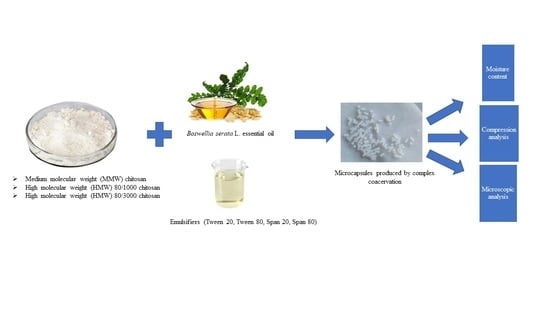
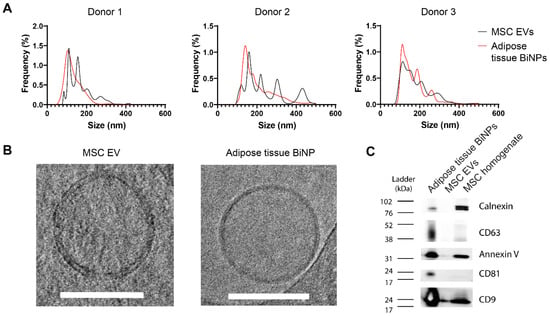
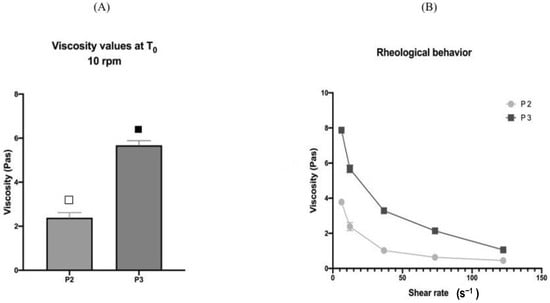
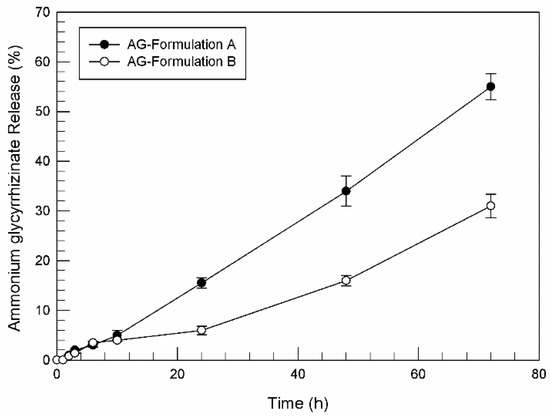
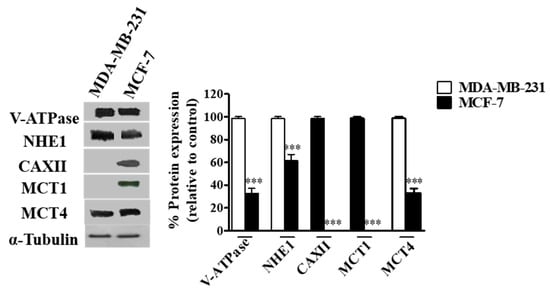
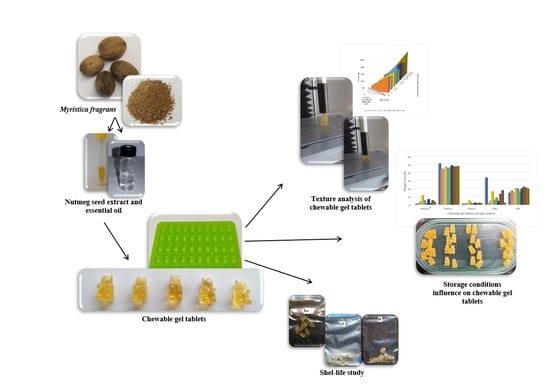
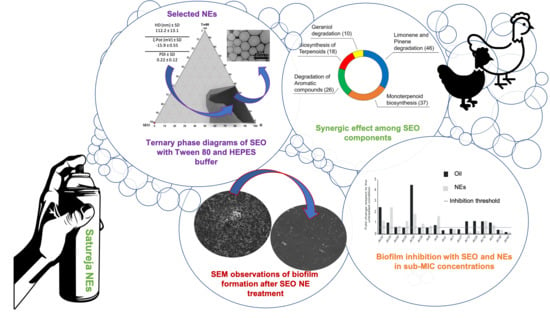
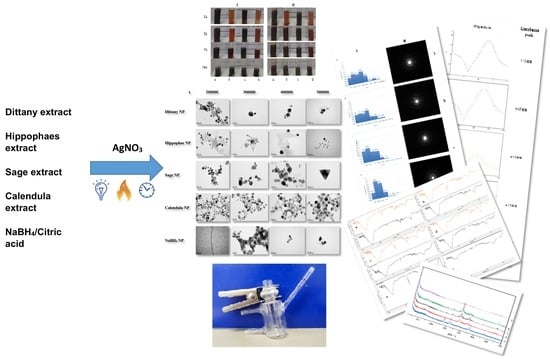
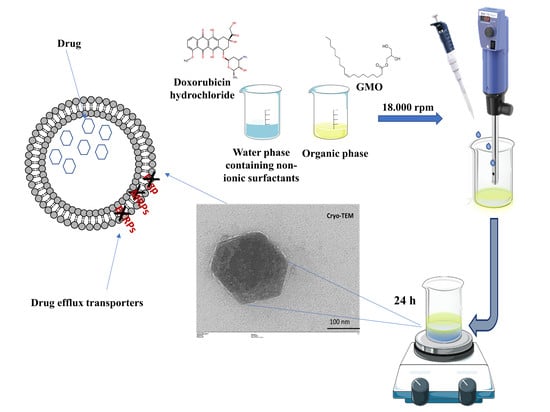
Women in Pharmaceutics
A topical collection in Pharmaceutics (ISSN 1999-4923). This collection belongs to the section "Nanomedicine and Nanotechnology".
Viewed by 201513Editors
Interests: nanomedicine; drug delivery; gene therapy; pharmaceutics; pharmaceutical education; topical delivery
Special Issues, Collections and Topics in MDPI journals
Interests: drug delivery; cyclodextrin; drug/cyclodextrin inclusion complexes; nanoparticles; nanostructures; supramolecular chemistry; anticancer therapy; antibacterial therapy; neurodegenerative disease; autoimmune pathologies
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
To date countless women have made historical contributions to science. Many didn’t have due scientific recognition but have spurred the birth of several generations of female scientists. Despite the struggles over time for gender discrimination, there are still too many problems facing women scientists as they advance their careers. Pharmaceutics wants to contribute to decreasing this gender discrimination launching a Special Issue titled “Women in Pharmaceutics.”
This Special Issue is intended to provide a forum for academic researchers and other professionals to share their recent works and contribution in the advancement of knowledge in several fields. Topics of interest for publication in this Special Issue include, but are not limited to, the following: innovative technologies and therapeutic strategies, nanomedicine, tissue engineering and regenerative medicine, selective targeting, drug release control, in vitro and in vivo evidences.
The only requirement is that the corresponding author is a woman.
Prof. Dr. Donatella Paolino
Prof. Dr. Cinzia Anna Ventura
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- nanomedicine
- innovative strategies
- regenerative medicine
- in vitro
- in vivo
- ex vivo
- pharmaceutical research
- encapsulation
- coating
- stem cells
- biologics delivery