- Review
Hepatocyte-Targeted Drug Delivery Strategies for Chronic Hepatitis B: Overcoming Delivery Barriers Toward Functional Cure
- Ayman Elbehiry and
- Musaad Aldubaib
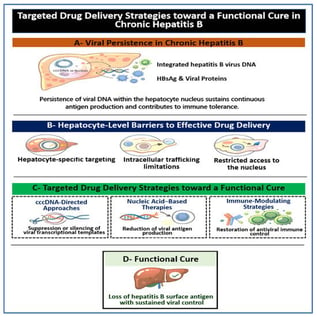
Chronic hepatitis B remains difficult to cure because viral persistence is maintained within hepatocytes through covalently closed circular DNA and integrated viral sequences that continue to drive antigen production even when viral replication is effectively suppressed. Although current antiviral therapies improve clinical outcomes and slow disease progression, they rarely achieve a durable functional cure, defined as sustained loss of hepatitis B surface antigen (HBsAg), with or without anti-HBs seroconversion. This limitation has shifted attention toward therapeutic strategies that depend on precise and reliable drug delivery to the liver. Several recent reviews have focused on antiviral mechanisms or immune modulation. However, the specific contribution of drug delivery to therapeutic success has not been systematically addressed. This review examines hepatocyte-targeted drug delivery as a central determinant of success for emerging hepatitis B therapies. Rather than cataloging individual therapeutic agents, this review adopts a delivery-centered framework that links viral persistence biology with translational feasibility across therapeutic classes. Recent advances in ligand-mediated hepatocyte targeting have demonstrated consistent liver specificity and clinical feasibility, enabling meaningful reductions in viral transcripts and antigens. At the same time, we discuss why more complex delivery platforms continue to face challenges related to intracellular access, immunogenicity, scalability, and safety during repeated dosing, particularly for strategies intended to act within the nucleus. Translational and clinical considerations, including differences between experimental models and human infection, manufacturing and regulatory constraints, and the demands of long-term treatment, are also addressed. Overall, this review supports a pragmatic path toward functional cure based on rational combination therapies, coordinated delivery strategies, and patient-tailored approaches, with delivery science serving as the critical link between biological insight and durable clinical benefit.
7 February 2026