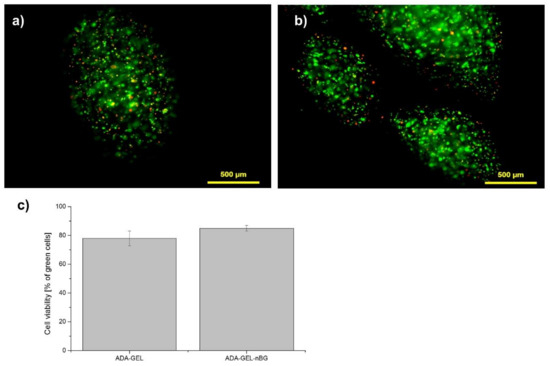
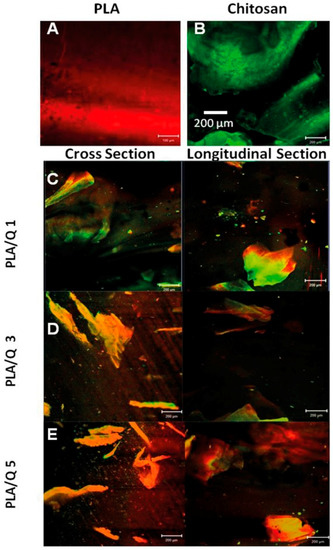
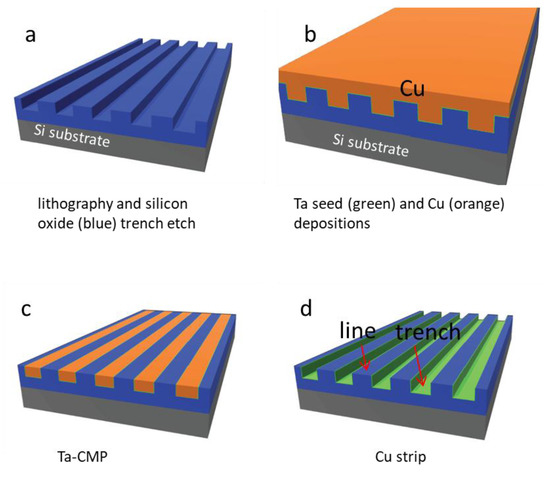
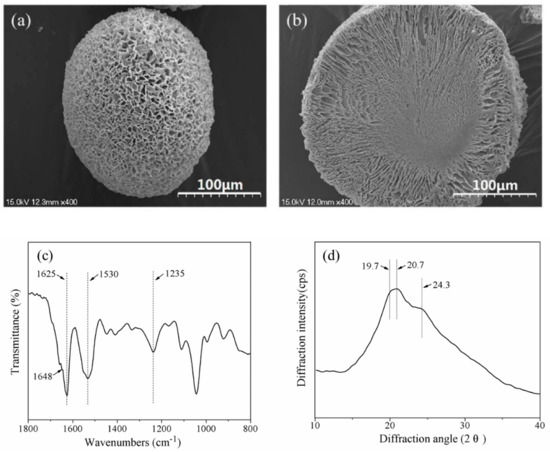
Advanced Biomaterials for Cells Adhesion, Proliferation and Differentiation
A topical collection in Materials (ISSN 1996-1944). This collection belongs to the section "Biomaterials".
Viewed by 132382Editor
Interests: scaffolds; porous materials; nanobiomaterials; biomimetic materials; extracellular matrices; composite materials; surface modification; micropatterning; hydrogels; tissue engineering
Topical Collection Information
Dear Colleagues,
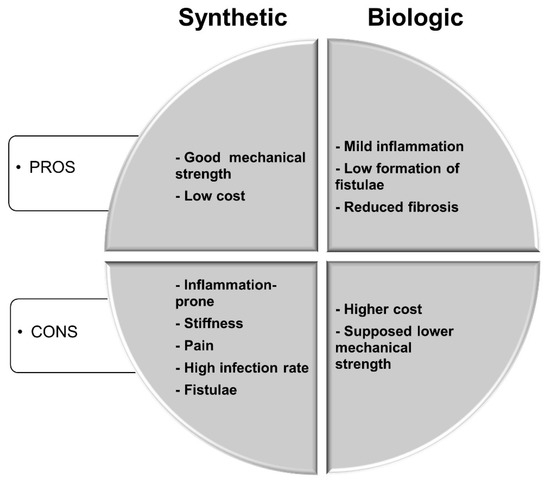
Cells adhesion, proliferation and differentiation are involved in various natural phenomena, such as embryogenesis, histogenesis, maintenance of tissue structure, immune response, metastasis, wound healing, as well as tissue integration of biomaterial. Lack of native tissue integration is one of frequent problems associated with the biomaterials surfaces of dental implants. It has prompted a significant body of research regarding the modification of these surfaces. Therefore, there is a constant need to find advanced biomaterials which are very closely related to cell behaviors and particularly to cell adhesion, proliferation and differentiation, that aim to restore a patient’s mobility and alleviate pains.
Properties of biomaterials and scaffolds, such as pore structures, mechanical properties and degradation, play a pivotal role in accomplishment of their functions for tissue repairing or regeneration. Surface characteristics of materials, whether their topography, chemistry or surface energy, play an essential part in cell–material interaction and implant integration.
This Special Issue will focus on the recent progress of biopolymers, biometals, bioceramics, biomimetic materials, nanobiomaterials, scaffolds, porous materials, composite materials, smart biomaterials for cell responses in terms of adhesion, spreading, viability, proliferation and differentiation.
Prof. Dr. Guoping Chen
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Materials is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Biopolymers
- biometals
- bioceramics
- biomimetic materials
- nanobiomaterials
- scaffolds
- porous materials
- composite materials
- smart biomaterials
- hydrogels
- extracellular matrices
- surface modification
- tissue engineering
- micropatterns
- nanopatterns
- regenerative medicine
- stem cells
- cell adhesion
- cell proliferation
- differentiation
- photothermal therapy
- cell–material interaction
- cell encapsulation
- biocompatibility
- biodegradation