The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels
Abstract
1. Introduction
2. Results and Discussions
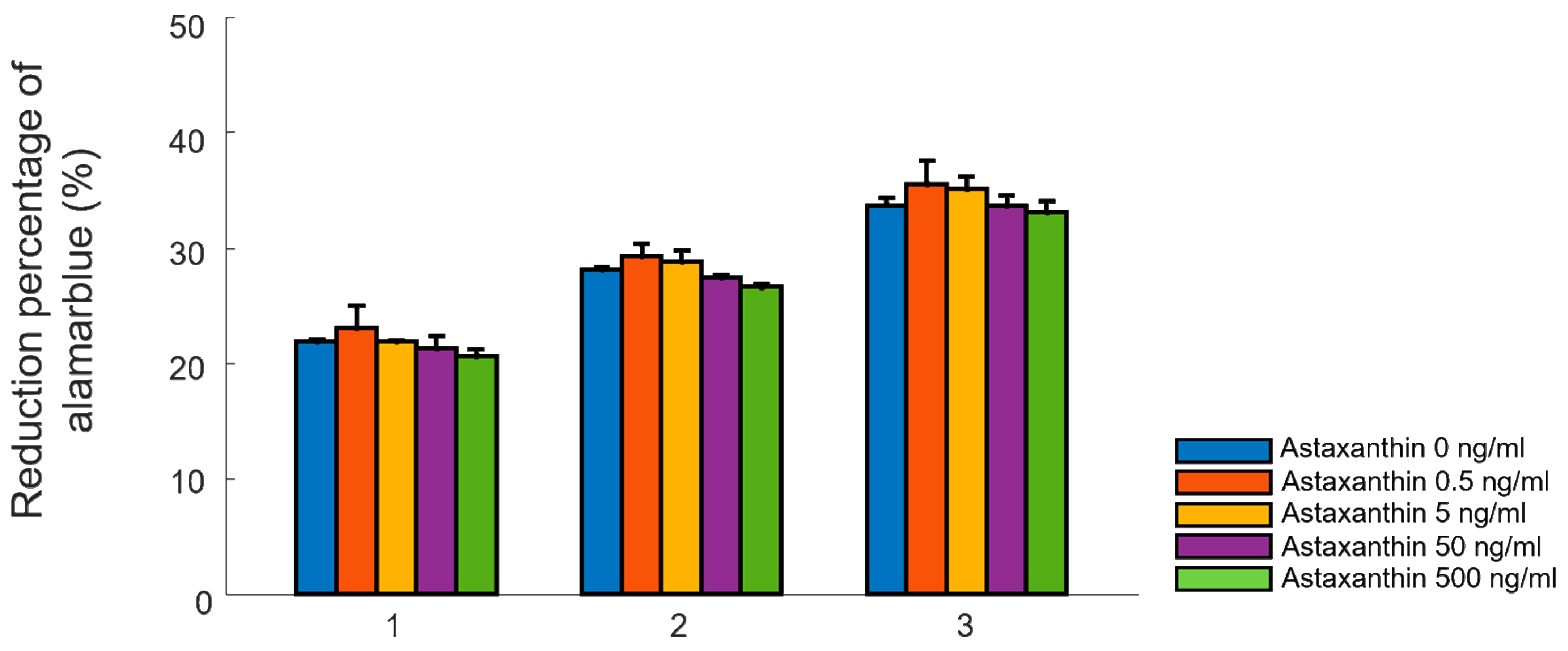
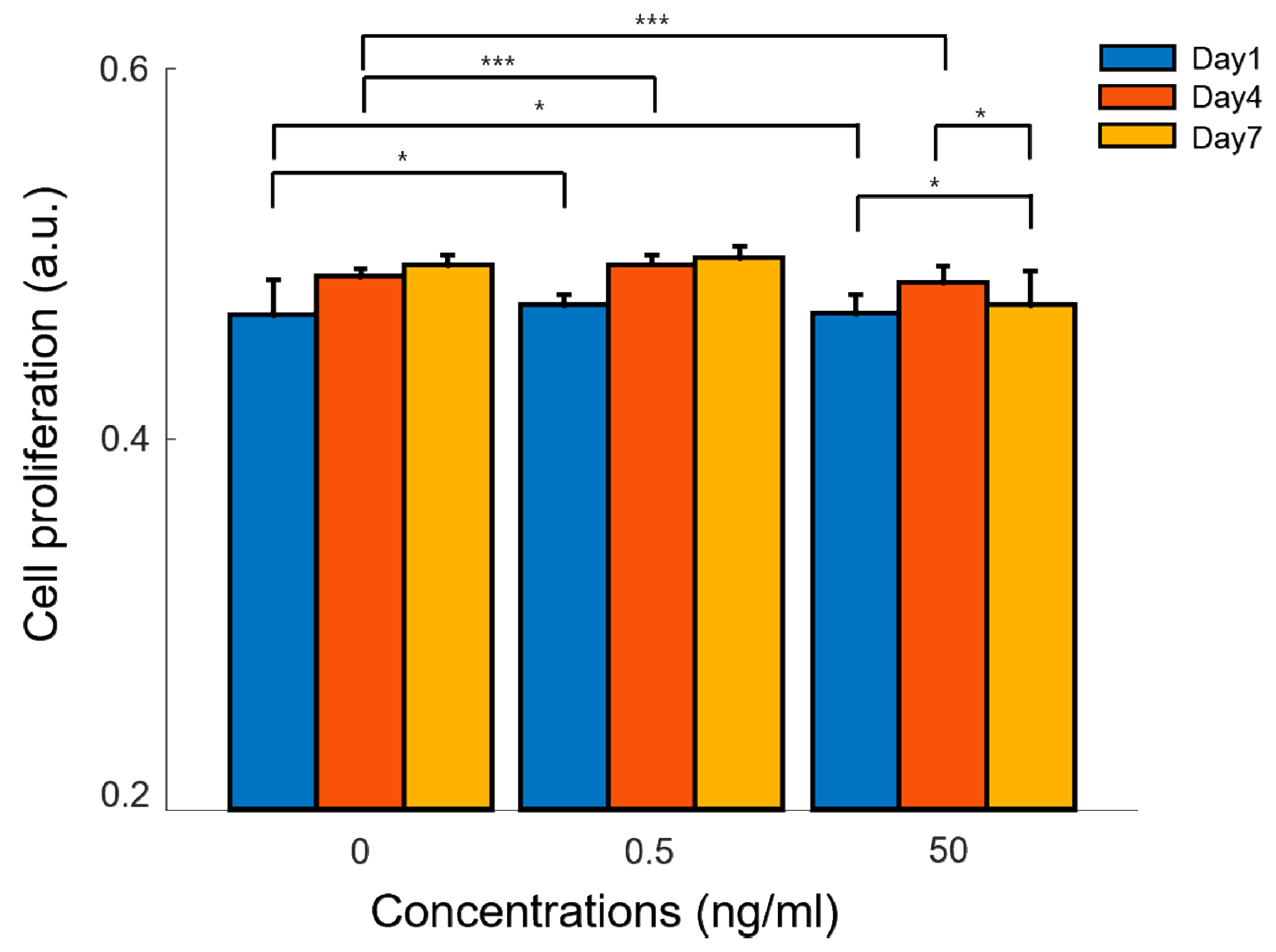
2.1. Cellular Proliferation Test in Two-Dimensional Environments
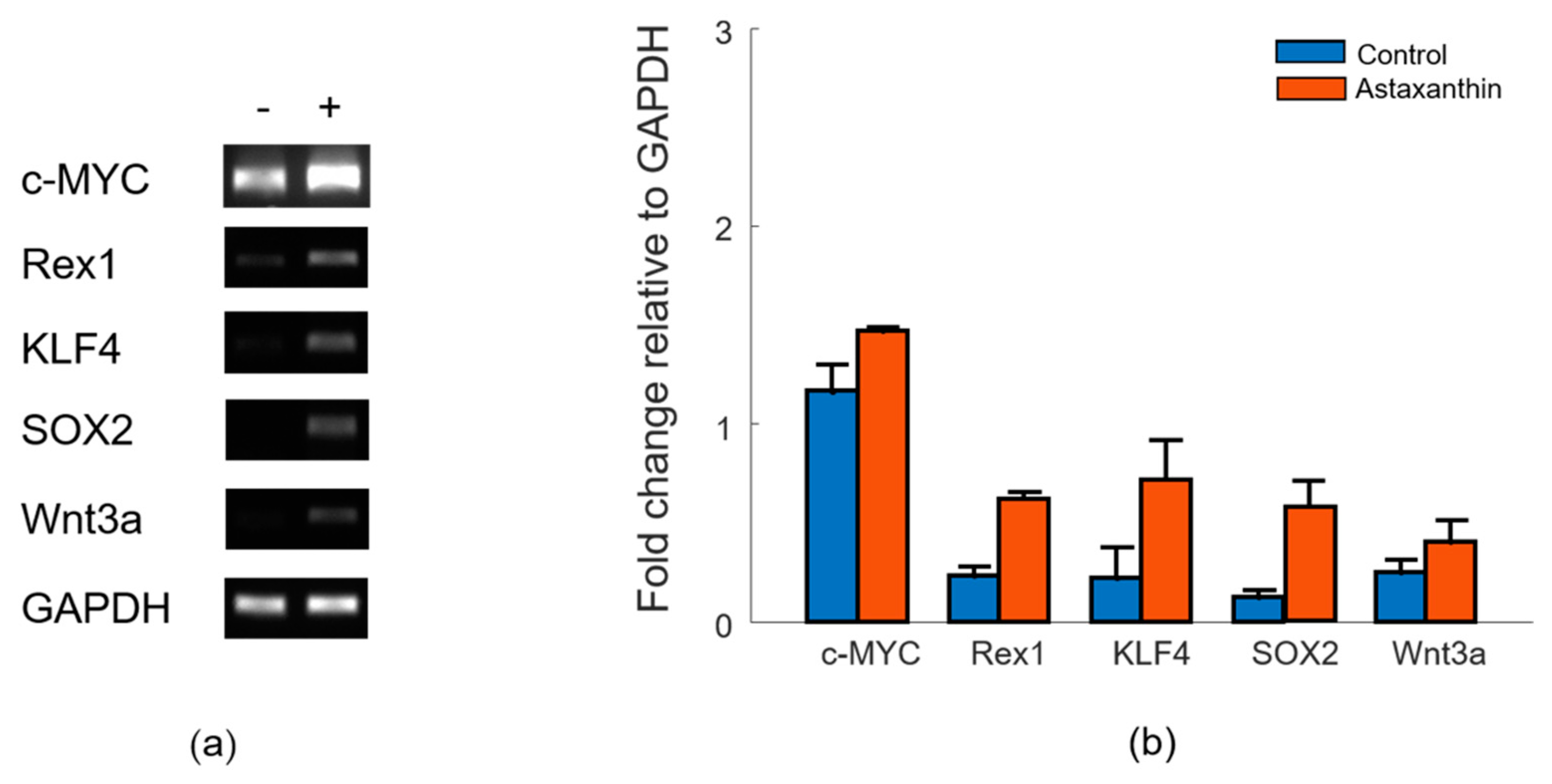
2.2. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.3. Characterization of Astaxanthin-Incorporated GelMA Scaffolds
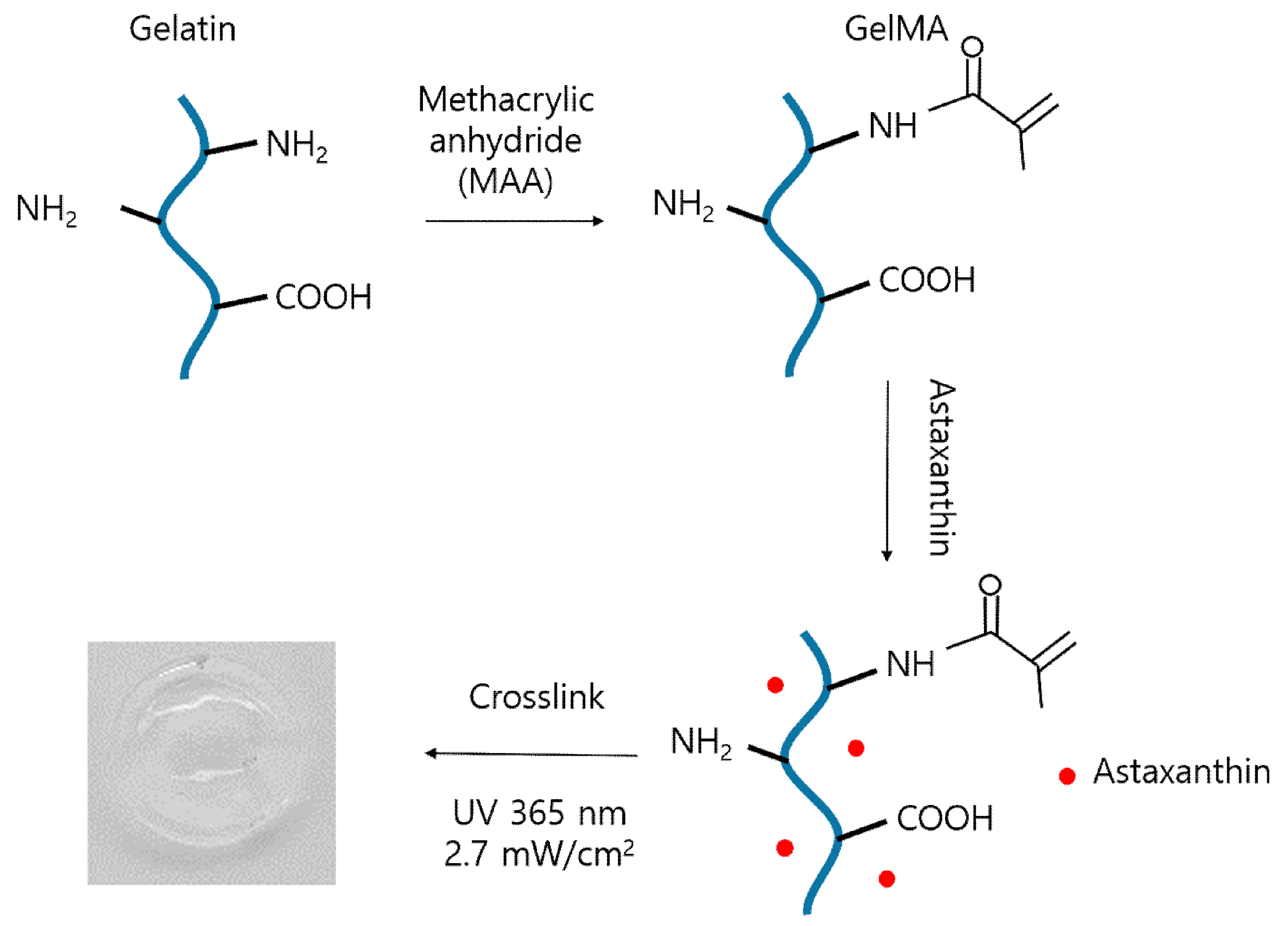
2.3.1. Fabrication of Astaxanthin-Incorporated GelMA Scaffolds
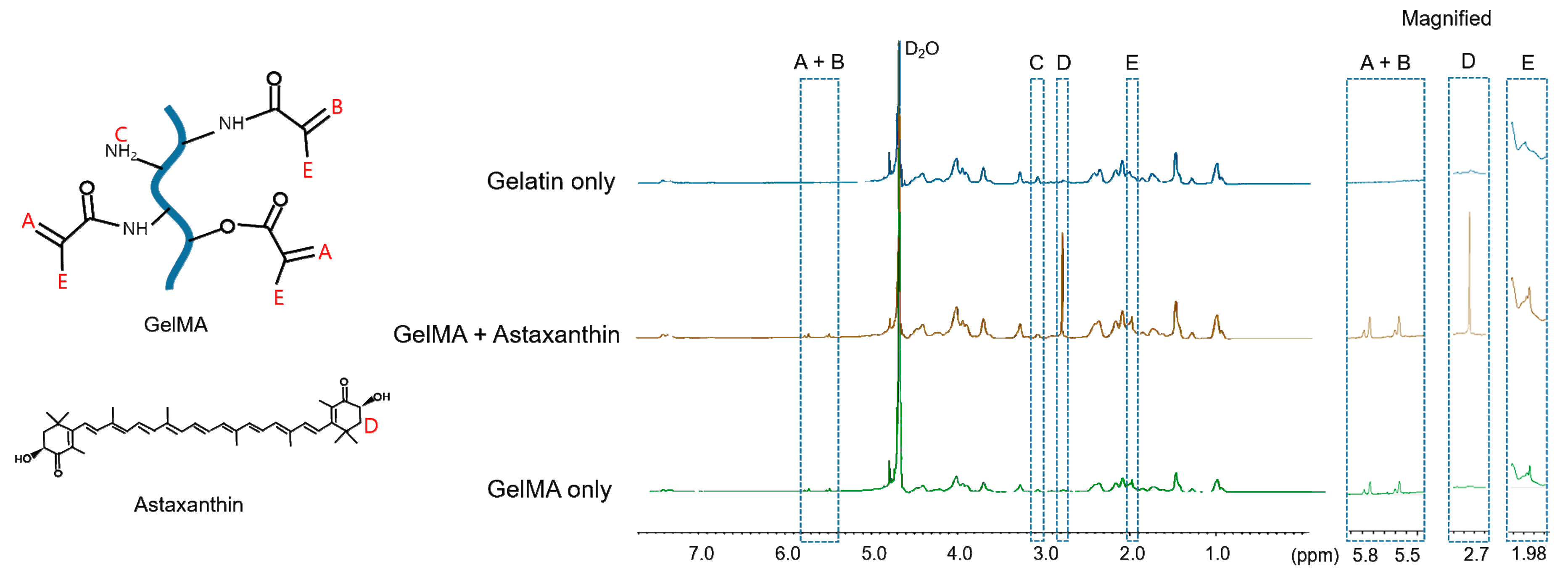
2.3.2. Degree of Methacrylation
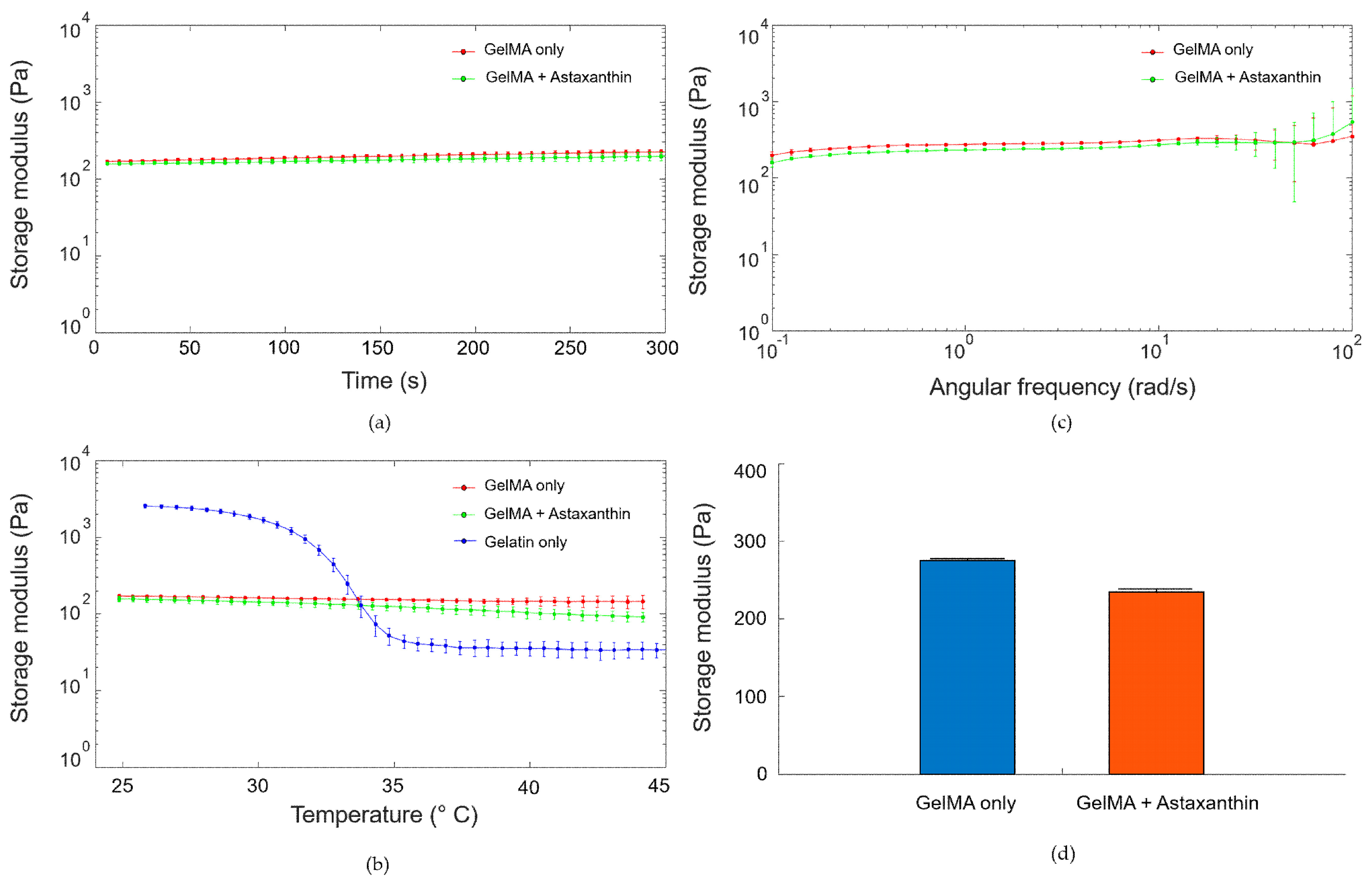
2.3.3. Rheological Characterization
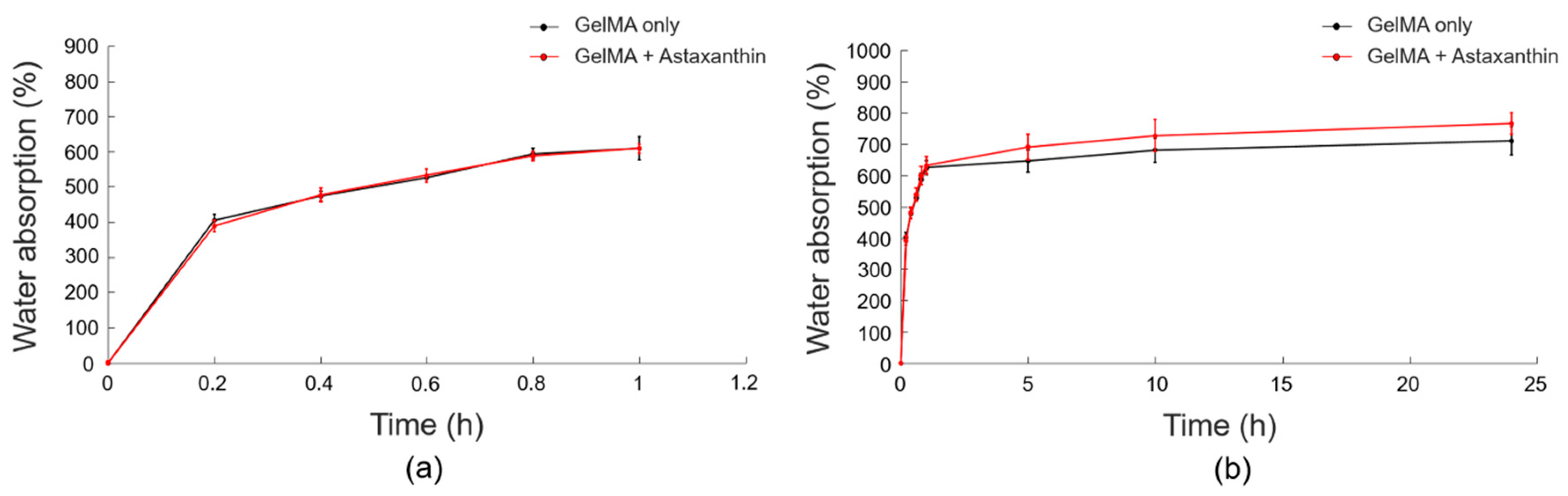
2.3.4. Swelling Behaviors
2.4. Cellular Proliferation in Astaxanthin-Incorporated GelMA Scaffolds
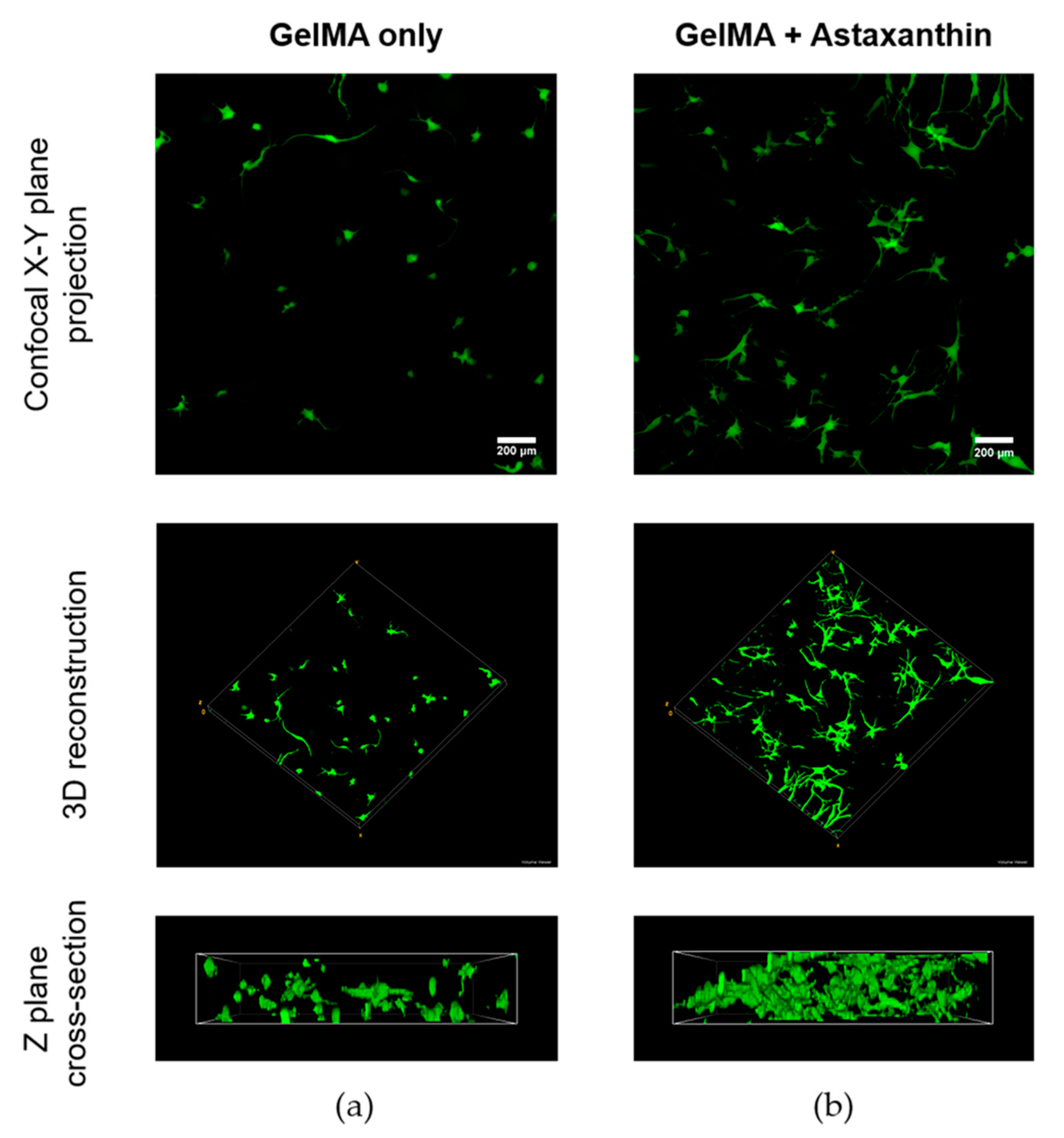
2.5. Confocal Imaging of ADMSCs in GelMA Hydrogels
3. Materials and Methods
3.1. Cell Culture with Astaxanthin Reagent
3.2. GelMA Synthesis
3.3. Fabrication of GelMA Scaffolds with Mesenchymal Stem Cells
3.4. Alamar Blue Assay
3.5. RNA Extraction and RT-PCR
3.6. Proton Nuclear Magnetic Resonance
3.7. Rheological Assessments
3.8. Swelling Ratio Test
3.9. Confocal Imaging Using Live/Dead Staining
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Kim, G.; Kang, M.; Lee, J.; Seo, J.; Do, J.; Hong, K.; Cha, J.; Shin, S.; Bae, H. Marine biomaterial-based bioinks for generating 3D printed tissue constructs. Mar. Drugs 2018, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nam, S.-W.; Kim, B.W.; Kim, W.-J.; Choi, Y.H. Astaxanthin improves the proliferative capacity as well as the osteogenic and adipogenic differentiation potential in neural stem cells. Food Chem. Toxicol. 2010, 48, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Nam, S.-W.; Kim, B.-W.; Choi, W.; Lee, J.-H.; Kim, W.-J.; Choi, Y.-H. Astaxanthin improves stem cell potency via an increase in the proliferation of neural progenitor cells. Int. J. Mol. Sci. 2010, 11, 5109–5119. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N. The evaluation of astaxanthin effects on differentiation of human adipose derived stem cells into oligodendrocyte precursor cells. Avicenna J. Med. Biotechonol. 2018, 10, 69–74. [Google Scholar]
- Yaghooti, H.; Mohammadtaghvaei, N.; Mahboobnia, K. Effects of palmitate and astaxanthin on cell viability and proinflammatory characteristics of mesenchymal stem cells. Int. Immunopharmacol. 2019, 68, 164–170. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, C.-A. Enhanced proliferation and differentiation of mesenchymal stem cells by astaxanthin-encapsulated polymeric micelles. PLoS ONE 2019, 14, e0216755. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727. [Google Scholar] [CrossRef]
- Kim, H.; Bae, C.; Kook, Y.-M.; Koh, W.-G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 51. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Yu, J.; Tsai, W. Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable crosslinking for controlled drug release. J. Mater. Chem. B 2016, 4.13, 2304–2313. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lum, N.; Seow, L.; Lim, P.; Tan, L. Synthesis and characterization of types a and b gelatin methacryloyl for bioink applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Bahney, C.; Lujan, T.; Hsu, C.; Bottlang, M.; West, J.; Johnstone, B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cells Mater. 2011, 22, 43. [Google Scholar] [CrossRef]
- Lin, C.H.; Su, J.J.M.; Lee, S.Y.; Lin, Y.M. Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2099–2111. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. In Mesenchymal Stem Cells-Basics and Clinical Application I; Weyand, B., Dominici, M., Hass, R., Jacobs, R., Kasper, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 59–71. [Google Scholar]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Pang, C.-Y.; Li, D.-K.; Liao, C.-H.; Huang, W.-C.; Wu, C.-C.; Chou, Y.-Y.; Li, W.W.; Chen, S.-Y.; Liu, H.-W. Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J. Biomed. Sci. 2013, 20, 53. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of epigallocatechin gallate and sulforaphane counteracts in vitro oxidative stress and delays stemness loss of amniotic fluid stem cells. Oxid. Med. Cell. Longev. 2018, 2018, 13. [Google Scholar] [CrossRef]
- Yu, J.; Tu, Y.-K.; Tang, Y.-B.; Cheng, N.-C. Stemness and transdifferentiation of adipose-derived stem cells using L-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials 2014, 35, 3516–3526. [Google Scholar] [CrossRef]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid. Med. Cell. Longev. 2017, 2017, 16. [Google Scholar] [CrossRef]
- Park, S.; Seo, K.W.; So, A.; Seo, M.; Yu, K.; Kang, S.; Kang, K. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012, 19, 534. [Google Scholar] [CrossRef]
- Bhandari, D.R.; Seo, K.-W.; Roh, K.-H.; Jung, J.-W.; Kang, S.-K.; Kang, K.-S. REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells. PLoS ONE 2010, 5, e10493. [Google Scholar] [CrossRef]
- Boland, G.M.; Perkins, G.; Hall, D.J.; Tuan, R.S. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J. Cell. Biochem. 2004, 93, 1210–1230. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI) MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Pacheco, C.; Prum, R.O.; Frank, H.A. Nuclear magnetic resonance analysis of carotenoids from the burgundy plumage of the Pompadour Cotinga (Xipholena punicea). Arch. Biochem. Biophys. 2013, 539, 133–141. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef]
- Chiellini, F.; Morelli, A. Ulvan: A versatile platform of biomaterials from renewable resources. Biomater. Phys. Chem. 2011, 75–98. [Google Scholar]
- Daubrawa, F.; Sies, H.; Stahl, W. Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. J. Nutr. 2005, 135, 2507–2511. [Google Scholar] [CrossRef]
- Packer, L. Carotenoids and Retinoids: Molecular Aspects and Health Issues; Lester Packer, U.O.-J., Kalus, K., Helmut, S., Eds.; AOCS Publishing: Champaign, IL, USA, 2005. [Google Scholar]
- Song, X.; Wang, B.; Lin, S.; Jing, L.; Mao, C.; Xu, P.; Lv, C.; Liu, W.; Zuo, J. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. J. Cell. Mol. Med. 2014, 18, 2198–2212. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. 2019, 98, 187–194. [Google Scholar] [CrossRef]
| Symbol | Forward | Reverse |
|---|---|---|
| SOX2 | GGCGGCAACCAGAAGAACAG | TCGATGAACGGCCGCTTCTC |
| KLF4 | AGC CCCAAGATGCACAACTC | AGGACGAGGAAGAGGCAGAC |
| Wnt3a | TTCCTCAAGGACAAGTACGACA | GAAGTTGGGGGAGTTCTCATAG |
| Rex1 | AGCCCAGCAGGCAGAAATGGAA | TGGTCAGTCTCACAGGGCACAT |
| c-MYC | TCGGACTCTCTGCTCTCCTC | CTTGTCGTTCTCCTCGGTGT |
| GAPDH | CAAGTTCCACGGCACGGTCA | CTCGGCACCAGCATCACCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.Y.; Chalisserry, E.P.; Kim, M.H.; Kang, H.W.; Choi, I.-W.; Nam, S.Y. The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels. Materials 2019, 12, 2416. https://doi.org/10.3390/ma12152416
Choi BY, Chalisserry EP, Kim MH, Kang HW, Choi I-W, Nam SY. The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels. Materials. 2019; 12(15):2416. https://doi.org/10.3390/ma12152416
Chicago/Turabian StyleChoi, Bo Young, Elna Paul Chalisserry, Myoung Hwan Kim, Hyun Wook Kang, Il-Whan Choi, and Seung Yun Nam. 2019. "The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels" Materials 12, no. 15: 2416. https://doi.org/10.3390/ma12152416
APA StyleChoi, B. Y., Chalisserry, E. P., Kim, M. H., Kang, H. W., Choi, I.-W., & Nam, S. Y. (2019). The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels. Materials, 12(15), 2416. https://doi.org/10.3390/ma12152416






