- 2.8Impact Factor
- 5.5CiteScore
- 15 daysTime to First Decision
CO2 Capture and Conversion Processes: Recent Trends and Future Perspectives
This special issue belongs to the section “Environmental and Green Processes“.
Special Issue Information
Dear Colleagues,
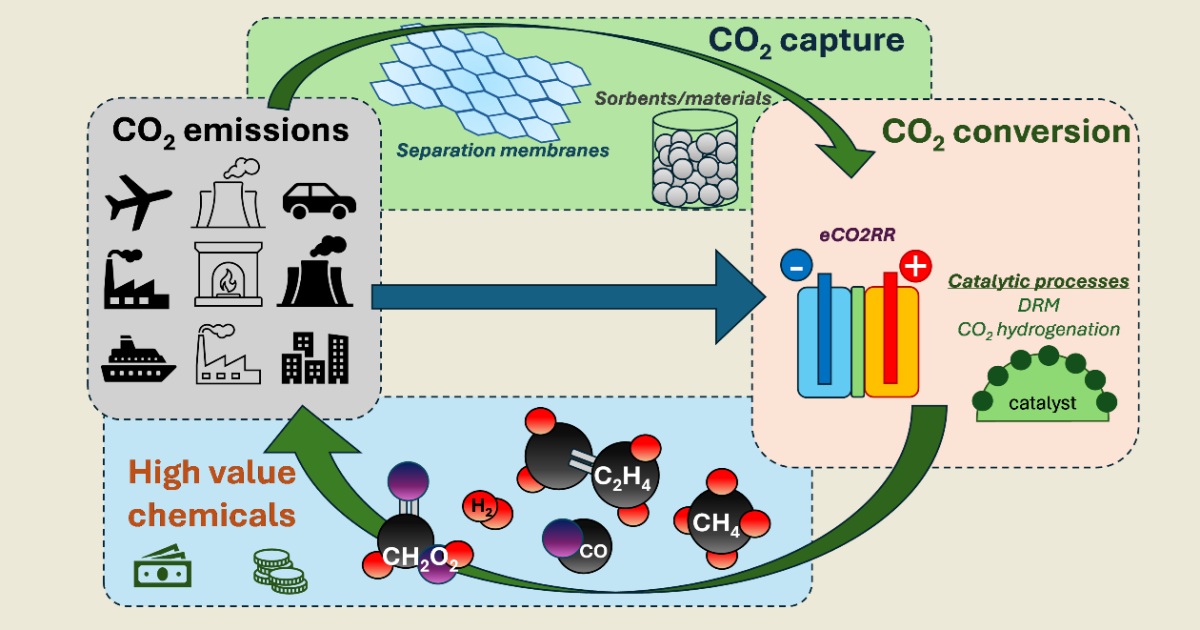
The amplification of energy demands due to global population growth and modern lifestyles result in increasing CO2 atmospheric levels, mostly attributed to intensifying fossil fuel industrial production. International initiatives, such as the Kyoto protocol and the Paris agreement, target the significant reduction of CO2 emissions in order to mitigate climate change. Towards this direction, various technologies have emerged, aiming to capture CO2 and transform it to useful products. Energy-efficient CO2 adsorption and absorption processes for capturing CO2 from various point emission sources and directly from air (DAC), employing innovative low-cost material solvents and membranes, as well as innovative conversion processes including electrocatalytic CO2 reduction reactions (CO2RRs) to useful products, are of major importance.
The present Special Issue seeks high quality works, focusing on CO2 capture and CO2 conversion technologies. The aim of the Issue is to collect recent research and review works related to the aforementioned processes targeting CO2 mitigation.
Topics include, but are not limited to, the following:
CO2 capture technologies:
- Direct air capture (DAC)
- Direct ocean capture (DOC)
- Post-combustion capture
- Pre-combustion capture
- Oxy-fuel combustion
- Chemical looping combustion
- Cryogenic separation
- Absorption
- Adsorption
- Membrane separation
- Hybrid processes
- Catalytic processes
- Dry reforming of methane (DRM) to sygas production
- CO2 hydrogenation to high-value products
- Electrocatalytic CO2 reduction reaction (CO2RR)
- Microbial electrosynthesis systems (MESs)
- Photocatalytic CO2 reduction
Dr. Georgios Bampos
Dr. Georgios Karanikolos
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Processes is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- greenhouse gases (GHGs)
- carbon emissions
- CO2
- CO2 capture
- carbon capture and storage (CCS)
- carbon capture and utilization (CCU)
- CO2 conversion
- CO2 reduction reaction (CO2RR)
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.


