by Schaffer et al. [303]. The cytoprotective effects were correlated to the content of hydroxytyrosol.
These studies showed the numerous beneficial and bioactive activities of polyphenols fraction obtained by olive by-products; for their use, it is often carried out an appropriate fractionation and/or purification to control their concentration and to avoid some antagonist effects.
Various valuable properties and the newest studies on the application of biologically active compounds derived form olive waste are presented in Table 21.

Table 21.
Biological activity and potential applications of phytochemicals obtained from olive waste.
3. Conclusions
The ever-increasing amount of processed food raw materials entails an increasing amount of biowaste. Their management has become a growing problem. The consulted literature shows that discussed waste still contains valuable ingredients, medicinally important phytochemicals, and good antioxidants, so it is very important to valorize them. Currently, the recovery of different valuable phytochemicals from agro-industrial waste has become an imperative research area among the scientific community because agro-industrial residues of plant materials are a cheap and natural source of bioactive compounds, which can be used in the prevention and treatment of various diseases. Despite many studies on the valuable properties and potential applications, still, not many solutions are implemented in the industry. This is probably caused by legislation that can affect the valorization of such waste biomass. There are not many regulatory and legal provisions for their use. In the European Union, the use of agricultural residues as food ingredients is regulated by the European Community Regulation (EC) No 178/2002. However, in order to use them as natural additives, proper authorization as a novel food is necessary (Regulation (EC) No 2015/2283) [304]. There is no doubt that the industrial application of the extracts needs to be regulated.
According to the circular bioeconomy and biorefinery concept, food waste should be recycled inside the whole food value chain from field to fork in order to formulate functional foods and nutraceuticals. Nonetheless, it is important to implement environmentally friendly industrial extraction procedures. Moreover, despite so many above reports, there is still a need for human and animal studies, as well as studies in the field in the case of plants, to confirm the protective effect of such phytochemicals against diseases.
Taking into account the European Union’s emphasis on the development of a circular economy and reducing the carbon footprint, it is expected that the effective application of these wastes will be carried out and that regulations will be developed in accordance with needs.
Author Contributions
Conceptualization, M.O., I.K. and W.O.; resources, W.O., I.K.; Visualisation, M.O., I.K. and T.B.; writing—original draft preparation, M.O., I.K. and T.B.; writing—review and editing, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- FAOSTAT (Statistics Division of Food and Agriculture Organization of the United Nations). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 June 2022).
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and vegetable co-products as functional feed ingredients in farm animal nutrition for improved product quality. Agriculture 2018, 5, 1020–1034. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Campos-Valdez, A.R.; Alcázar-Valle, M.; Barrera-Martínez, I. Comparison of Extraction Techniques for the Recovery of Sugars, Antioxidant and Antimicrobial Compounds from Agro-Industrial Wastes. Sustainability 2022, 14, 5956. [Google Scholar] [CrossRef]
- Ngwasiri, P.N.; Ambindei, W.A.; Adanmengwi, V.A.; Ngwi, P.; Mah, A.T.; Ngangmou, N.T.; Fonmboh, D.J.; Ngwabie, N.M.; Ngassoum, M.B.; Aba, E.R. Review Paper on Agro-food Waste and Food by-Product Valorization into Value Added Products for Application in the Food Industry: Opportunities and Challenges for Cameroon Bioeconomy. Asian J. Biotechnol. Bioresour. Technol. 2022, 8, 32–61. [Google Scholar] [CrossRef]
- Rodrigues, F.; Nunes, M.A.; Alves, R.C.; Oliveira, M.B.P. Applications of recovered bioactive compounds in cosmetics and other products. In Handbook of Coffee Processing By-Products; Academic Press: London, UK, 2017; pp. 195–220. [Google Scholar]
- Pestana-Bauer, V.R.; Zambiazi, R.C.; Mendonça, C.R.; Beneito-Cambra, M.; Ramis-Ramos, G. γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012, 134, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhuang, D.; Fu, J.; Huang, Y.; Wen, K. Bioenergy potential from crop residues in China: Availability and distribution. Renew. Sustain. Energy Rev. 2012, 16, 1377–1382. [Google Scholar] [CrossRef]
- Searle, S.; Malins, C. Availability of Cellulosic Residues and Wastes in the EU 2013; The International Council on Clean Transportation: Washington, DC, USA, 2013; p. 11. [Google Scholar]
- Ben Taher, I.; Fickers, P.; Chniti, S.; Hassouna, M. Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol. Prog. 2017, 33, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Yanli, Y.; Peidong, Z.; Wenlong, Z.; Yongsheng, T.; Yonghong, Z.; Lisheng, W. Quantitative appraisal and potential analysis for primary biomass resources for energy utilization in China. Renew. Sustain. Energy Rev. 2010, 14, 3050–3058. [Google Scholar] [CrossRef]
- Oleszek, M.; Tys, J.; Wiącek, D.; Król, A.; Kuna, J. The possibility of meeting greenhouse energy and CO2 demands through utilisation of cucumber and tomato residues. BioEnergy Res. 2016, 9, 624–632. [Google Scholar] [CrossRef]
- Gabhane, J.; William, S.P.; Gadhe, A.; Rath, R.; Vaidya, A.N.; Wate, S. Pretreatment of banana agricultural waste for bio-ethanol production: Individual and interactive effects of acid and alkali pretreatments with autoclaving, microwave heating and ultrasonication. Waste Manag. 2014, 34, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.G.; Bastos, R.; Pinto, M.; Ferreira, J.M.; Santos, J.F.; Wessel, D.F.; Coelho, E.; Coimbra, M.A. Waste mitigation: From an effluent of apple juice concentrate industry to a valuable ingredient for food and feed applications. J. Clean. Prod. 2018, 193, 652–660. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the residues recovery: Orange waste as raw material for new products. Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Kusbiantoro, A.; Embong, R.; Aziz, A.A. Strength and microstructural properties of mortar containing soluble silica from sugarcane bagasse ash. Key Eng. Mater. 2018, 765, 269–274. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Zhou, H.; Yan, H.; Ye, J.; Zhao, Z.; You, L.; Fu, X. Antioxidant/antihyperglycemic activity of phenolics from sugarcane (Saccharum officinarum L.) bagasse and identification by UHPLC-HR-TOFMS. Ind. Crops Prod. 2017, 101, 104–114. [Google Scholar] [CrossRef]
- Ishak NA, I.M.; Kamarudin, S.K.; Timmiati, S.N.; Sauid, S.M.; Karim, N.A.; Basri, S. Green synthesis of platinum nanoparticles as a robust electrocatalyst for methanol oxidation reaction: Metabolite profiling and antioxidant evaluation. J. Clean. Prod. 2023, 382, 135111. [Google Scholar] [CrossRef]
- Rocha, G.J.; Nascimento, V.M.; Goncalves, A.R.; Silva, V.F.; Martin, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Mohan, P.R.; Ramesh, B.; Redyy, O.V. Production and optimization of ethanol from pretreated sugarcane bagasse using Sacchromyces bayanus in simultaneous saccharification and fermentation. Microbiol. J. 2012, 2, 52–63. [Google Scholar] [CrossRef]
- Xi, Y.L.; Dai, W.Y.; Xu, R.; Zhang, J.H.; Chen, K.Q.; Jiang, M.; Wei, P.; Ouyang, P.K. Ultrasonic pretreatment and acid hydrolysis of sugarcane bagasse for succinic acid production using Actinobacillus succinogenes. Bioprocess Biosyst. Eng. 2013, 36, 1779–1785. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, H.; Zheng, R.; Khan, M.S.; Fu, X.; Tao, Z.; Zhang, Z. Anthocyanins characterization and antioxidant activities of sugarcane (Saccharum officinarum L.) rind extracts. Ind. Crops Prod. 2018, 113, 38–45. [Google Scholar] [CrossRef]
- Nieder-Heitmann, M.; Haigh, K.F.; Görgens, J.F. Process design and economic analysis of a biorefinery co-producing itaconic acid and electricity from sugarcane bagasse and trash lignocelluloses. Bioresour. Technol. 2018, 262, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef]
- Al Arni, S.; Drake, A.F.; Del Borghi, M.; Converti, A. Study of aromatic compounds derived from sugarcane bagasse. Part I: Effect of pH. Chem. Eng. Technol. 2010, 33, 895–901. [Google Scholar] [CrossRef]
- González-Bautista, E.; Santana-Morales, J.C.; Ríos-Fránquez, F.J.; Poggi-Varaldo, H.M.; Ramos-Valdivia, A.C.; Cristiani-Urbina, E.; Ponce-Noyola, T. Phenolic compounds inhibit cellulase and xylanase activities of Cellulomonas flavigena PR-22 during saccharification of sugarcane bagasse. Fuel 2017, 196, 32–35. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Li, J.; Zhao, Z.; Wei, J.; Fu, X.; Liu, R.H. Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Ind. Crops Prod. 2017, 107, 360–369. [Google Scholar] [CrossRef]
- Van der Pol, E.; Bakker, R.; Van Zeeland, A.; Garcia, D.S.; Punt, A.; Eggink, G. Analysis of by-product formation and sugar monomerization in sugarcane bagasse pretreated at pilot plant scale: Differences between autohydrolysis, alkaline and acid pretreatment. Bioresour. Technol. 2015, 181, 114–123. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S.; Lou, R.; Yang, Q. Analytical pyrolysis characteristics of enzymatic/mild acidolysis lignin from sugarcane bagasse. Cellulose Chemistry and Technology 2010, 44, 335–342. [Google Scholar]
- Michelin, M.; Ximenes, E.; Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Juttuporn, W.; Thiengkaew, P.; Rodklongtan, A.; Rodprapakorn, M.; Chitprasert, P. Ultrasound-assisted extraction of antioxidant and antibacterial phenolic compounds from steam-exploded sugarcane bagasse. Sugar Technol. 2018, 20, 599–608. [Google Scholar] [CrossRef]
- Treedet, W.; Suntivarakorn, R. Design and operation of a low cost bio-oil fast pyrolysis from sugarcane bagasse on circulating fluidized bed reactor in a pilot plant. Fuel Process. Technol. 2018, 179, 17–31. [Google Scholar] [CrossRef]
- Krishnan, C.; Sousa, L.C.; Jin, M.; Chang, L.; Dale, B.E.; Balan, V. Alkalibased AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnol. Bioeng. 2010, 107, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.S.; Zhu, M.J.; Xu, W.X.; Liang, L. Production of bioethanol from sugarcane bagasse using NH4OH-H2O2 pretreatment and simultaneous saccharification and co-fermentation. Biotechnol. Bioprocess Eng. 2012, 17, 316–325. [Google Scholar] [CrossRef]
- Guilherme, A.A.; Dantas, P.V.; Santos, E.S.; Fernandes, F.A.; Macedo, G.R. Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugarcane bagasse. Braz. J. Chem. Eng. 2015, 32, 23–33. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Wang, W.; Liu, T.; Xin, Z. Isolation and identification of bound compounds from corn bran and their antioxidant and angiotensin I-converting enzyme inhibitory activities. Eur. Food Res. Technol. 2015, 241, 37–47. [Google Scholar] [CrossRef]
- Bujang, J.S.; Zakaria, M.H.; Ramaiya, S.D. Chemical constituents and phytochemical properties of floral maize pollen. PLoS ONE 2021, 16, e0247327. [Google Scholar] [CrossRef]
- Dong, J.; Cai, L.; Zhu, X.; Huang, X.; Yin, T.; Fang, H.; Ding, Z. Antioxidant activities and phenolic compounds of cornhusk, corncob and stigma maydis. J. Braz. Chem. Soc. 2014, 25, 1956–1964. [Google Scholar] [CrossRef]
- Li, Q.; Somavat, P.; Singh, V.; Chatham, L.; Gonzalez de Mejia, E. A comparative study of anthocyanin distribution in purple and blue corn coproducts from three conventional fractionation processes. Food Chem. 2017, 231, 332–339. [Google Scholar] [CrossRef]
- Haslina, H.; Eva, M. Extract corn silk with variation of solvents on yield, total phenolics, total flavonoids and antioxidant activity. Indones. Food Nutr. Prog. 2017, 14, 21–28. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z. Extraction of flavonoids from corn silk and biological activities in vitro. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Mou, H.; Kong, Q. Ultrasound-assisted extraction and characterization of anthocyanins from purple corn bran. J. Food Preserv. 2017, 42, e13377. [Google Scholar] [CrossRef]
- Barba, F.J.; Rajha, H.N.; Debs, E.; Abi-Khattar, A.M.; Khabbaz, S.; Dar, B.N.; Simirgiotis, M.J.; Castagnini, J.M.; Maroun, R.G.; Louka, N. Optimization of Polyphenols’ Recovery from Purple Corn Cobs Assisted by Infrared Technology and Use of Extracted Anthocyanins as a Natural Colorant in Pickled Turnip. Molecules 2022, 27, 5222. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and identification of anthocyanins in corn cob and corn husk from Cacahuacintle maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.J.; Berhow, M.A. Bioactives derived from ripe corn tassels: A possible new natural skin whitener, 4-hydroxy-1-oxindole-3-acetic acid. Curr. Bioact. Compd. 2011, 7, 126–134. [Google Scholar] [CrossRef]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic variation in anthocyanins, phenolic compounds, and antioxidant activity in cob and husk of purple field corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef]
- Brobbey, A.A.; Somuah-Asante, S.; Asare-Nkansah, S.; Boateng, F.O.; Ayensu, I. Preliminary phytochemical screening and scientific validation of the antidiabetic effect of the dried husk of Zea mays L. (Corn, Poaceae). Int. J. Phytopharm. 2017, 7, 1–5. [Google Scholar]
- Thapphasaraphong, S.; Rimdusit, T.; Priprem, A.; Puthongking, P. Crops of waxy purple corn: A valuable source of antioxidative phytochemicals. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 73–77. [Google Scholar]
- Simla, S.; Boontang, S.; Harakotr, B. Anthocyanin content, total phenolic content, and antiradical capacity in different ear components of purple waxy corn at two maturation stages. Aust. J. Crop Sci. 2016, 10, 675–682. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sidorov, A.N.; Deineka, L.A. Determination of purple corn husk anthocyanins. J. Anal. Chem. 2016, 71, 1145–1150. [Google Scholar] [CrossRef]
- Suryanto, E.; Momuat, L.I.; Rotinsulu, H.; Mewengkang, D.S. Anti-photooxidant and photoprotective activities of ethanol extract and solvent fractions from corn cob (Zea mays). Int. J. ChemTech Res. 2018, 11, 25–37. [Google Scholar]
- Duangpapeng, P.; Lertrat, K.; Lomthaisong, K.; Scott, M.P.; Suriharn, B. Variability in anthocyanins, phenolic compounds and antioxidant capacity in the tassels of collected waxy corn germplasm. Agronomy 2019, 9, 158. [Google Scholar] [CrossRef]
- Duangpapeng, P.; Ketthaisong, D.; Lomthaisong, K.; Lertrat, K.; Scott, M.P.; Suriharn, B. Corn tassel: A new source of phytochemicals and antioxidant potential for value-added product development in the agro-industry. Agronomy 2018, 8, 242. [Google Scholar] [CrossRef]
- Žilić, S.; Vančetović, J.; Janković, M.; Maksimović, V. Chemical composition, bioactive compounds, antioxidant capacity and stability of floral maize (Zea mays L.) pollen. J. Funct. Foods 2014, 10, 65–74. [Google Scholar] [CrossRef]
- Sarepoua, E.; Tangwongchai, R.; Suriharn, B.; Lertrat, K. Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chem. 2015, 169, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rasane, P.; Nanda, V.; Kaur, S. Bioactive compounds of corn silk and their role in management of glycaemic response. J. Food Sci. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Ren, S.C.; Qiao, Q.Q.; Ding, X.L. Antioxidative activity of five flavones glycosides from corn silk (Stigma maydis). Czech J. Food Sci. 2013, 31, 148–155. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of food components and emerging technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Roh, K.B.; Kim, H.; Shin, S.; Kim, Y.S.; Lee, J.A.; Kim, M.O.; Jung, E.; Lee, J.; Park, D. Anti-inflammatory effects of Zea mays L. husk extracts. BMC Complement. Altern. Med. 2016, 16, 298–306. [Google Scholar] [CrossRef]
- Boeira, C.P.; Flores, D.C.B.; Lucas, B.N.; Santos, D.; Flores, E.M.M.; Reis, F.L.; Morandini, M.L.B.; Morel, A.F.; Rosa, C.S.D. Extraction of antioxidant and antimicrobial phytochemicals from corn stigma: A promising alternative to valorization of agricultural residues. Ciência Rural. 2022, 52, e20210535. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Fang, M.; Zhan, C.; Pan, H.; Wu, Y.; Gong, Z. Antioxidant and antigenotoxic activity of bioactive extracts from corn tassel. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2014, 34, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Habeebullah, S.F.; Grejsen, H.D.; Jacobsen, C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chem. 2012, 131, 843–851. [Google Scholar]
- Mohdaly, A.A.; Hassanien, M.F.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics extracted from potato, sugar beet, and sesame processing by-products. Int. J. Food Prop. 2013, 16, 1148–1168. [Google Scholar] [CrossRef]
- Lappalainen, K.; Kärkkäinen, J.; Joensuu, P.; Lajunen, M. Modification of potato peel waste with base hydrolysis and subsequent cationization. Carbohydr. Polym. 2015, 132, 97–103. [Google Scholar] [CrossRef]
- Chang, K. Polyphenol antioxidants from potato peels: Extraction optimization and application to stabilizing lipid oxidation in foods. In Proceedings of the National Conference on Undergraduate Research (NCUR) 2019, New York, NY, USA, 11–13 April 2019. [Google Scholar]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullod, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Patar, G. Optimization of pulsed electric fields-assisted extraction of polyphenols from potato peels using response surface methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste-its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Samarin, A.M.; Poorazarang, H.; Hematyar, N.; Elhamirad, A. Phenolics in potato peels: Extraction and utilization as natural antioxidants. World Appl. Sci. J. 2012, 18, 191–195. [Google Scholar]
- Chamorro, S.; Cueva-Mestanza, R.; de Pascual-Teresa, S. Effect of spray drying on the polyphenolic compounds present in purple sweet potato roots: Identification of new cinnamoylquinic acids. Food Chem. 2021, 345, 128679. [Google Scholar] [CrossRef]
- Paniagua-García, A.I.; Hijosa-Valsero, M.; Garita-Cambronero, J.; Coca, M.; Díez-Antolínez, R. Development and validation of a HPLC-DAD method for simultaneous determination of main potential ABE fermentation inhibitors identified in agro-food waste hydrolysates. Microchem. J. 2019, 150, 104147. [Google Scholar] [CrossRef]
- Sarwari, G.; Sultana, B.; Sarfraz, R.A.; Zia, M.A. Cytotoxicity, antioxidant and antimutagenic potential evaluation of peels of edible roots and tubers. Int. Food Res. J. 2019, 26, 1773–1779. [Google Scholar]
- Wu, Z.G.; Xu, H.Y.; Ma, Q.; Cao, Y.; Ma, J.N.; Ma, C.M. Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem. 2012, 135, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltran, N.P.; Chaidez-Quiroz, C.; Lopez-Cuevas, O.; Ruiz-Cruz, S.; Lopez-Mata, M.A.; Del-Toro-Sanchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J. Phenolic compounds of potato peel extracts: Their antioxidant activity and protection against human enteric viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef]
- Ji, X.; Rivers, L.; Zielinski, Z.; Xu, M.; MacDougall, E.; Jancy, S.; Zhang, S.; Wang, Y.; Chapman, R.G.; Keddy, P.; et al. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2012, 133, 1177–1187. [Google Scholar] [CrossRef]
- Hossain, M.B.; Aguilo-Aguayo, I.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Effect of pulsed electric field and pulsed light pre-treatment on the extraction of steroidal alkaloids from potato peels. Innov. Food Sci. Emerg. Technol. 2015, 29, 9–14. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and recovery of valuable bioactive compounds from potato peels: A comprehensive review. Antioxidants 2021, 10, 1630. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Alves-Filho, E.G.; Sousa, V.M.; Ribeiro, P.R.; Rodrigues, S.; de Brito, E.S.; Tiwari, B.K.; Fernandes, F.A. Single-stage ultrasound-assisted process to extract and convert α-solanine and α-chaconine from potato peels into β-solanine and β-chaconine. Biomass Convers. Biorefinery 2018, 8, 689–697. [Google Scholar] [CrossRef]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochemistry 2014, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Rytel, E.; Czopek, A.T.; Aniolowska, M.; Hamouz, K. The influence of dehydrated potatoes processing on the glycoalkaloids content in coloured-fleshed potato. Food Chem. 2013, 141, 2495–2500. [Google Scholar] [CrossRef]
- Singh, L.; Kaur, S.; Aggarwal, P. Techno and bio functional characterization of industrial potato waste for formulation of phytonutrients rich snack product. Food Biosci. 2022, 49, 101824. [Google Scholar] [CrossRef]
- Hillebrand, S.; Husing, B.; Schliephake, U.; Trautz, D.; Herrmann, M.E.; Winterhalter, P. Effect of thermal processing on the content of phenols in pigmented potatoes (Solanum tuberosum L.). Ernaehrungs-Umsch. 2011, 58, 349–353. [Google Scholar]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S. Microwave-assisted extraction of phenolic antioxidants from potato peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef]
- Maldonado, A.F.; Mudge, E.; Gänzle, M.G.; Scheber, A. Extraction and fractionation of phenolic acids and glycoalkaloids from potato peels using acidified water/ethanol-based solvents. Food Res. Int. 2014, 65, 27–34. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its by-products: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato peels as a source of novel green extracts suitable as antioxidant additives for fresh-cut fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef]
- Gebrechristos, H.Y.; Chen, W. Utilization of potato peel as eco-friendly products: A review. Food Sci. Nutr. 2018, 6, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Amado, I.R.; Franco, D.; Sanchez, M.; Zapata, C.; Vazques, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Yeh, Y.H.; Lee, Y.T.; Huang, C.Y. Dietary potato peel extract reduces the toxicity of cholesterol oxidation products in rats. J. Funct. Foods 2016, 27, 461–471. [Google Scholar] [CrossRef]
- Yang, G.; Cheon, S.Y.; Chung, K.S.; Lee, S.J.; Hong, C.H.; Lee, K.T.; Jang, D.S.; Jeong, J.C.; Kwon, O.K.; Nam, J.H.; et al. Solanum tuberosum L. young epidermis extract inhibits mite antigen-induced atopic dermatitis in NC/Nga mice by regulating the Th1/Th2 balance and expression of filaggrin. J. Med. Food 2015, 18, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Khawla, B.J.; Sameh, M.; Imen, G.; Donyes, F.; Dhouha, G.; Raoudha, E.G.; Oumèma, N.E. Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind. Crops Prod. 2014, 52, 144–149. [Google Scholar] [CrossRef]
- Wu, D. Recycle technology for potato peel waste processing: A review. Procedia Environ. Sci. 2016, 31, 103–107. [Google Scholar] [CrossRef]
- Liang, S.; Han, Y.; Wei, L.; McDonald, A.G. Production and characterization of bio-oil and bio-char from pyrolysis of potato peel wastes. Biomass Convers. Biorefin. 2015, 5, 237–246. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Elkahoui, S.; Levin, C.; Bartley, G.; Yokoyama, W.; Friedman, M. Dietary supplementation of potato peel powders prepared from conventional and organic russet and nonorganic gold and red potatoes reduces weight gain in mice on a high-fat diet. J. Agric. Food Chem. 2018, 66, 6064–6072. [Google Scholar] [CrossRef]
- Chimonyo, M. A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed. Sci. Technol. 2017, 227, 107–117. [Google Scholar]
- Apel, C.; Lyng, J.G.; Papoutsis, K.; Harrison, S.M.; Brunton, N.P. Screening the effect of different extraction methods (ultrasound-assisted extraction and solid–liquid extraction) on the recovery of glycoalkaloids from potato peels: Optimization of the extraction conditions using chemometric tools. Food Bioprod. Process. 2019, 119, 277–286. [Google Scholar] [CrossRef]
- Khan, M.T.; Shah, A.S.; Safdar, N.; Rani, S.; Bilal, H.; Hashim, M.M.; Basir, A.; Rahman ZShah, S.A. Polyphenoles extraction from the potato peel and their utilization in biscuit. Pure Appl. Biol. 2017, 6, 1269–1275. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, F.; Yang, Y.; Li, M. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food Chem. 2013, 141, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Rana, S.; Dasila, K.; Agnihotri, V.; Pandey, A.; Pande, V. Comparative nutritional and antimicrobial analysis of Himalayan black and yellow soybean and their okara. J. Sci. Food Agric. 2022, 102, 5358–5367. [Google Scholar] [CrossRef]
- Park, J.; Choi, I.; Kim, Y. Cookies formulated from fresh okara using starch, soy flour and hydroxypropyl methylcellulose have high quality and nutritional value. LWT-Food Sci. Technol. 2015, 63, 660–666. [Google Scholar] [CrossRef]
- Ostermann-Porcel, M.V.; Quiroga-Panelo, N.; Rinaldoni, A.N.; Campderrós, M.E. Incorporation of okara into gluten-free cookies with high quality and nutritional value. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin MC, F.; da Silva MA, P.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Šibul, F.; Orčić, D.; Vasić, M.; Anačkov, G.; Nađpal, J.; Savić, A.; Mimica-Dukić, N. Phenolic profile, antioxidant and anti-inflammatory potential of herb and root extracts of seven selected legumes. Ind. Crops Prod. 2016, 83, 641–653. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.X.; Wu, Z.L.; Wang, Y.J.; Wang, L.J. Recovery of isoflavone aglycones from soy whey wastewater using foam fractionation and acidic hydrolysis. J. Agric. Food Chem. 2013, 61, 7366–7372. [Google Scholar] [CrossRef]
- Kumar, V.; Chauhan, S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Marciniak, B.; Kontek, R. Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Appl. Sci. 2021, 11, 10233. [Google Scholar] [CrossRef]
- Singh, P.; Krishnaswamy, K. Sustainable zero-waste processing system for soybeans and soy by-product valorization. Trends Food Sci. Technol. 2022, 128, 331–344. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Funari, C.S.; Ibáñez, E.; Cifuentes, A. Metabolomics as a tool to study underused soy parts: In search of bioactive compounds. Foods 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Chen, S.Y.; Lin, J.H.; Yen, G.C. Application of saponins extract from food byproducts for the removal of pesticide residues in fruits and vegetables. Food Control 2022, 136, 108877. [Google Scholar] [CrossRef]
- Freitas, S.C.; Alves da Silva, G.; Perrone, D.; Vericimo, M.A.; dos S. Baião, D.; Pereira, P.R.; Paschoalin, V.M.F.; Del Aguila, E.M. Recovery of antimicrobials and bioaccessible isoflavones and phenolics from soybean (Glycine max) meal by aqueous extraction. Molecules 2018, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.D.O.; Perrone, D. Characterization and Stability of Bioactive Compounds from Soybean Meal. LWT Food Sci. Technol. 2015, 63, 992–1000. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, P.; Wang, W.; Zhou, J.; Raj, R.; Du, Z.; Xu, S.; Wang, W.; Yu, B.; Zhang, J. Derivatization of Soyasapogenol A through Microbial Transformation for Potential Anti-inflammatory Food Supplements. J. Agric. Food Chem. 2021, 69, 6791–6798. [Google Scholar] [CrossRef]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable valorization of tomato by-products to obtain bioactive compounds: Their potential in inflammation and cancer management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M. Chemical composition and ameliorative effect of tomato on isoproterenol-induced myocardial infarction in rats. Asian J. Clin. Nutr. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Padalino, L.; Conte, A.; Lecce, L.; Likyova, D.; Sicari, V.; Pellicano, T.M.; Poiana, M.; Del Nobile, M.A. Functional pasta with tomato by-product as a source of antioxidant compounds and dietary fibre. Czech J. Food Sci. 2017, 35, 48–56. [Google Scholar]
- Bakic, M.T.; Pedisic, S.; Zoric, Z.; Dragovic-Uzelac, V.; Grassino, A.N. Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim. Slov. 2019, 66, 367–377. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Plant Nutraceuticals as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Valta, K.; Damala, P.; Panaretou, V.; Orli, E.; Moustakas, K.; Loizidou, M. Review and assessment of waste and wastewater treatment from fruits and vegetables processing industries in Greece. Waste Biomass Valorization 2017, 8, 1629–1648. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Perea-Dominguez, X.P.; Hernandez-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato by-product: Free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Trandafir, I.; Corbu, A.R. Nutritional and bioactive compounds in dried tomato processing waste. CyTA J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Ćetković, G.; Savatović, S.; Čanadović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Reuse potential of vegetable wastes (broccoli, green bean and tomato) for the recovery of antioxidant phenolic acids and flavonoids. Int. J. Food Sci. Technol. 2017, 52, 98–107. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, M.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing by-products. LWT-Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jimenez, J.M.; de Pascual, T.S.; Poli, A.; Nicolaus, B. Biological properties of polyphenols extracts from agro industry’s wastes. Waste Biomass Valorization 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- García-Valverde, V.; Navarro-González, I.; García Alonso, J.; Periago, M. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Catoi, A.F.; Vodnar, D.C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds and by-products. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sa, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Singh, S.; et al. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Palomo, I.; Plaza, A.; Gadioli Tarone, A.; Junior MR, M.; Sáyago-Ayerdi, S.G.; Fuentes, E. Platelet anti-aggregant activity and bioactive compounds of ultrasound-assisted extracts from whole and seedless tomato pomace. Foods 2020, 9, 1564. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Michiu, D.; Biriş, S.; Tofană, M. Tomato waste as a source of biologically active compounds. Bull. UASVM Food Sci. Technol. 2019, 76, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Markovic, K.; Krbavcic, I.P.; Krpan, M.; Bicanic, D.; Vahcic, N. The lycopene content in pulp and peel of five fresh tomato cultivars. Acta Aliment. 2010, 39, 90–98. [Google Scholar] [CrossRef]
- Stoica, R.M.; Tomulescu, C.; Cășărică, A.; Soare, M.G. Tomato by-products as a source of natural antioxidants for pharmaceutical and food industries—A mini-review. Sci. Bull. Ser. F Biotechnol. 2018, 22, 200–204. [Google Scholar]
- Górecka, D.; Wawrzyniak, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Hamułka, J.; Kowalczewski, P.Ł.; Walkowiak, J. Lycopene in tomatoes and tomato products. Open Chem. 2020, 18, 752–756. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.; Calhelha, R.C.; Ferreira, I.C.; Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Djakovic, S.; Bosiljkov, T.; Halambek, J.; Zorić, Z.; Dragović-Uzelac, V.; Petrović, M.; Brnčić, S.R. Valorisation of tomato peel waste as a sustainable source for pectin, polyphenols and fatty acids recovery using sequential extraction. Waste Biomass Valorization 2019, 11, 4593–4611. [Google Scholar] [CrossRef]
- Grassino, A.N.; Pedistić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into high-hydrostatic pressure extraction of polyphenols from tomato peel waste. Plant Foods Hum. Nutr. 2020, 75, 427–433. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and vegetable by-products to fortify spreadable cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef]
- Lim, W.; Li, J. Co-expression of onion chalcone isomerase in Del/Ros1-expressing tomato enhances anthocyanin and flavonol production. Plant Cell Tissue Organ Cult. 2017, 128, 113–124. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 2013, 6, 3499–3509. [Google Scholar] [CrossRef]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and analysis of tomato seed oil. J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2019, 40, 692–697. [Google Scholar] [CrossRef]
- Marti, R.; Rosello, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Savatović, S.; Cetkovic, G.; Canadanovic-Brunet, J.; Djilas, S. Tomato waste: A potential source of hydrophilic antioxidants. Int. J. Food Sci. Nutr. 2012, 63, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bread enriched in lycopene and other bioactive compounds by addition of dry tomato waste. J. Food Sci. Technol. 2015, 52, 8260–8267. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Jridi, M.; Khemakhem, I.; Bouaziz, M.; Attia, H. Storage stability of traditional Tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem. 2017, 15, 476–482. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Ho, K.K.; Ferruzzi, M.G.; Liceaga, A.M.; San Martin-Gonzales, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT-Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Horuz, T.I.; Belibagli, K.B. Encapsulation of tomato peel extract into nanofibers and its application in model food. Food Process. Preserv. 2019, 43, e14090. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Afsharnezhad, M.; Shahangian, S.S.; Panahi, E.; Sariri, R. Evaluation of the antioxidant activity of extracts from some fruit peels. Casp. J. Environ. Sci. 2017, 15, 213–222. [Google Scholar]
- Kabir, M.R.; Hasan, M.M.; Islam, M.R.; Haque, A.R.; Hasan, S.K. Formulation of yogurt with banana peel extracts to enhance storability and bioactive properties. J. Food Process. Preserv. 2021, 45, e15191. [Google Scholar] [CrossRef]
- Chaudhry, F.; Ahmad, M.L.; Hayat, Z.; Ranjha MM, A.N.; Chaudhry, K.; Elboughdiri, N.; Asmari, M.; Uddin, J. Extraction and Evaluation of the Antimicrobial Activity of Polyphenols from Banana Peels Employing Different Extraction Techniques. Separations 2022, 9, 165. [Google Scholar] [CrossRef]
- Rebello LP, G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; El-Hefny, M.; Salem, M.Z.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z. Antifungal and antibacterial activities of Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Kandasamy, S.; Ramu, S.; Aradhya, S.M. In vitro functional properties of crude extracts and isolated compounds from banana pseudostem and rhizome. J. Sci. Food Agric. 2016, 96, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Avram, I.; Gatea, F.; Vamanu, E. Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes 2022, 10, 248. [Google Scholar] [CrossRef]
- Nofianti, T.; Ahmad, M.; Irda, F. Comparison of antihyperglycemic activity of different parts of klutuk banana (Musa balbisiana colla). Int. J. Appl. Pharm. 2021, 13, 57–61. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Encapsulation of phenolic-rich extract from banana (Musa cavendish) peel. J. Food Sci. Technol. 2020, 57, 2089–2098. [Google Scholar] [CrossRef]
- Buendía-Otero, M.J.; Jiménez-Corzo, D.J.; Caamaño De Ávila, Z.I.; Restrepo, J.B. Chromatographic analysis of phytochemicals in the peel of Musa paradisiaca to synthesize silver nanoparticles. Rev. Fac. De Ing. Univ. De Antioq. 2022, 103, 130–137. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed]
- Vani, R.; Bhandari, A.; Jain, Y.A. Inhibition Effects Of Banana And Orange Peel Extract On The Corrosion Of Bright Steel In Acidic Media. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1065, p. 012029. [Google Scholar] [CrossRef]
- CSO (Central Statistical Office in Poland). Production of Agricultural and Horticultural Crops in 2021. 2022. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agricultural-and-horticultural-crops/production-of-agricultural-and-horticultural-crops-in-2021,2,6.html (accessed on 29 June 2022).
- Fernandes, P.A.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple pomace extract as a sustainable food ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, M.; Vandezande, P.; Van Dael, M.; Vloemans, S.; Noten, B.; Bongers, B.; Porto-Carrero, M.; Unamunzaga, M.M.; Bulut, M.; Lemmens, B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a techno-economic assessment. J. Food Process Eng. 2018, 41, e12629. [Google Scholar] [CrossRef]
- Barreira, J.C.; Arraibi, A.A.; Ferreira, I.C. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple pomace as potential source of natural active compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of apple pomace flour obtained industrially by dehydration as a source of biomolecules with antioxidant, antidiabetic and antiobesity effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of apple pomace by extraction of valuable compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Ż.; Oszust, K.; Frąc, M. Phytochemicals of apple pomace as prospect bio-fungicide agents against mycotoxigenic fungal species—In vitro experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef]
- Ramirez-Ambrosi, M.; Abad-Garcia, B.; Viloria-Bernal, M.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B. A new ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry analytical strategy for fast analysis and improved characterization of phenolic compounds in apple products. J. Chromatogr. A 2013, 1316, 78–91. [Google Scholar]
- Mohammed, E.T.; Mustafa, Y.F. Coumarins from Red Delicious apple seeds: Extraction, phytochemical analysis, and evaluation as antimicrobial agents. Syst. Rev. Pharm. 2020, 11, 64–70. [Google Scholar]
- Khalil, R.R.; Mustafa, Y.F. Phytochemical, antioxidant and antitumor studies of coumarins extracted from Granny Smith apple seeds by different methods. Syst. Rev. Pharm. 2020, 11, 57–63. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of triterpenic acids and phytosterols from apple pomace with supercritical carbon dioxide: Impact of process parameters, modelling of kinetics, and scaling-up study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological activity of phenolics enriched extracts from industrial apple pomace. Ind. Crops Prod. 2021, 160, 113158. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Silva, G.N.; Maria, N.R.; Schuck, D.C.; Cruz, L.N.; de Moraes, M.S.; Nakabashi, M.; Graebin, C.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C. Two series of new semisynthetic triterpene derivatives: Differences in anti-malarial activity, cytotoxicity and mechanism of action. Malar. J. 2013, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arraibi, A.A.; Liberal, Â.; Dias, M.I.; Alves, M.J.; Ferreira, I.C.; Barros, L.; Barreira, J.C. Chemical and bioactive characterization of Spanish and Belgian apple pomace for its potential use as a novel dermocosmetic formulation. Foods 2021, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Rezaei, K. Designing an all-apple-pomace-based functional dessert formulation. Br. Food J. 2013, 115, 409–424. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Zhang, C.; Liu, J.; Jiao, Z. Enzymatic preparation and antioxidant activity of the phloridzin oxidation product. J. Food Biochem. 2018, 42, e12475. [Google Scholar] [CrossRef]
- Vera, R.; Figueredo, F.; Díaz-Gómez, A.; Molinari, A. Evaluation of Fuji apple peel extract as a corrosion inhibitor for carbon steel in a saline medium. Int. J. Electrochem. Sci. 2018, 13, 4139–4159. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; Kačániová, M.; Ivanišhová, E.; Mareček, J.; Gambuś, H. Industrial Apple Pomace By-Products As A Potential Source Of Pro-Health Compounds In Functional Food. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 22–26. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Luo, H.; Li, L.; Tang, J.; Zhang, F.; Zhao, F.; Sun, D.; Zheng, F.; Wang, X. Amygdalin inhibits HSC-T6 cell proliferation and fibrosis through the regulation of TGF-β/CTGF. Mol. Cell. Toxicol. 2016, 12, 265–271. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10, 3–7. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles MA, A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Wang, X.; Tong, H.; Chen, F.; Gangemi, J.D. Chemical characterization and antioxidant evaluation of muscadine grape pomace extract. Food Chem. 2010, 123, 1156–1162. [Google Scholar] [CrossRef]
- Daniel, T.; Ben-Shachar, M.; Drori, E.; Hamad, S.; Permyakova, A.; Ben-Cnaan, E.; Tam, J.; Kerem, Z.; Rosenzweig, T. Grape pomace reduces the severity of non-alcoholic hepatic steatosis and the development of steatohepatitis by improving insulin sensitivity and reducing ectopic fat deposition in mice. J. Nutr. Biochem. 2021, 98, 108867. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.; Barros, L.; Haminiuk, C.W.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Rodríguez-Pulido, F.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Determination of phenolic substances of seeds, skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Iora, S.R.; Maciel, G.M.; Zielinski, A.A.; da Silva, M.V.; Pontes PV, D.A.; Haminiuk, C.W.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Silva DS, M.E.; Grisi CV, B.; da Silva, S.P.; Madruga, M.S.; da Silva, F.A.P. The technological potential of agro-industrial residue from grape pulping (Vitis spp.) for application in meat products: A review. Food Biosci. 2022, 49, 101877. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Muniz, P. From winery by-product to healthy product: Bioavailability, redox signaling and oxidative stress modulation by wine pomace product. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT-Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Álvarez, E.; Rodiño-Janeiro, B.K.; Jerez, M.; Ucieda-Somoza, R.; Núñez, M.J.; González-Juanatey, J.R. Procyanidins from grape pomace are suitable inhibitors of human endothelial NADPH oxidase. J. Cell. Biochem. 2012, 113, 1386–1396. [Google Scholar] [CrossRef]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crops Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Alibade, A.; Kaltsa, O.; Bozinou, E.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.; Makris, D.P. Stability of microemulsions containing red grape pomace extract obtained with a glycerol/sodium benzoate deep eutectic solvent. OCL 2022, 29, 28. [Google Scholar] [CrossRef]
- Soares SC, S.; de Lima, G.C.; Laurentiz, A.C.; Féboli, A.; Dos Anjos, L.A.; de Paula Carlis, M.S.; da Silva Filardi, R.; de Laurentiz RD, S. In vitro anthelmintic activity of grape pomace extract against gastrointestinal nematodes of naturally infected sheep. Int. J. Vet. Sci. Med. 2018, 6, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Gutiérrez-Docio, A.; Moreno-Fernandez, S.; Alarcón-Cavero, T.; Prodanov, M.; Martinez-Rodriguez, A.J. Procyanidin-rich extract from grape seeds as a putative tool against Helicobacter pylori. Foods 2020, 9, 1370. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Della Vedova, L.; Ferrario, G.; Gado, F.; Altomare, A.; Carini, M.; Morazzoni, P.; Aldini, G.; Baron, G. Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS) Profiling of Commercial Enocianina and Evaluation of Their Antioxidant and Anti-Inflammatory Activity. Antioxidants 2022, 11, 1187. [Google Scholar] [CrossRef]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.; Kato, E.T.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and photoprotective efficacy of a sunscreen system based on grape pomace (Vitis vinifera L.) phenolics from winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Gavrilaș, S.; Calinovici, I.; Chiș, S.; Ursachi, C.Ș.; Raț, M.; Munteanu, F.D. White Grape Pomace Valorization for Remediating Purposes. Appl. Sci. 2022, 12, 1997. [Google Scholar] [CrossRef]
- Olt, V.; Báez, J.; Jorcin, S.; López, T.; Fernández, A.; Medrano, A. Development of a potential functional yogurt using bioactive compounds obtained from the by-product of the production of Tannat red wine. Biol. Life Sci. Forum 2021, 6, 93. [Google Scholar]
- Asmat-Campos, D.; Bravo Huivin, E.; Avalos-Vera, V. Valorization of agro-industrial waste in a circular economy environment: Grape pomace as a source of bioactive compounds for its application in nanotechnology. In Proceedings of the 19th LACCEI International Multi-Conference for Engineering, Education, and Technology: “Prospective and Trends in Technology and Skills for Sustainable Social Development” “Leveraging Emerging Technologies to Construct the Future”, Buenos Aires, Argentina, 21–23 July 2021. [Google Scholar] [CrossRef]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity–a review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Deng, Z.; Pan, X.; Xie, X.; Peng, C. Effective utilization of food wastes: Bioactivity of grape seed extraction and its application in food industry. J. Funct. Foods 2020, 73, 104113. [Google Scholar] [CrossRef]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. Int. J. Food Sci. Technol. 2019, 54, 952–958. [Google Scholar] [CrossRef]
- Gárcia-Lomillo, J.; González-SanJosé, M. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Yaqoob, M.; Aggarwal, P.; Rasool, N.; Baba, W.N.; Ahluwalia, P.; Abdelrahman, R. Enhanced functional properties and shelf stability of cookies by fortification of kinnow derived phytochemicals and residues. J. Food Meas. Charact. 2021, 15, 2369–2376. [Google Scholar] [CrossRef]
- Karetha, K.; Gadhvi, K.; Vyas, S. Peelings of citrus fruits as a precious resource of phytochemical and vital bioactive medicines during Covid: 19 periods. Int. J. Bot. Stud. 2020, 5, 342–344. [Google Scholar]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical profile, α-glucosidase, and α-amylase inhibition potential and toxicity evaluation of extracts from Citrus aurantium (L) peel, a valuable by-product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.D.P.M.; Ruviaro, A.R.; Macedo, G.A. Comparison of different Brazilian citrus by-products as source of natural antioxidants. Food Sci. Biotechnol. 2018, 27, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Sharifudin SA, B. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Silva AC, D.; Aranha, C.P. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. An. Da Acad. Bras. De Ciên. 2016, 88, 951–958. [Google Scholar] [CrossRef]
- Olfa, T.; Gargouri, M.; Akrouti, A.; Brits, M.; Gargouri, M.; Ben Ameur, R.; Pieters, L.; Foubert, K.; Magné, C.; Soussi, A.; et al. A comparative study of phytochemical investigation and antioxidative activities of six citrus peel species. Flavour Fragr. J. 2021, 36, 564–575. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, S.Y.; Kang, N.G.; Jin, M.H. A multi-faceted comparison of phytochemicals in seven citrus peels and improvement of chemical composition and antioxidant activity by steaming. LWT 2022, 160, 113297. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green extraction techniques for obtaining bioactive compounds from mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, J.; Hu, C.; Wang, N.; Zhang, L.; Mo, X.; Li, G.; Liao, H.; Huang, H.; Ji, S.; et al. Integrative analyses of transcriptome and carotenoids profiling revealed molecular insight into variations in fruits color of Citrus Reticulata Blanco induced by transplantation. Genomics 2022, 114, 110291. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Convers. Biorefinery 2021, 11, 2157–2165. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Petrillo, F.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Calabrò, A. A non-conventional method to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction. Sep. Purif. Technol. 2014, 137, 13–20. [Google Scholar] [CrossRef]
- Okino Delgado, C.H.; Fleuri, L.F. Orange and mango by-products: Agro-industrial waste as source of bioactive compounds and botanical versus commercial description—A review. Food Rev. Int. 2016, 32, 1–14. [Google Scholar] [CrossRef]
- Yang, X.; Kang, S.M.; Jeon, B.T.; Kim, Y.D.; Ha, J.H.; Kim, Y.T.; Jeon, Y.J. Isolation and identification of an antioxidant flavonoid compound from citrus-processing by-product. J. Sci. Food Agric. 2011, 91, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Zanaroli, G.; Vannini, L.; Guerzoni, E.; Bordoni, A.; Viaggi, D.; Robertson, J.; Waldron, K.; Bald, C.; Esturo, A.; et al. New advances in the integrated management of food processing by-products in Europe: Sustainable exploitation of fruit and cereal processing by-products with the production of new food products (NAMASTE EU). New Biotechnol. 2013, 30, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Zhang, L.; Zhao, H.; Ho, C.T.; Li, S. Recent study on the anticancer activity of nobiletin and its metabolites. J. Food Bioact. 2021, 14. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.Y.; Guo, Y.; Chu, C.; Zheng, G.D.; Liu, E.H.; Li, F. Enrichment of polymethoxyflavones from Citrus reticulata ‘Chachi’peels and their hypolipidemic effect. J. Chromatogr. B 2019, 1124, 226–232. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Li, S.-Z.; Xiao, P.-T.; Cai, Y.-Y.; Chu, C.; Chen, B.-Z.; Li, P.; Li, J.; Liu, E.-H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef]
- Chiechio, S.; Zammataro, M.; Barresi, M.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P. A standardized extract prepared from red orange and lemon wastes blocks high-fat diet-induced hyperglycemia and hyperlipidemia in mice. Molecules 2021, 26, 4291. [Google Scholar] [CrossRef]
- Barbosa PD, P.M.; Ruviaro, A.R.; Martins, I.M.; Macedo, J.A.; LaPointe, G.; Macedo, G.A. Enzyme-assisted extraction of flavanones from citrus pomace: Obtention of natural compounds with anti-virulence and anti-adhesive effect against Salmonella enterica subsp. enterica serovar Typhimurium. Food Control 2021, 120, 107525. [Google Scholar] [CrossRef]
- Lamine, M.; Gargouri, M.; Rahali, F.Z.; Mliki, A. Recovering and characterizing phenolic compounds from citrus by-product: A way towards agriculture of subsistence and sustainable bioeconomy. Waste Biomass Valorization 2021, 12, 4721–4731. [Google Scholar] [CrossRef]
- Gunwantrao, B.B.; Bhausaheb, S.K.; Ramrao, B.S.; Subhash, K.S. Antimicrobial activity and phytochemical analysis of orange (Citrus aurantium L.) and pineapple (Ananas comosus (L.) Merr.) peel extract. Ann. Phytomed. 2016, 5, 156–160. [Google Scholar] [CrossRef]
- Khan, N.H.; Qian, C.J.; Perveen, N. Phytochemical screening, antimicrobial and antioxidant activity determination of citrus maxima peel. Pharm. Pharmacol. Int. J. 2018, 6, 279–285. [Google Scholar]
- Khan, J.; Sakib, S.A.; Mahmud, S.; Khan, Z.; Islam, M.N.; Sakib, M.A.; Emran, T.B.; Simal-Gandara, J. Identification of potential phytochemicals from Citrus limon against main protease of SARS-CoV-2: Molecular docking, molecular dynamic simulations and quantum computations. J. Biomol. Struct. Dyn. 2021, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Achimón, F.; Leal, L.E.; Pizzolitto, R.P.; Brito, V.D.; Alarcón, R.; Omarini, A.B.; Zygadlo, J.A. Insecticidal and antifungal effects of lemon, orange, and grapefruit peel essential oils from Argentina. Agriscientia 2022, 39, 71–82. [Google Scholar]
- Liu, Y.; Benohoud, M.; Yamdeu JH, G.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, H.; Jin, C.; Mo, J.; Wang, H. Nobiletin flavone inhibits the growth and metastasis of human pancreatic cancer cells via induction of autophagy, G0/G1 cell cycle arrest, and inhibition of NF-kB signalling pathway. J. Buon 2020, 25, 1070–1075. [Google Scholar]
- Ozkan, A.D.; Kaleli, S.; Onen, H.I.; Sarihan, M.; Eskiler, G.G.; Yigin, A.K.; Akdogan, M. Anti-inflammatory effects of nobiletin on TLR4/TRIF/IRF3 and TLR9/IRF7 signaling pathways in prostate cancer cells. Immunopharmacol. Immunotoxicol. 2020, 42, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, S.M.; El-Shafea, A.; Mohamed, Y.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Martakos, I.; Katsianou, P.; Koulis, G.; Efstratiou, E.; Nastou, E.; Nikas, S.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Development of Analytical Strategies for the Determination of Olive Fruit Bioactive Compounds Using UPLC-HRMS and HPLC-DAD. Chemical Characterization of Kolovi Lesvos Variety as a Case Study. Molecules 2021, 26, 7182. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Darvishzadeh, P.; Orsat, V. Microwave-assisted extraction of antioxidant compounds from Russian olive leaves and flowers: Optimization, HPLC characterization and comparison with other methods. J. Appl. Res. Med. Aromat. Plants 2022, 27, 100368. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of Olive Mill Waste Waters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Zhao, H.; Avena-Bustillos, R.J.; Wang, S.C. Extraction, Purification and In Vitro Antioxidant Activity Evaluation of Phenolic Compounds in California Olive Pomace. Foods 2022, 11, 174. [Google Scholar] [CrossRef]
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2022, 8, 782693. [Google Scholar] [CrossRef]
- Ladhari, A.; Zarrelli, A.; Ghannem, M.; Ben Mimoun, M. Olive wastes as a high-potential by-product: Variability of their phenolic profiles, antioxidant and phytotoxic properties. Waste Biomass Valorization 2021, 12, 3657–3669. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Baskyr, I. Organic compounds in olive mill wastewater and in solutions resulting from hydrothermal carbonization of the wastewater. Chemosphere 2013, 92, 1472–1482. [Google Scholar] [CrossRef]
- Uribe, E.; Pasten, A.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Ortiz, J.; Di Scala, K. Comparison of Chemical Composition, Bioactive Compounds and Antioxidant Activity of Three Olive-Waste Cakes. J. Food Biochem. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of Polyphenols from Olive Leaves Employing Deep Eutectic Solvents: The Application of Chemometrics to a Quantitative Study on Antioxidant Compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Taamalli, A.; Arraez-Roman, D.; Barrajon-Catalan, E.; Ruiz-Torres, V.; Perez-Sanchez, A.; Herrero, M.; Ibanñez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: Phenolic composition and cytotoxicity against human breast cancer cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Servian-Rivas, L.D.; Pachón, E.R.; Rodríguez, M.; González-Miquel, M.; González, E.J.; Díaz, I. Techno-economic and environmental impact assessment of an olive tree pruning waste multiproduct biorefinery. Food Bioprod. Process. 2022, 134, 95–108. [Google Scholar] [CrossRef]
- Yeniçeri, M.; Filik, A.G.; Filik, G. The Effect of Some Selected Fruit Wastes for Poultry Feed on Growth Performance of Broilers. Palandöken J. Anim. Sci. Technol. Econ. 2022, 1, 33–41. [Google Scholar]
- Kreatsouli, K.; Fousteri, Z.; Zampakas, K.; Kerasioti, E.; Veskoukis, A.S.; Mantas, C.; Gkoutsidis, P.; Ladas, D.; Petrotos, K.; Kouretas, D.; et al. A Polyphenolic Extract from Olive Mill Wastewaters Encapsulated in Whey Protein and Maltodextrin Exerts Antioxidant Activity in Endothelial Cells. Antioxidants 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, G. Antioxidant and other biological activities of olive mill waste waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic Fraction from Olive MillWastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef]
- Benincasa, C.; La Torre, C.; Plastina, P.; Fazio, A.; Perri, E.; Caroleo, M.C.; Gallelli, L.; Cannataro, R.; Cione, E. Hydroxytyrosyl Oleate: Improved Extraction Procedure from Olive Oil and By-Products, and In Vitro Antioxidant and Skin Regenerative Properties. Antioxydants 2019, 8, 233. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Yangui, T.; Sayadi, S.; Gargoubi, A.; Dhouib, A. Fungicidal effect of hydroxytyrosol rich preparations from olive mill wastewater against Verticillium dahliae. Crop Prot. 2010, 29, 1208–1213. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.; Shehata, G. Characterization of Olive Oil By-products: Antioxidant Activity, Its Ability to Reduce Aflatoxigenic Fungi Hazard and Its Aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Abi-Khattar, A.M.; Rajha, N.; Abdel-Massih, M.; Maroun, G.; Louka, N.; Debs, E. Intensification of Polyphenol Extraction from Olive Leaves Using Ired-Irrad, an Environmentally-Friendly Innovative Technology. Antioxidants 2019, 8, 227. [Google Scholar] [CrossRef]
- Bavaro, S.L.; D’Antuono, I.; Cozzi, G.; Haidukowski, M.; Cardinali, A.; Logrieco, A.F. Inhibition of aflatoxin B1 production by verbascoside and other olive polyphenols. World Mycotoxin J. 2016, 9, 545–553. [Google Scholar] [CrossRef]
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill waste water extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Fernández MY, G.; Burrieza, J.E.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining green extracts rich in phenolic compounds from underexploited food by-products using natural deep eutectic solvents. Opportunities and challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).