- Communication
Short-Term Influence of Administering Janus Kinase Inhibitor on Renal Function in Patients with Rheumatoid Arthritis
- Ichiro Yoshii,
- Tatsumi Chijiwa and
- Naoya Sawada
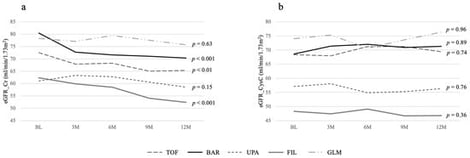
Background/Objectives: The short-term effect of Janus kinase inhibitors (JAKis) on renal function in patients with rheumatoid arthritis (RA) was examined in a hypothesis-generating, exploratory study. Methods: RA patients treated with JAK inhibitors and, as a control group, those receiving golimumab and continuing treatment for one or more years were enrolled. They were monitored every 3 months for disease activity using the Simplified Disease Activity Index (SDAI), functional capacity using the Health Assessment Questionnaire Disability Index (HAQ), and renal function using the estimated glomerular filtration rate (eGFR) calculated from creatinine (Cr) and cystatin C (CysC). Patients were categorized by medication, and average values were computed. Two groups for each drug were then compared statistically. Results: A total of 144 patients were analyzed: 24 on tofacitinib, 43 on baricitinib, 21 on upadacitinib, 21 on filgotinib, and 35 on golimumab. Background factors did not differ significantly among groups. Improvements in CDAI and HAQ at any time point also showed no significant differences. eGFR based on Cr showed a significant decline in the baricitinib and filgotinib groups at one year after starting JAKi treatment compared with the other JAKi groups; however, there was no significant difference when using CysC. Conclusions: These results indicate that there is no significant difference in renal function decline among the JAKi drugs over a short period, despite differences in their metabolic pathways and renal excretion patterns.
13 February 2026