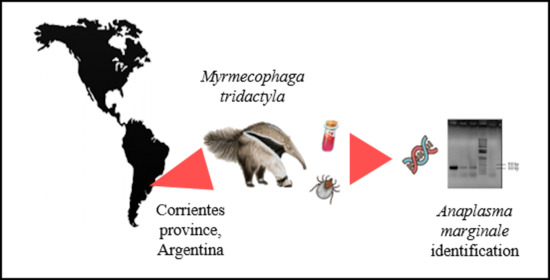
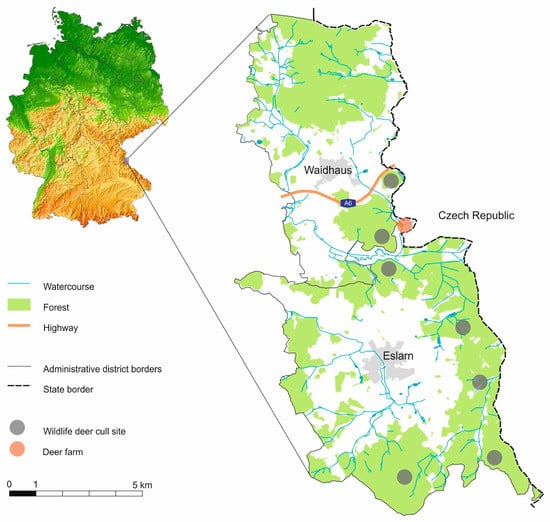
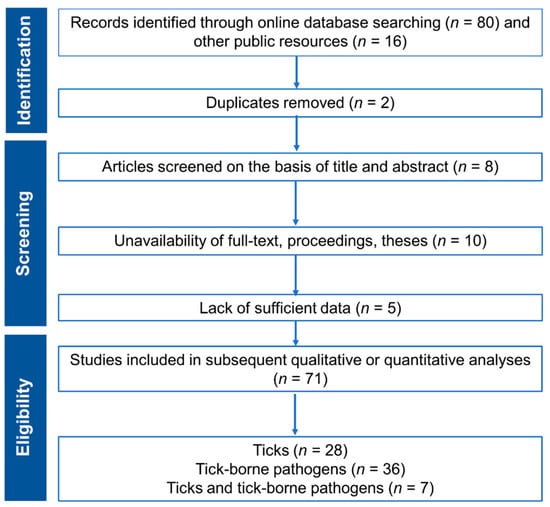
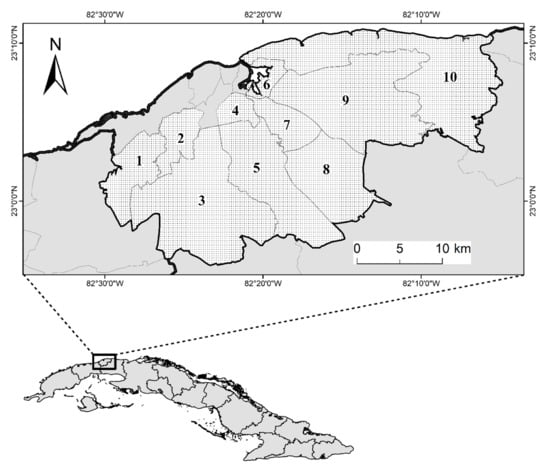
Advances in Tick Research
A topical collection in Pathogens (ISSN 2076-0817). This collection belongs to the section "Ticks".
Viewed by 153586Editors
Interests: tick-host-pathogen interactions; emerging tick-borne pathogens; Anaplasma; Ehrlichia; epidemiology; tick microbiome; α-Gal
Special Issues, Collections and Topics in MDPI journals
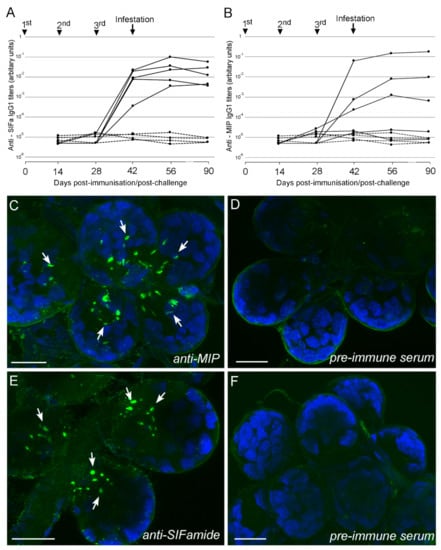
Interests: tick neuro-physiology; signal transductions; neuropeptides; neurotransmitters; GPCRs
Special Issues, Collections and Topics in MDPI journals
Veterinary Research Institute, Hudcova 70, CZ-62100, Brno, Czech Republic
Interests: Thermodynamics of drug therapy against vector-based diseases
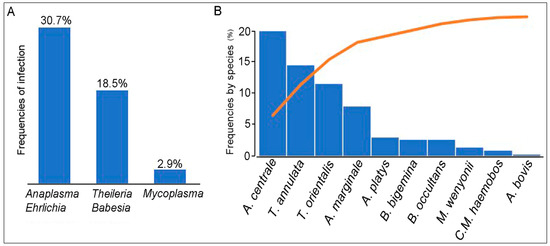
Interests: Preventive veterinary medicine; microbial ecology; vector-borne pathogens; host-pathogen interaction; functional metagenomics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
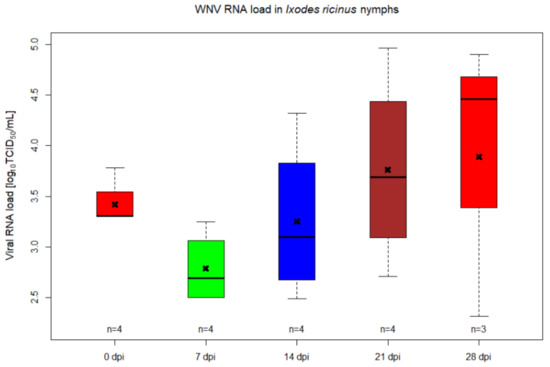
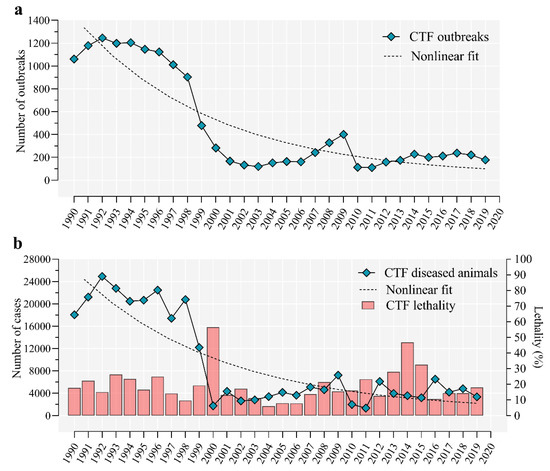
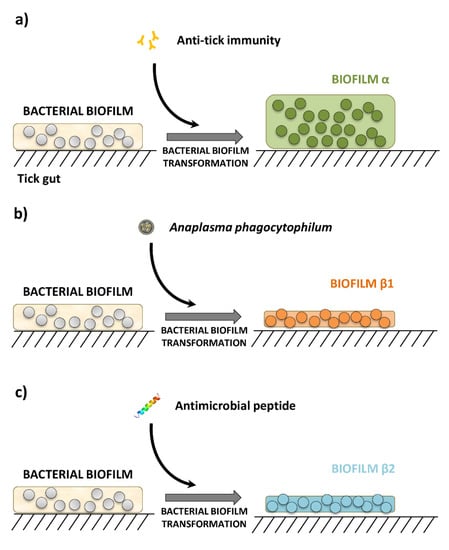
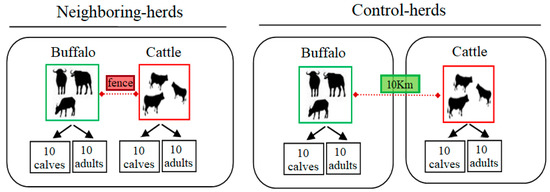
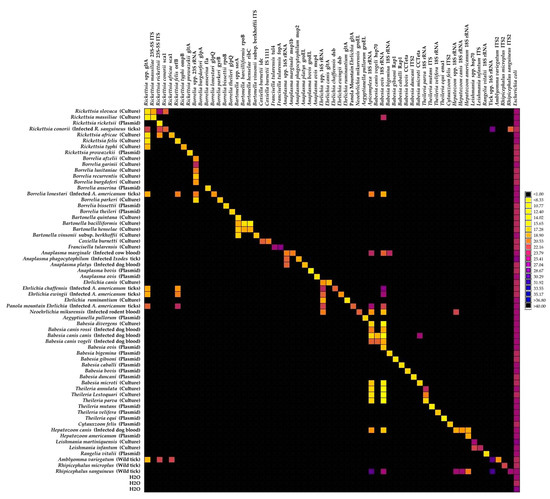
Ticks, along with mites, are arachnids that constitute the subclass Acari. Molecular clock estimates that ticks originated in the Carboniferous era, approximately 300 million years ago. Fossil records also support that ticks were blood-suckers of dinosaurs 100 million years ago. The unusual adaptation of tick physiology that directly reflects the challenges of their fluctuating environment is incomparable to any other blood feeding arthropod. The pharmacopeia of tick salivary proteins is an arsenal for ticks to counteract host defense mechanisms. Although tick salivary proteins belong to known structural families, the function of these salivary proteins has diversified throughout tick evolution. Besides causing direct damage associated with blood feeding, and in some cases toxicity, ticks transmit a wide variety of pathogens, including bacteria, viruses, protozoa, and helminths. New genetic variants of these pathogens frequently emerge with an unforeseen impact on human and animal health. In addition to pathogens, ticks harbor some complex microbial communities that influence tick-pathogen interactions and potentially tick physiology. Currently, tick control overuse acaricides with substantial drawbacks that include environmental damage, human poisonings and the emergence of multiacaricide-resistant ticks. Anti-tick vaccines are an alternative for the control of one-host ticks (e.g., Rhipicephalus microplus). Implementing vaccination, however, has significant limitations - specifically against ticks that fed on multiple hosts during their life cycle. The aim of this Topical Collection, ‘New Frontiers in Tick Research’, is to explore the research landscape to find novel developments that may impact tick biology, tick-borne pathogen epidemiology, and strategies for controlling ticks and tick-borne pathogens.
Dr. Alejandro Cabezas-Cruz
Dr. Ladislav Šimo
Dr. James J. Valdés
Dr. Dasiel Obregón
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pathogens is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Vector physiology
- Structural biology of vector/viral proteins
- Functional vector metagenomics
- Factors shaping the structure of vector microbiome
- Emerging vector-borne pathogens
- Vector-host-pathogen interactions
- Challenges in vector-borne pathogen detection
- Drug discovery in vector-borne pathogens
- Biocontrol of vectors
- Advanced tools in vector researh