Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ti Particles
2.2. Cell Culture
2.3. MTT Assay
2.4. Lactate Dehydrogenase Activity (LDH) Assay
2.5. Protein Isolation and Western Blotting
2.6. RNA Isolation and Real-Time RT-PCR
2.7. Luciferase Assay
2.8. ELISA
2.9. Statistical Analysis
3. Results
3.1. Ti Particles Induce Inflammation in FLS
3.2. Ti Particles Induced the Expression of Pro-Inflammatory Cytokines in FLS
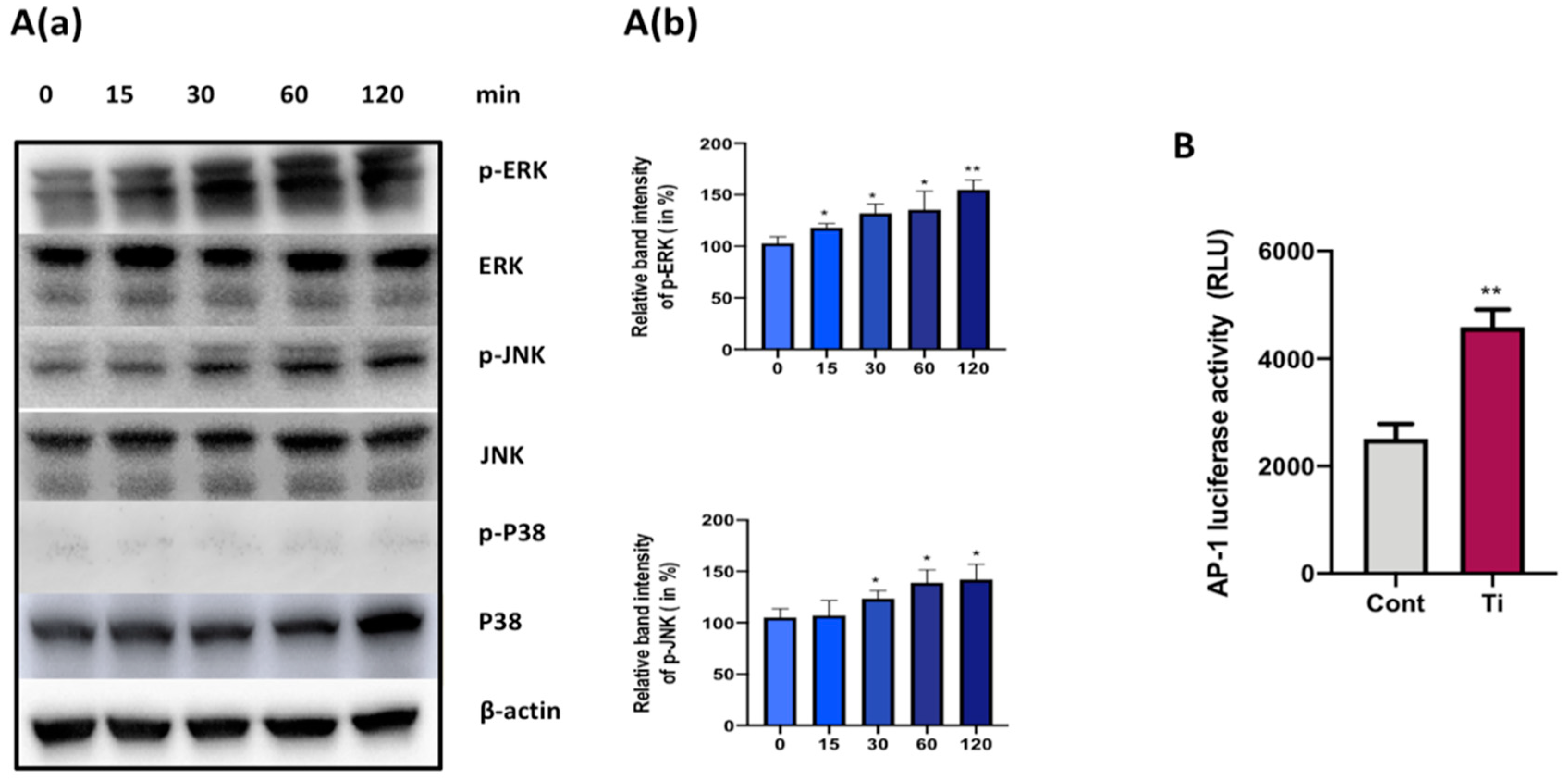
3.3. Ti Particles Activate ERK and JNK Signaling Pathways in FLS
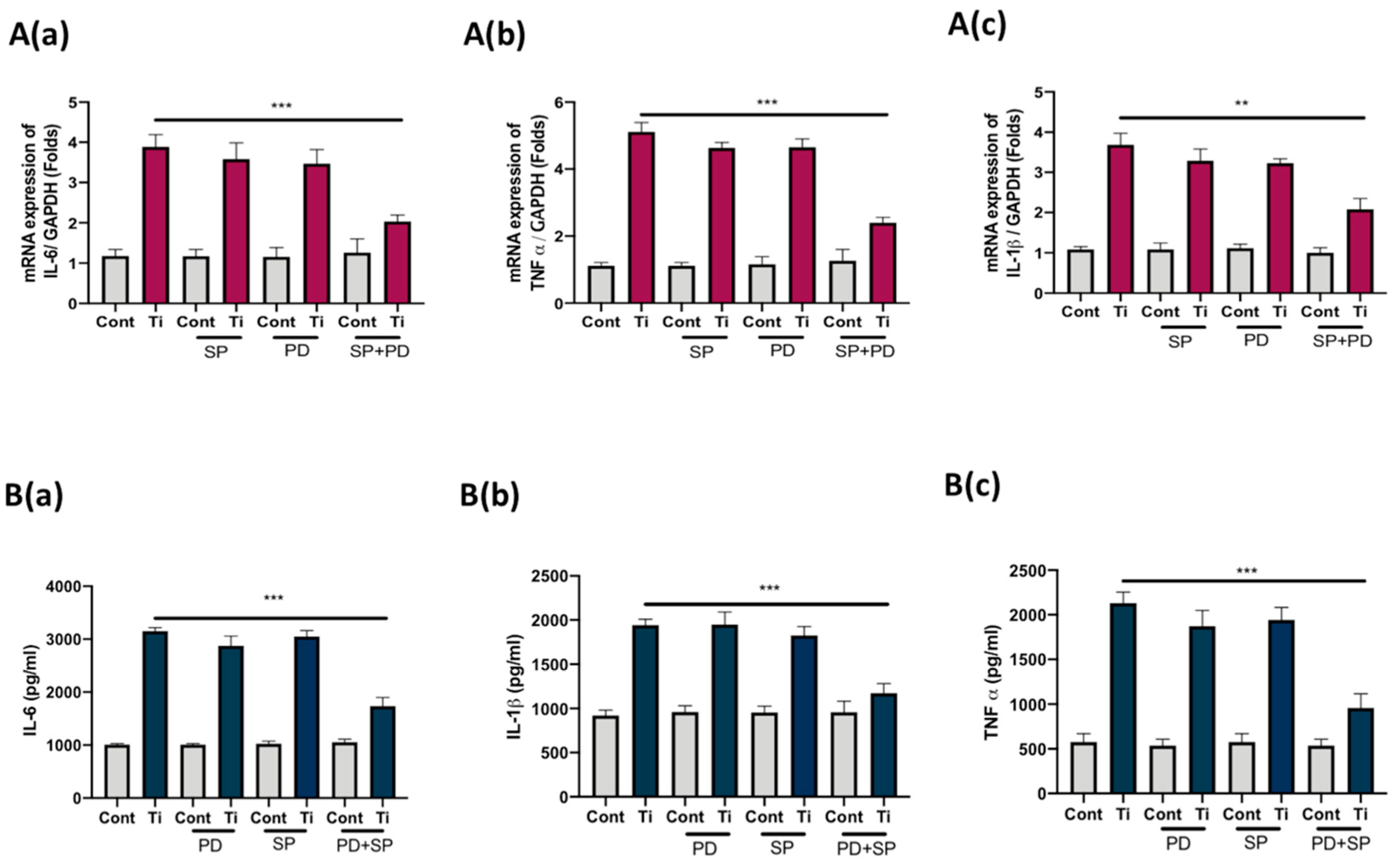
3.4. Co-Inhibition of ERK and JNK Signaling Pathways Suppressed Secretion of IL-6, IL1β, and TNFα from FLS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wroblewski, B.M.; Fleming, P.A.; Siney, P.D. Charnley low-frictional torque arthroplasty of the hip. 20-to-30 year results. J. Bone Jt. Surg. Br. Vol. 1999, 81, 427–430. [Google Scholar] [CrossRef]
- Shon, W.Y.; Park, B.Y.; Rajsankar, N.R.; Park, P.S.; Im, J.T.; Yun, H.H. Total Hip Arthroplasty: Past, Present, and Future. What Has Been Achieved? Hip Pelvis 2019, 31, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Tschon, M.; Fini, M. Gene Expression in Osteolysis: Review on the Identification of Altered Molecular Pathways in Preclinical and Clinical Studies. Int. J. Mol. Sci. 2017, 18, 499. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.; Kim, J.S. Osteolysis in well-functioning fixed- and mobile-bearing TKAs in younger patients. Clin. Orthop. Relat. Res. 2010, 468, 3084–3093. [Google Scholar] [CrossRef]
- Fraser, J.F.; Werner, S.; Jacofsky, D.J. Wear and loosening in total knee arthroplasty: A quick review. J. Knee Surg. 2015, 28, 139–144. [Google Scholar]
- Goodman, S.B.; Gibon, E.; Yao, Z. The basic science of periprosthetic osteolysis. Instr. Course Lect. 2013, 62, 201–206. [Google Scholar]
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef]
- Xu, S.; Lim, W.J.; Chen, J.Y.; Lo, N.N.; Chia, S.L.; Tay, D.K.J.; Hao, Y.; Yeo, S.J. The influence of obesity on clinical outcomes of fixed-bearing unicompartmental knee arthroplasty: A ten-year follow-up study. Bone Jt. J. 2019, 101, 213–220. [Google Scholar] [CrossRef]
- Maloney, W.; Rosenberg, A. What is the outcome of treatment for osteolysis? J. Am. Acad. Orthop. Surg. 2008, 16 (Suppl. S1), S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. Osteolysis and particle disease in hip replacement. A review. Acta Orthop. Scand. 1994, 65, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Jagga, S.; Sharma, A.R.; Bhattacharya, M.; Chakraborty, C.; Lee, S.S. Influence of single nucleotide polymorphisms (SNPs) in genetic susceptibility towards periprosthetic osteolysis. Genes Genom. 2019, 41, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Lee, F.Y.I.; Konttinen, Y.; Wilkinson, J.M.; Smith, R.L. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J. Am. Acad. Orthop. Surg. 2008, 16 (Suppl. S1), S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, T.H.; Chong, A.C.; Bai, L.; Yu, H.; Gong, W.; Wooley, P.H.; Yang, S.Y. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther. 2010, 17, 1262–1269. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Raska, M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013, 19, 213–224. [Google Scholar] [CrossRef]
- Purdue, P.E.; Koulouvaris, P.; Nestor, B.J.; Sculco, T.P. The central role of wear debris in periprosthetic osteolysis. Hss J. Musculoskelet. J. Hosp. Spec. Surg. 2006, 2, 102–113. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef]
- Broeren, M.G.A.; Waterborg, C.E.J.; Wiegertjes, R.; Thurlings, R.M.; Koenders, M.I.; Van Lent, P.; Van der Kraan, P.M.; Van de Loo, F.A.J. A three-dimensional model to study human synovial pathology. ALTEX 2019, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, K.; Qiu, L.; Li, S.; Niu, H.; Hao, M.; Yang, S.; Zhao, Z.; Lai, Y.; Anderson, J.L.; et al. Critical role of AKT protein in myeloma-induced osteoclast formation and osteolysis. J. Biol. Chem. 2013, 288, 30399–30410. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Buly, R.L.; Huo, M.H.; Salvati, E.; Brien, W.; Bansal, M. Titanium wear debris in failed cemented total hip arthroplasty. An analysis of 71 cases. J. Arthroplast. 1992, 7, 315–323. [Google Scholar] [CrossRef]
- Nam, J.S.; Jagga, S.; Sharma, A.R.; Lee, J.H.; Park, J.B.; Jung, J.S.; Lee, S.S. Anti-inflammatory effects of traditional mixed extract of medicinal herbs (MEMH) on monosodium urate crystal-induced gouty arthritis. Chin. J. Nat. Med. 2017, 15, 561–575. [Google Scholar] [CrossRef]
- Chang, J.H.; Lee, K.J.; Kim, S.K.; Yoo, D.H.; Kang, T.Y. Validity of SW982 synovial cell line for studying the drugs against rheumatoid arthritis in fluvastatin-induced apoptosis signaling model. Indian J. Med Res. 2014, 139, 117–124. [Google Scholar]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A review of UHMWPE wear-induced osteolysis: The role for early detection of the immune response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef]
- Greidanus, N.V.; Peterson, R.C.; Masri, B.A.; Garbuz, D.S. Quality of Life Outcomes in Revision Versus Primary Total Knee Arthroplasty. J. Arthroplast. 2011, 26, 615–620. [Google Scholar] [CrossRef]
- Korovessis, P.; Repanti, M. Evolution of aggressive granulomatous periprosthetic lesions in cemented hip arthroplasties. Clin. Orthop. Relat. Res. 1994, 300, 155–161. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Roebuck, K.A.; Archibeck, M.; Hallab, N.J.; Glant, T.T. Osteolysis: Basic science. Clin. Orthop. Relat. Res. 2001, 393, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.J.; Anoushiravani, A.A.; Sayeed, Z.; Chambers, M.C.; El-Othmani, M.M.; Saleh, K.J. Osteolysis Complicating Total Knee Arthroplasty. JBJS Rev. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Koreny, T.; Tunyogi-Csapo, M.; Gal, I.; Vermes, C.; Jacobs, J.J.; Glant, T.T. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006, 54, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Niida, S.; Yasunaga, Y.; Yamasaki, A.; Ochi, M. Wear debris stimulates bone-resorbing factor expression in the fibroblasts and osteoblasts. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2011, 21, 231–237. [Google Scholar]
- Park, J.B.; Duong, C.T.; Chang, H.G.; Sharma, A.R.; Thompson, M.S.; Park, S.; Kwak, B.C.; Kim, T.Y.; Lee, S.S. Role of hyaluronic acid and phospholipid in the lubrication of a cobalt-chromium head for total hip arthroplasty. Biointerphases 2014, 9, 031007. [Google Scholar] [CrossRef]
- Nam, J.S.; Sharma, A.R.; Jagga, S.; Lee, D.H.; Sharma, G.; Nguyen, L.T.; Lee, Y.H.; Chang, J.D.; Chakraborty, C.; Lee, S.S. Suppression of osteogenic activity by regulation of WNT and BMP signaling during titanium particle induced osteolysis. J. Biomed. Mater. Res. Part. A 2017, 105, 912–926. [Google Scholar] [CrossRef]
- Seo, S.W.; Lee, D.; Cho, S.K.; Kim, A.D.; Minematsu, H.; Celil Aydemir, A.B.; Geller, J.A.; Macaulay, W.; Yang, J.; Lee, F.Y. ERK signaling regulates macrophage colony-stimulating factor expression induced by titanium particles in MC3T3.E1 murine calvarial preosteoblastic cells. Ann. New York Acad. Sci. 2007, 1117, 151–158. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Y.; Wang, Q.; Sun, Y.; Xiong, C.; Cao, L.; Wang, B.; Bao, N.; Zhao, J. Tumor necrosis factor-like weak inducer of apoptosis regulates particle-induced inflammatory osteolysis via the p38 mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2015, 12, 1499–1505. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Li, X.; Wang, J.; Yan, L.; Zhang, Z.; Wang, D.; Dai, J.; He, J.; Wang, S. Scutellarin inhibits RANKL-mediated osteoclastogenesis and titanium particle-induced osteolysis via suppression of NF-kappaB and MAPK signaling pathway. Int. Immunopharmacol. 2016, 40, 458–465. [Google Scholar] [CrossRef]
- Lee, H.G.; Minematsu, H.; Kim, K.O.; Celil Aydemir, A.B.; Shin, M.J.; Nizami, S.A.; Chung, K.J.; Hsu, A.C.; Jacobs, C.R.; Lee, F.Y. Actin and ERK1/2-CEBPbeta signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells. Biomaterials 2011, 32, 9197–9206. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, C.; Dai, H.; Li, J.; Liu, W.; Luo, Y.; Zhang, X.; Wang, Q. Crocin inhibits titanium particle-induced inflammation and promotes osteogenesis by regulating macrophage polarization. Int. Immunopharmacol. 2019, 76, 105865. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, Y.; Mao, X.; Zhang, X. Inhibition of p38 mitogen-activated protein kinase down-regulates the inflammatory osteolysis response to titanium particles in a murine osteolysis model. Inflammation 2012, 35, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Sharma, A.R.; Choi, B.S.; Jung, J.S.; Chang, J.D.; Park, S.; Salvati, E.A.; Purdue, E.P.; Song, D.K.; Nam, J.S. The effect of TNFalpha secreted from macrophages activated by titanium particles on osteogenic activity regulated by WNT/BMP signaling in osteoprogenitor cells. Biomaterials 2012, 33, 4251–4263. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, D.S.; Ly, K.; Sengupta, T.K.; Nestor, B.J.; Sculco, T.P.; Ivashkiv, L.B.; Purdue, P.E. Wear debris inhibition of anti-osteoclastogenic signaling by interleukin-6 and interferon-gamma. Mechanistic insights and implications for periprosthetic osteolysis. J. Bone Jt. Surg. Am. Vol. 2006, 88, 788–799. [Google Scholar]
- Kitanaka, T.; Nakano, R.; Kitanaka, N.; Kimura, T.; Okabayashi, K.; Narita, T.; Sugiya, H. JNK activation is essential for activation of MEK/ERK signaling in IL-1beta-induced COX-2 expression in synovial fibroblasts. Sci. Rep. 2017, 7, 39914. [Google Scholar] [CrossRef]


| Target | Forward Primer (5′–>3′) | Reverse primer (3′–>5′) |
|---|---|---|
| Cox-2 | CCAAATCCTTGCTGTTCCCACCCAT | GTGCACTGTGTTTGGAGTGGGTTT |
| IL-6 | CCAGCTATGAACTCCTTCTC | GCTTGTTCCTCACATCTCTC |
| IL-8 | AAGAAACCACCGGAAGGAACCATCT | AGAGCTGCAGAAATCAGGAAGGCT |
| IL-11 | AGATATCCTGACATTGGCCAGGCA | ACTTCAGTGATCCACTCGCTTCGT |
| IL-1β | AACCAGGCTGCTCTGGGATTCTCTT | ATTTCACTGGCGAGCTCAGGTACT |
| TNF-α | AAGGACGAACATCCAACCTTCCCAA | TTTGAGCCAGAAGAGGTTGAGGGT |
| GAPDH | TCGACAGTCAGCCGCATCTTCTTT | ACCAAATCCGTTGACTCCGACCTT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.R.; Jagga, S.; Chakraborty, C.; Lee, S.-S. Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis. Materials 2020, 13, 3628. https://doi.org/10.3390/ma13163628
Sharma AR, Jagga S, Chakraborty C, Lee S-S. Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis. Materials. 2020; 13(16):3628. https://doi.org/10.3390/ma13163628
Chicago/Turabian StyleSharma, Ashish Ranjan, Supriya Jagga, Chiranjib Chakraborty, and Sang-Soo Lee. 2020. "Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis" Materials 13, no. 16: 3628. https://doi.org/10.3390/ma13163628
APA StyleSharma, A. R., Jagga, S., Chakraborty, C., & Lee, S.-S. (2020). Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis. Materials, 13(16), 3628. https://doi.org/10.3390/ma13163628






