RGD-Dendrimer-Poly(L-lactic) Acid Nanopatterned Substrates for the Early Chondrogenesis of Human Mesenchymal Stromal Cells Derived from Osteoarthritic and Healthy Donors
Abstract
1. Introduction
2. Materials and Methods
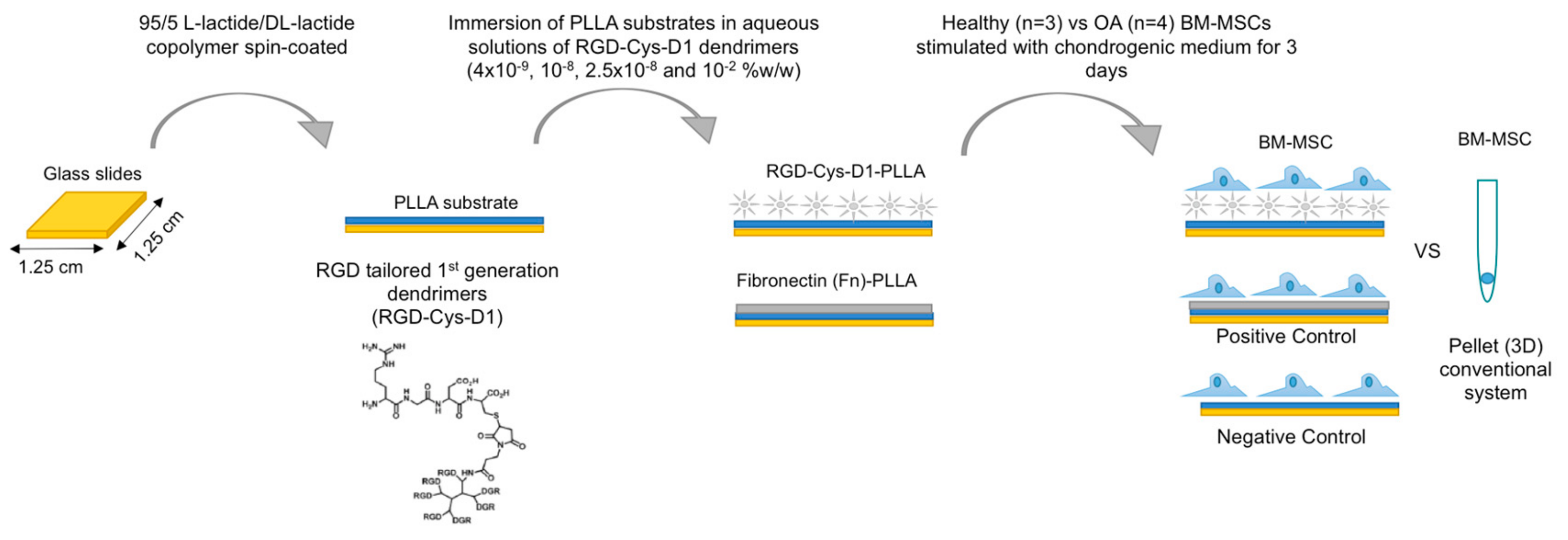
2.1. RGD-Cys-D1- Dendrimer PLLA Nanopatterned Substrate Production
2.2. Bone Marrow-Mesenchymal Stromal Cells Isolation and Chondrogenic Differentiation
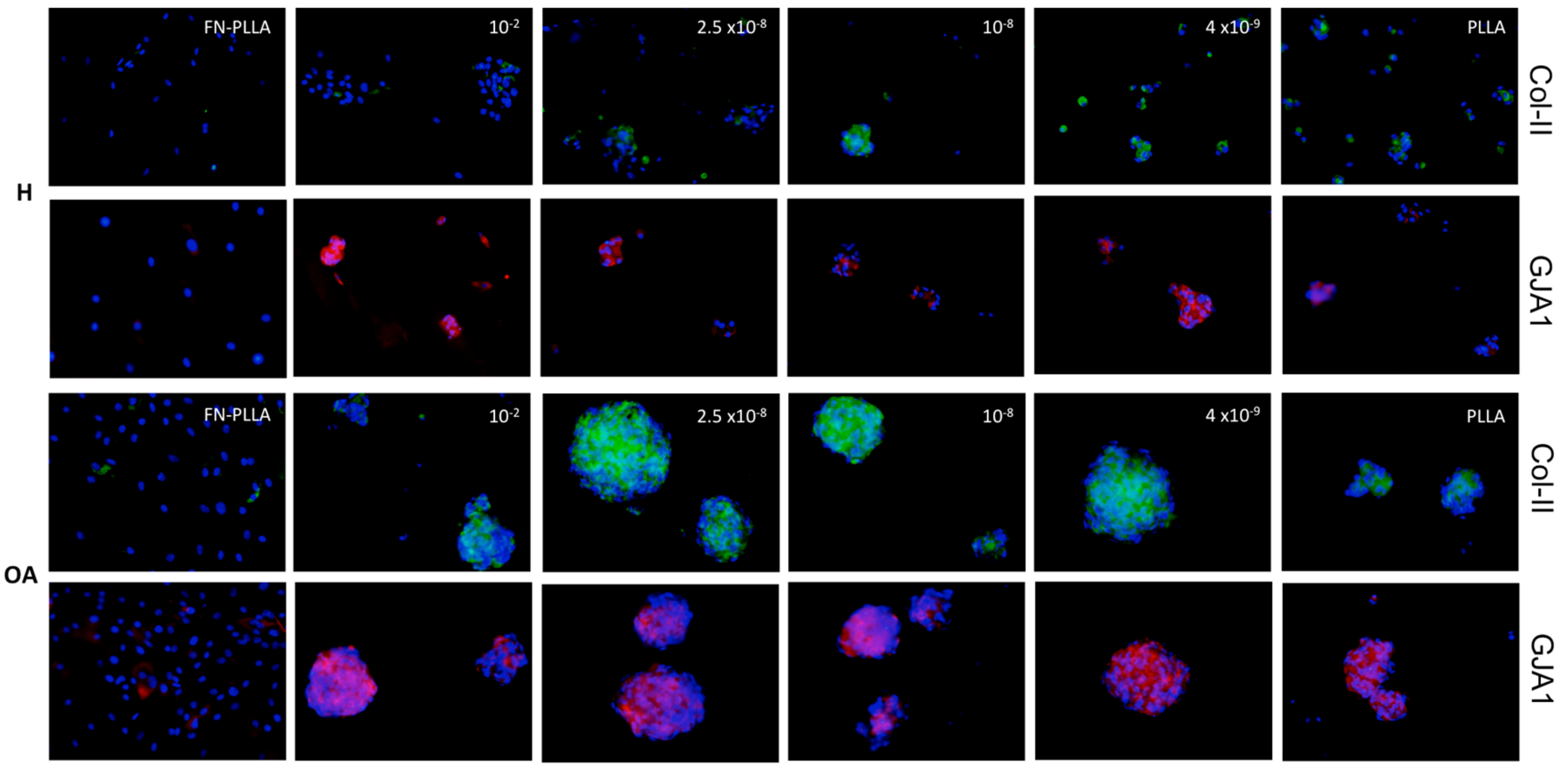
2.3. Immunofluorescence and Histology
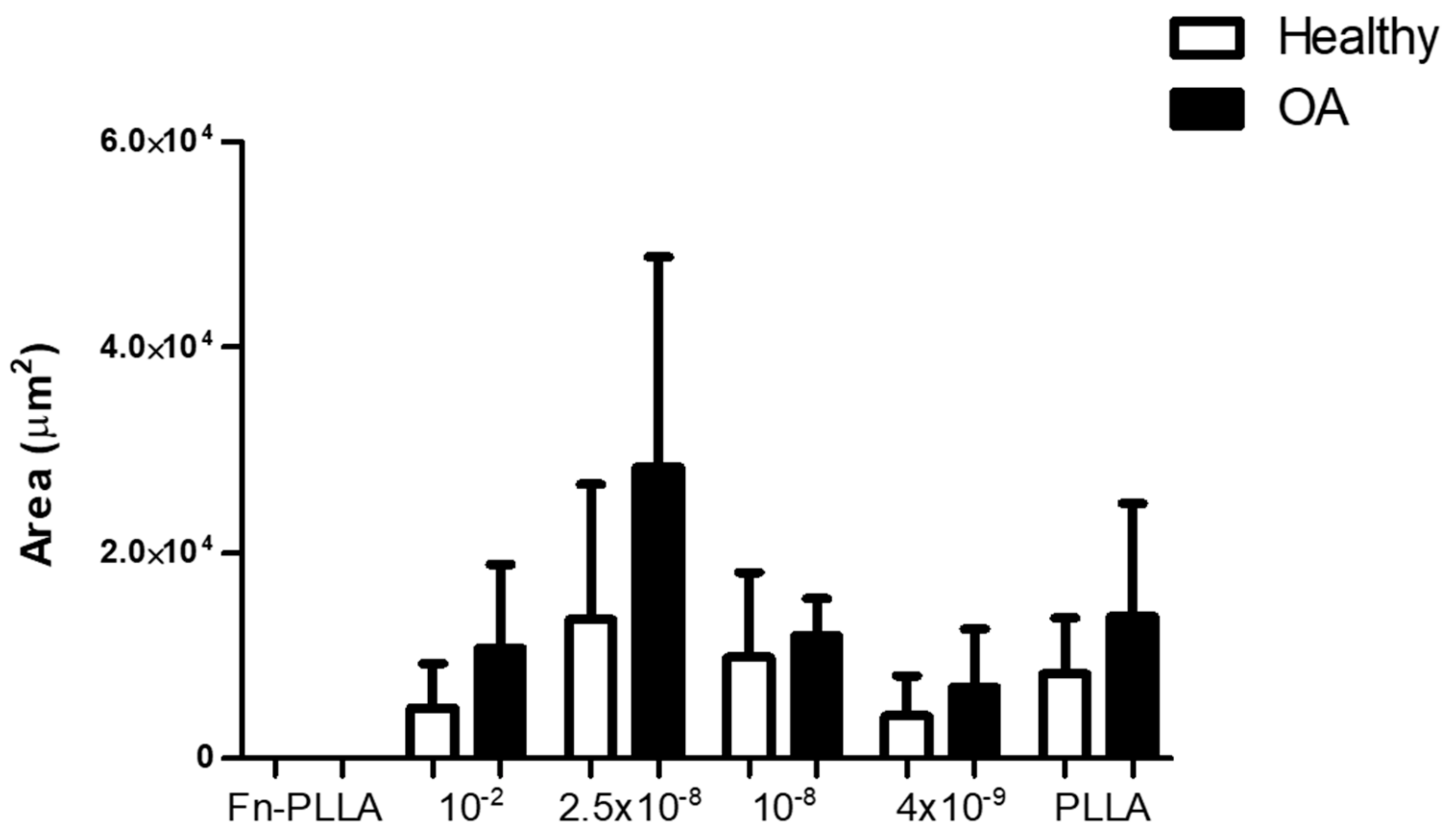
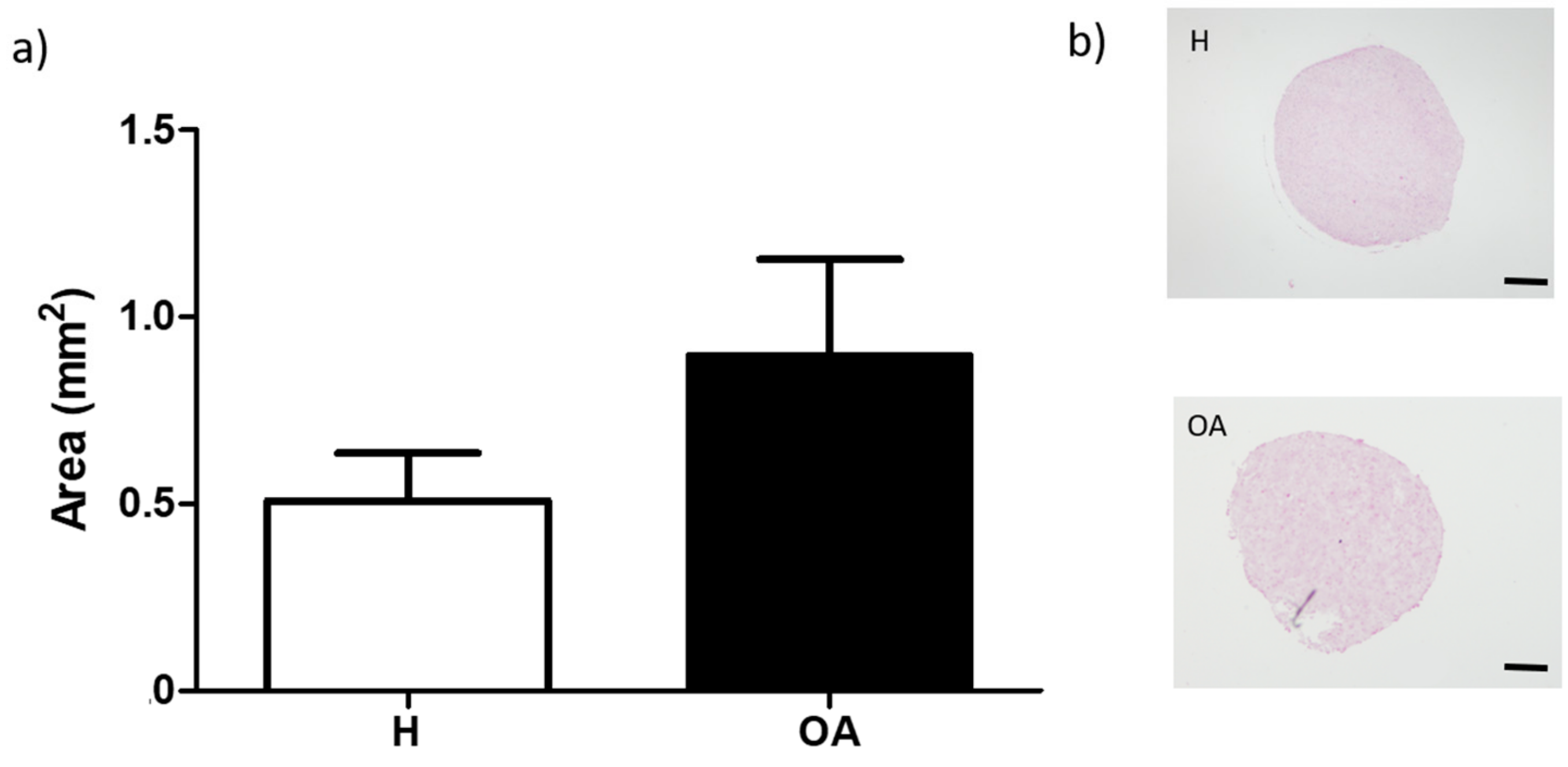
2.4. BM-MSC Aggregates and Pellet Areas
2.5. Molecular Analysis
2.6. Statistical Analysis
3. Results
3.1. BM-MSC Aggregates Formation and Areas
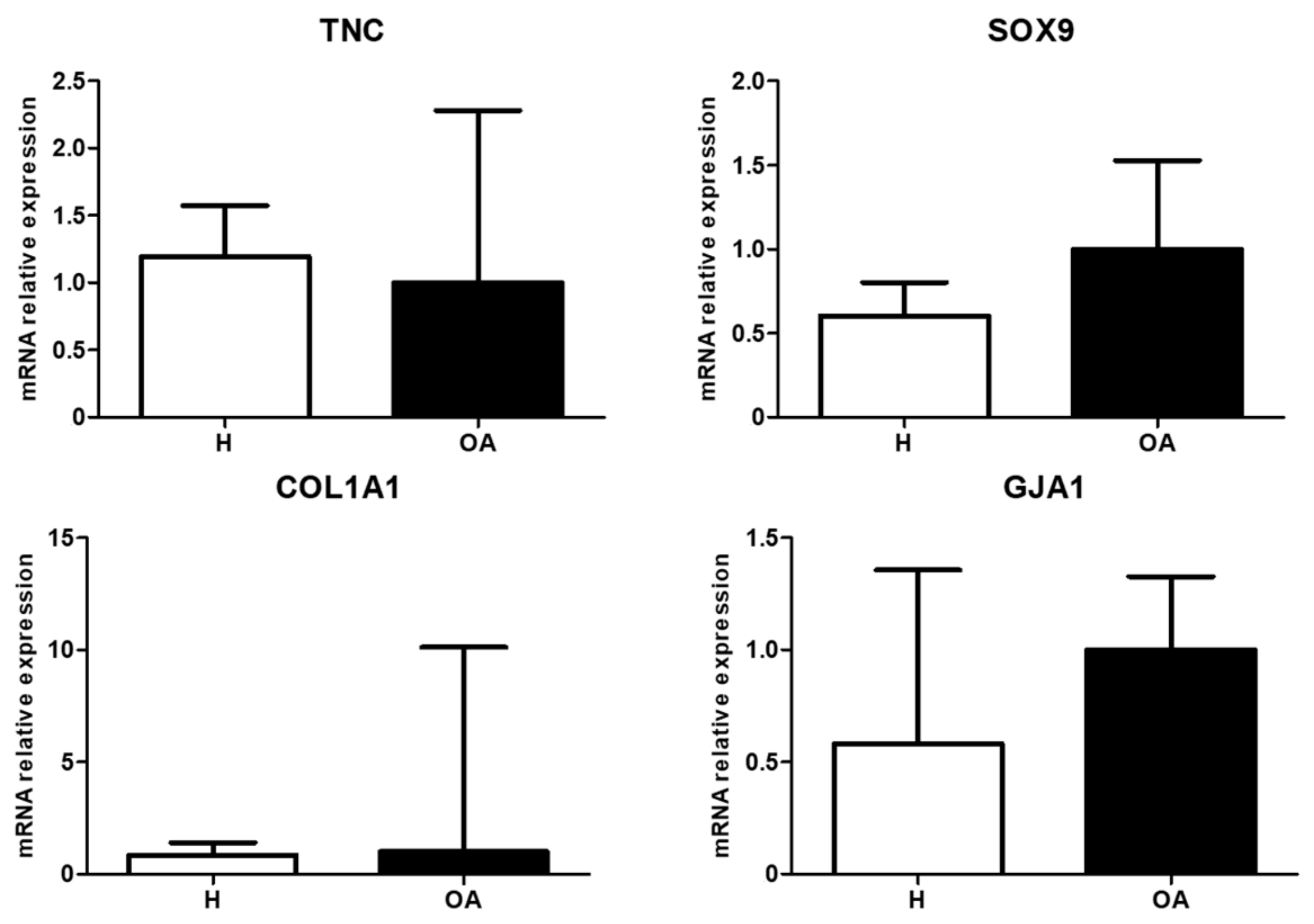
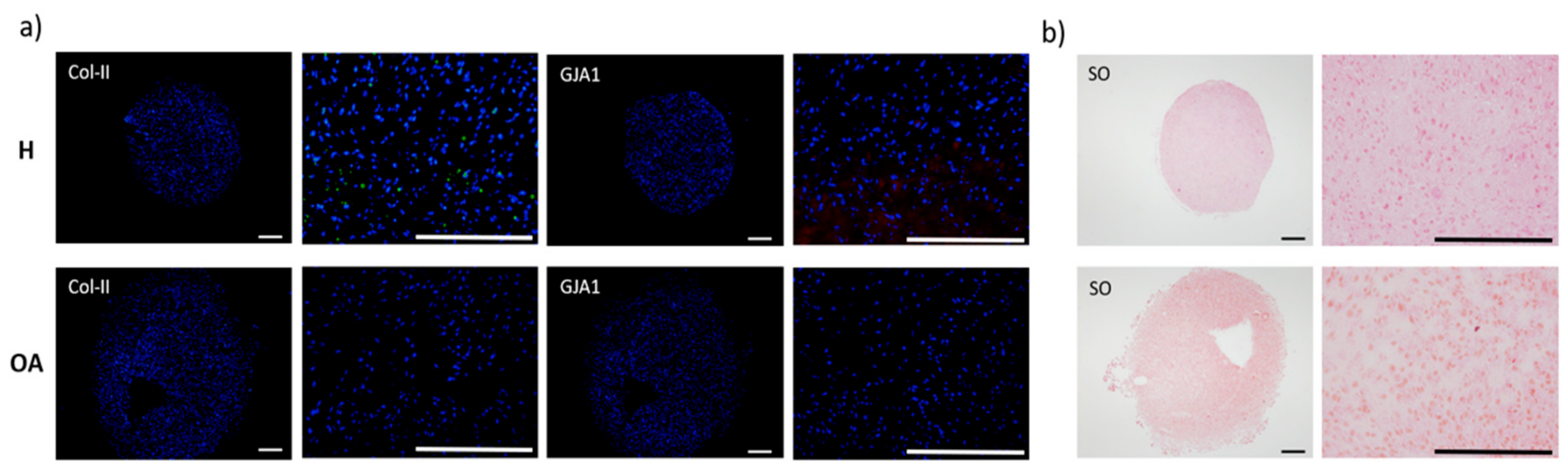
3.2. Molecular Expression and Protein Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- McMurray, R.J.; Dalby, M.J.; Tsimbouri, P.M. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 528–539. [Google Scholar] [CrossRef]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-induced osteogenic differentiation of stem cells – A systematic review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mashinchian, O.; Turner, L.A.; Dalby, M.J.; Laurent, S.; Shokrgozar, M.A.; Bonakdar, S.; Imani, M.; Mahmoudi, M. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine 2015, 10, 829–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Ye, K.; He, Y.; Li, Z.; Ding, J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials 2013, 34, 2865–2874. [Google Scholar] [CrossRef]
- Kang, J.; Park, H.M.; Kim, Y.W.; Kim, Y.; Varghese, S.; Seok, H.K.; Kim, Y.G.; Kim, Y.H. Control of mesenchymal stem cell phenotype and differentiation depending on cell adhesion mechanism. Eur. Cells Mater. 2016, 28, 387–403. [Google Scholar] [CrossRef]
- Medda, R.; Helth, A.; Herre, P.; Pohl, D.; Rellinghaus, B.; Perschmann, N.; Neubauer, S.; Kessler, H.; Oswald, S.; Eckert, J. Investigation of early cell-surface interactions of human mesenchymal stem cells on nanopatterned-type titanium-niobium alloy surfaces. Interface Focus 2013, 4, 20130046-20130046. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Williams, R.; Dixon, S.; Murray, P. Induction of mesenchymal stem cell chondrogenesis by polyacrylate substrates. Acta Biomater. 2013, 9, 6041–6051. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.H. Enhanced Chondrogenic Differentiation of Dental Pulp Stem Cells Using Nanopatterned PEG-GelMA-HA Hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Pericet-Camara, R.; Cahill, B.P.; Papastavrou, G.; Borkovec, M. Nano-patterning of solid substrates by adsorbed dendrimers. Chem. Commun. 2007, 266–268. [Google Scholar] [CrossRef]
- Lagunas, A.; Castaño, A.G.; Artés, J.M.; Vida, Y.; Collado, D.; Pérez-Inestrosa, E.; Gorostiza, P.; Claros, S.; Andrades, J.A.; Samitier, J. Large-scale dendrimer-based uneven nanopatterns for the study of local arginine-glycine-aspartic acid (RGD) density effects on cell adhesion. Nano Res. 2014, 7, 399–409. [Google Scholar] [CrossRef]
- Xiong, J.P. Crystal Structure of the Extracellular Segment of Integrin alpha Vbeta 3 in Complex with an Arg-Gly-Asp Ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, A.; Tsintzou, I.; Vida, Y.; Collado, D.; Pérez-Inestrosa, E.; Rodríguez Pereira, C.; Magalhaes, J.; Andrades, J.A.; Samitier, J. Tailoring RGD local surface density at the nanoscale toward adult stem cell chondrogenic commitment. Nano Res. 2017, 10, 1959–1971. [Google Scholar] [CrossRef]
- Singh, P.; Schwarzbauer, J.E. Fibronectin and stem cell differentiation-Lessons from chondrogenesis. J. Cell Sci. 2012, 125, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Ohmiya, Y.; Honma, K.I.; Honma, S.; Nagai, T.; Saito, K.; Yasuda, K. Synchronized ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis. Cell Death Dis. 2012, 3, e278. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Stains, J.P. Connexins and pannexins in the skeleton: Gap junctions, hemichannels and more. Cell. Mol. Life Sci. 2015, 72, 2853–2867. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Zhao, S.C.; Zhu, Z.S.; Wang, Y.F.; Liu, J.X.; Zhang, Z.C.; Xue, F. Cx43-and Smad-Mediated TGF-β/BMP Signaling Pathway Promotes Cartilage Differentiation of Bone Marrow Mesenchymal Stem Cells and Inhibits Osteoblast Differentiation. Cell. Physiol. Biochem. 2017, 42, 1277–1293. [Google Scholar] [CrossRef]
- Loty, S.; Foll, C.; Forest, N.; Sautier, J.M. Association of enhanced expression of gap junctions with in vitro chondrogenic differentiation of rat nasal septal cartilage-released cells following their dedifferentiation and redifferentiation. Arch. Oral Biol. 2000, 45, 843–856. [Google Scholar] [CrossRef]
- Mayan, M.D.; Carpintero-Fernandez, P.; Gago-Fuentes, R.; Martinez-De-Ilarduya, O.; Wang, H.Z.; Valiunas, V.; Brink, P.; Blanco, F.J. Human articular chondrocytes express multiple gap junction proteins: Differential expression of connexins in normal and osteoarthritic cartilage. Am. J. Pathol. 2013, 182, 1337–1346. [Google Scholar] [CrossRef]
- Schrobback, K.; Klein, T.J.; Woodfield, T.B.F. The Importance of Connexin Hemichannels During Chondroprogenitor Cell Differentiation in Hydrogel Versus Microtissue Culture Models. Tissue Eng. Part A 2015, 21, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Fife, R.S.; Braunstein, E.M.; Katz, B. Radiographic grading of the severity of knee osteoarthritis: Relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991, 34, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Gómez, T.; Fuentes-Boquete, I.; Gimeno-Longas, M.J.; Muiños-López, E.; Díaz-Prado, S.; de Toro, F.J.; Blanco, F.J. Bone Marrow Cells Immunomagnetically Selected For CD271+ Antigen Promote In Vitro the Repair of Articular Cartilage Defects. Tissue Engineering Part A 2011, 17, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Tips and Tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open source platform for biological image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Casanellas, I.; Lagunas, A.; Tsintzou, I.; Vida, Y.; Collado, D.; Pérez-Inestrosa, E.; Rodríguez-Pereira, C.; Magalhaes, J.; Gorostiza, P.; Andrades, J.A.; et al. Dendrimer-based Uneven Nanopatterns to Locally Control Surface Adhesiveness: A Method to Direct Chondrogenic Differentiation. J. Vis. Exp. 2018, 131. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Krishnan, H.; Miller, W.T.; Blanco, F.J.; Goldberg, G.S. Src and podoplanin forge a path to destruction. Drug Discov. Today 2019, 24, 241–249. [Google Scholar] [CrossRef]
- Dolatshahi-Pirouz, A.; Jensen, T.; Kraft, D.C.; Foss, M.; Kingshott, P.; Hansen, J.L.; Larsen, A.N.; Chevallier, J.; Besenbacher, F. Fibronectin adsorption, cell adhesion, and proliferation on nanostructured tantalum surfaces. ACS Nano 2010, 4, 2874–2882. [Google Scholar] [CrossRef]
- Lin, M.; Mao, S.; Wang, J.; Xing, J.; Wang, Y.; Cai, K.; Luo, Y. Adsorption force of fibronectin controls transmission of cell traction force and subsequent stem cell fate. Biomaterials 2018, 162, 170–182. [Google Scholar] [CrossRef]
- Grigoriou, E.; Cantini, M.; Dalby, M.J.; Petersen, A.; Salmeron-Sanchez, M. Cell migration on material-driven fibronectin microenvironments. Biomater. Sci. 2017, 5, 1326–1333. [Google Scholar] [CrossRef]
- Brammer, K.S.; Choi, C.; Frandsen, C.J.; Oh, S.; Jin, S. Hydrophobic nanopillars initiate mesenchymal stem cell aggregation and osteo-differentiation. Acta Biomater. 2011, 7, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B: Rev. 2013, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Maddox, N.C.; Wong, A.W.; Rahnama, R.; Kuo, A.C. Comparison of gene expression patterns in articular cartilage and dedifferentiated articular chondrocytes. J. Orthop. Res. 2012, 30, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Murphy, L.I. The role of tenascin-C and related glycoproteins in early chondrogenesis. Microsc. Res. Tech. 1998, 43, 102–110. [Google Scholar] [CrossRef]
- Murphy, L.I.; Fischer, D.; Chiquet-Ehrismann, R.; Mackie, E.J. Tenascin-C induced stimulation of chondrogenesis is dependent on the presence of the C-terminal fibrinogen-like globular domain. FEBS Lett. 2000, 480, 189–192. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Eton, R.E.; Oungoulian, S.R.; Wan, L.Q.; Ateshian, G.A.; Vunjak-Novakovic, G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc. Natl. Acad. Sci. USA 2014, 111, 6940–6945. [Google Scholar] [CrossRef]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef]
- Lefebvre, V.; Smits, P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. Part C - Embryo Today: Rev. 2005, 75, 200–212. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Ludeman, M.; Cheng, K.; Hayami, T.; Lotz, J.C.; Kapila, S. Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Three-Dimensional Alginate Gels. Tissue Eng. Part A 2008, 14, 667–680. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Bemis, A.; Wang, E.; Qiu, Y.; Morris, E.A.; Flannery, C.R.; Yang, Z. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007, 56, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Lourido, L.; Calamia, V.; Mateos, J.; Fernández-Puente, P.; Fernández-Tajes, J.; Blanco, F.J.; Ruiz-Romero, C. Quantitative proteomic profiling of human articular cartilage degradation in osteoarthritis. J. Proteome Res. 2014, 13, 6096–6106. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhi, L.; Zhang, Z.; Bian, W.; Qiu, Y. Identification of potential target genes associated with the pathogenesis of osteoarthritis using microarray based analysis. Mol. Med. Rep. 2017, 16, 2799–2806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brady, K.; Dickinson, S.C.; Hollander, A.P. Changes in Chondrogenic Progenitor Populations Associated with Aging and Osteoarthritis. Cartilage 2015, 6 (Suppl. 2), 30S–35S. [Google Scholar] [CrossRef]

| Genes | Forward | Reverse | Probe | GeneBank Accession n° |
|---|---|---|---|---|
| CX43 | gcctgaacttgccttttcat | ctccagtcacccatgttgc | 88 | NM_000165.4 |
| COL2A1 | tggtgctaatggcgagaag | cccagtctctccacgttcac | 4 | NM_001844.4 |
| SOX9 | gtacccgcacttgcacaac | tcgctctcgttcagaagtctc | 61 | NM_000346.3 |
| COL1A1 | ctggccccattggtaatgt | accagggaaaccagtagcac | 1 | NM_000088.3 |
| TNC | ggtacagtgggacagcaggt | cccctttgtaggacagagca | 9 | NM_002160.3 |
| RPL13A | caagcggatgaacaccaac | tgtggggcagcatacctc | 28 | NM_012423.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pereira, C.; Lagunas, A.; Casanellas, I.; Vida, Y.; Pérez-Inestrosa, E.; Andrades, J.A.; Becerra, J.; Samitier, J.; Blanco, F.J.; Magalhães, J. RGD-Dendrimer-Poly(L-lactic) Acid Nanopatterned Substrates for the Early Chondrogenesis of Human Mesenchymal Stromal Cells Derived from Osteoarthritic and Healthy Donors. Materials 2020, 13, 2247. https://doi.org/10.3390/ma13102247
Rodríguez-Pereira C, Lagunas A, Casanellas I, Vida Y, Pérez-Inestrosa E, Andrades JA, Becerra J, Samitier J, Blanco FJ, Magalhães J. RGD-Dendrimer-Poly(L-lactic) Acid Nanopatterned Substrates for the Early Chondrogenesis of Human Mesenchymal Stromal Cells Derived from Osteoarthritic and Healthy Donors. Materials. 2020; 13(10):2247. https://doi.org/10.3390/ma13102247
Chicago/Turabian StyleRodríguez-Pereira, Cristina, Anna Lagunas, Ignasi Casanellas, Yolanda Vida, Ezequiel Pérez-Inestrosa, José A. Andrades, José Becerra, Josep Samitier, Francisco J. Blanco, and Joana Magalhães. 2020. "RGD-Dendrimer-Poly(L-lactic) Acid Nanopatterned Substrates for the Early Chondrogenesis of Human Mesenchymal Stromal Cells Derived from Osteoarthritic and Healthy Donors" Materials 13, no. 10: 2247. https://doi.org/10.3390/ma13102247
APA StyleRodríguez-Pereira, C., Lagunas, A., Casanellas, I., Vida, Y., Pérez-Inestrosa, E., Andrades, J. A., Becerra, J., Samitier, J., Blanco, F. J., & Magalhães, J. (2020). RGD-Dendrimer-Poly(L-lactic) Acid Nanopatterned Substrates for the Early Chondrogenesis of Human Mesenchymal Stromal Cells Derived from Osteoarthritic and Healthy Donors. Materials, 13(10), 2247. https://doi.org/10.3390/ma13102247







