- Article
Benzodiazepine (BZD) Use and Patient Safety: Opportunities for Community Pharmacy Involvement in the Management of Drug Interactions
- Juan Ramón Santana Ayala,
- Daida Alberto Armas and
- Veronica Hernández García
- + 5 authors
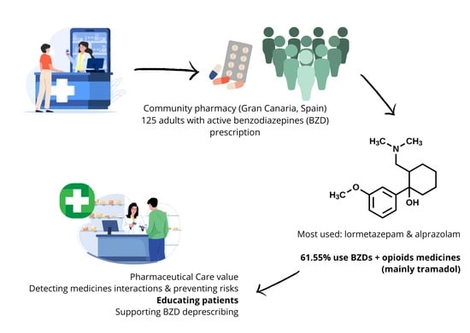
Introduction: During pharmaceutical care, community pharmacists play a crucial role by carrying out interventions aimed at preventing, detecting, and resolving drug-related problems (DRPs) and negative outcomes associated with medication (NOM), simultaneously enhancing patients’ knowledge about their treatments. The chronic use of Benzodiazepines (BZDs) is known to be associated with risks such as tolerance, dependence, and cognitive impairment. Furthermore, the combined use of BZDs with other medications or alcohol may expose patients to significant drug interactions. Objectives: This study aimed to characterize and describe the clinical profile of patients using BZDs, to evaluate the extent of polypharmacy and potential drug interactions, to investigate their level of knowledge regarding BZD treatment, and ultimately, to propose evidence-based interventions from the community pharmacy to contribute to improving patient safety and minimizing risks associated with BZD use. Method: A cross-sectional, descriptive study was conducted in a single community pharmacy in Gran Canaria (Canary Islands, Spain). The study population comprised 125 adult patients with active BZD prescriptions. Data collection was performed through pharmacist–patient structured interviews using a questionnaire that included sociodemographic, clinical, and BZD knowledge variables. Results: Lormetazepam and alprazolam were the BZDs most frequently prescribed and dispensed. Potential drug interactions with other medications were detected in 38.4% of BZD users. Notably, 61.5% of patients using BZDs also reported the concurrent use of opioid analgesics, with tramadol being the most common opioid (48.1% of BZD users were also treated with tramadol). Statistically significant differences were observed between patients with and without BZD and other drug interactions in several adverse outcome variables, including the risk of falls (p = 0.003), cognitive impairment (p = 0.047), and urinary incontinence (p = 0.016). Existing BZD dependence is detected in 25% and 22.1% of cases, respectively. Patients’ knowledge of their BZD treatment revealed critical gaps, which are identified as a challenge and a clear opportunity for intervention through pharmaceutical care services. Conclusions: The findings underscore the essential and proactive role of community pharmacists in identifying and managing drug interactions, as well as in supporting deprescribing strategies through collaborative and interprofessional care models.
11 December 2025