In Vitro Study of the Interaction of Innate Immune Cells with Liquid Silicone Rubber Coated with Zwitterionic Methyl Methacrylate and Thermoplastic Polyurethanes
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer Functionalization
2.2. Endotoxin Measurement
2.3. Human Monocyte Isolation and Cultivation
2.4. Adhesion and Morphology of Monocytes/Macrophages
2.5. Viability and Metabolic Activity
2.6. Cytokine Quantification
2.7. Gene Expression Quantification
2.8. Statistics
3. Results
3.1. Decreased Monocyte Adhesion on Thermoplastic Polyurethane (TPU) and Liquid Silicone Rubber (LSR) with Only Minor Effects on Cell Viability and Metabolic Activity
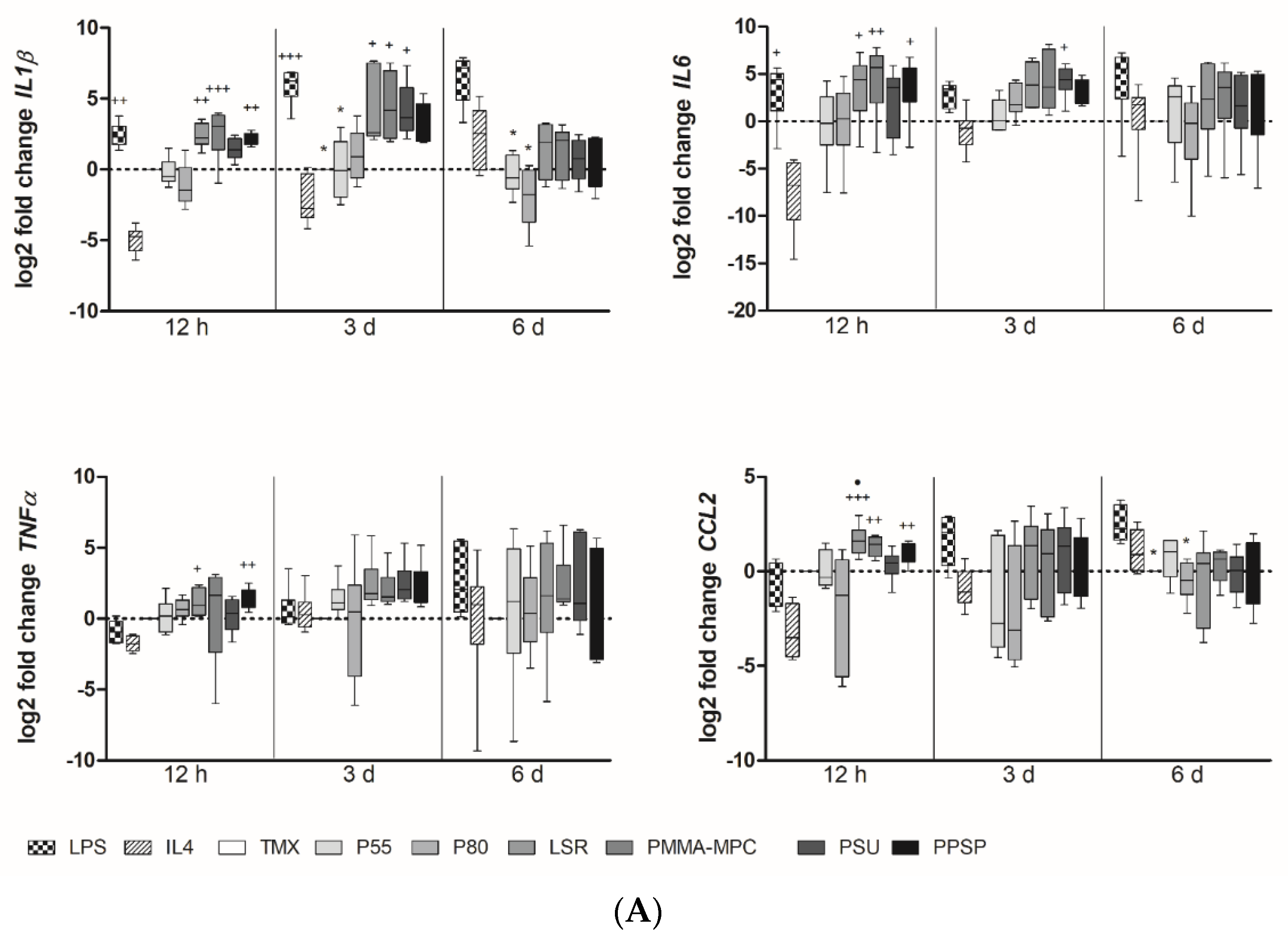
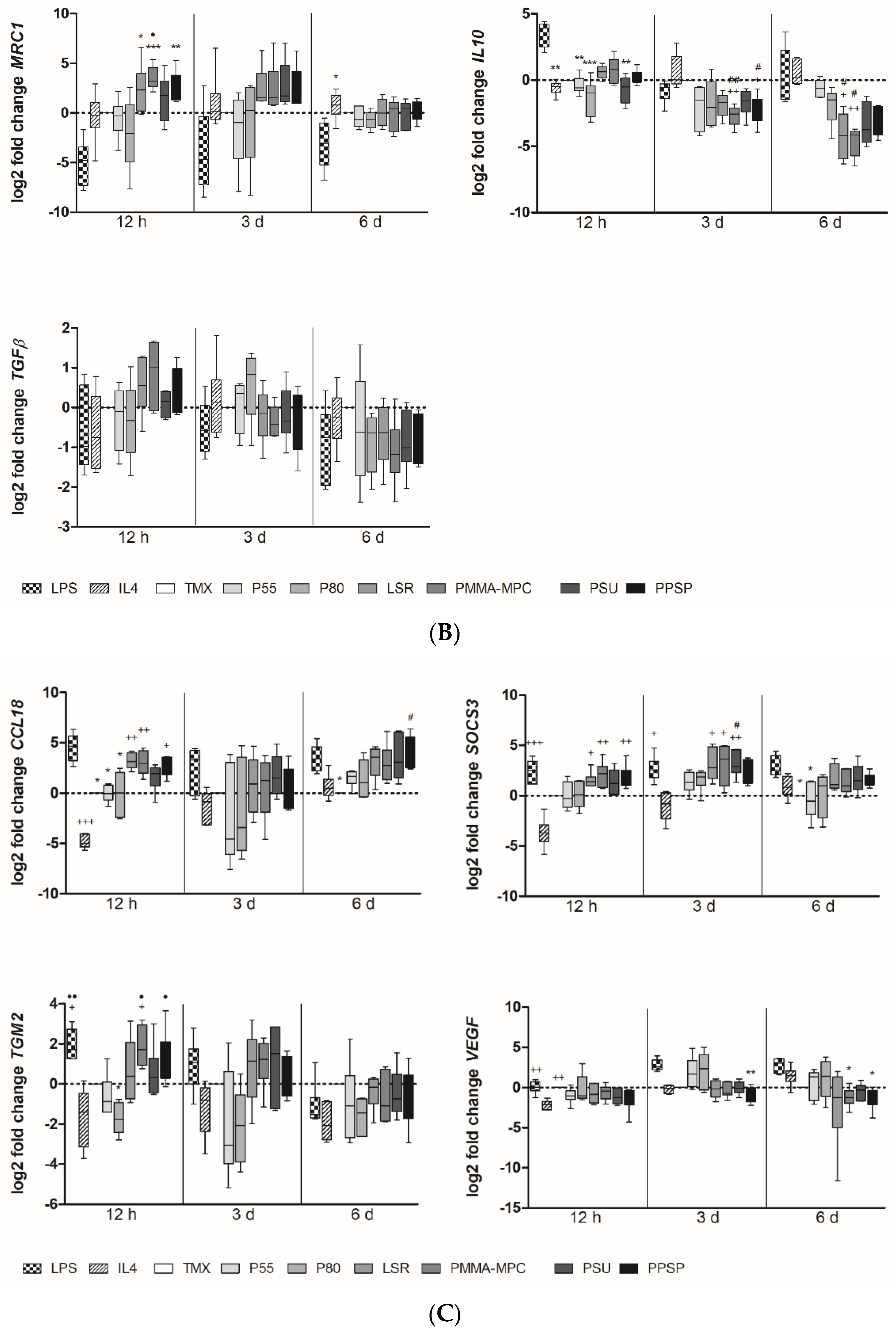
3.2. Unmodified and Modified Liquid Silicon Rubbers (LSR) Trigger an Unpolarized Immune Response
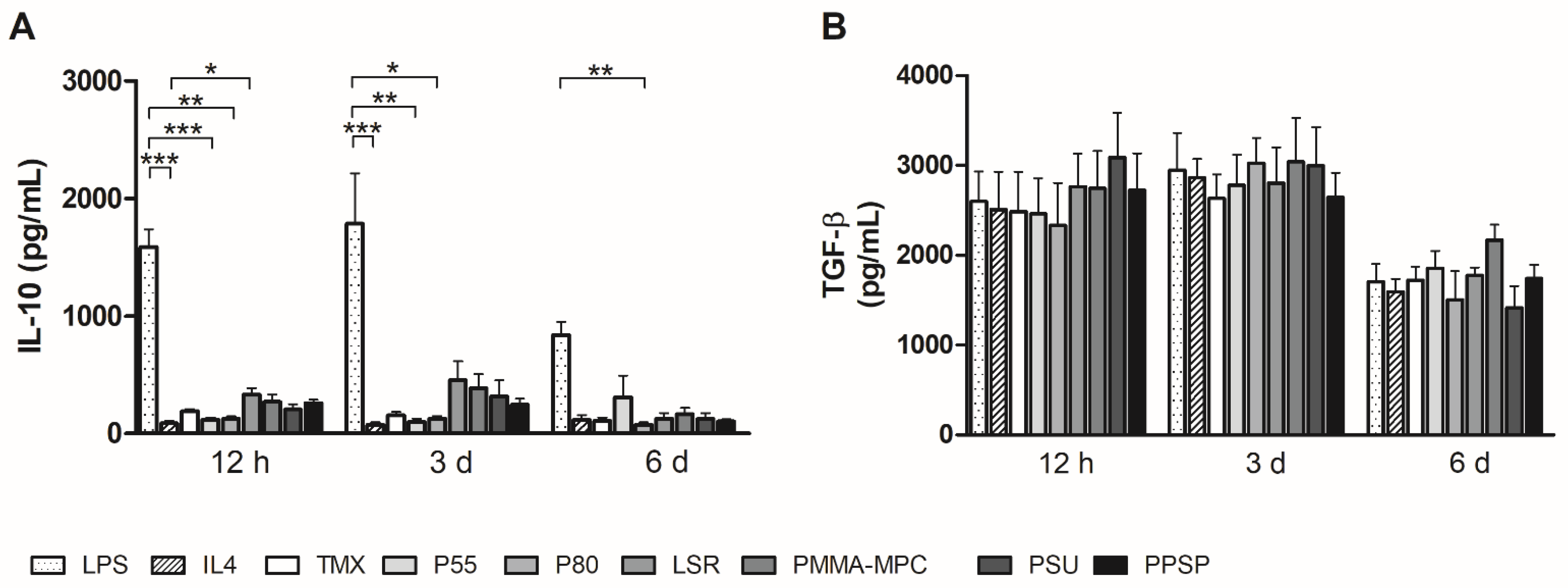
3.3. Unmodified and Modified Liquid Silicone Rubber (LSR) Stimulates Macrophages to Ssecrete Proinflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polym. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Schutte, R.J.; Parisi-Amon, A.; Reichert, W.M. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J. Biomed. Mater. Res. A 2009, 88, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Ziats, N.P.; Miller, K.M.; Anderson, J.M. In vitro and in vivo interactions of cells with biomaterials. Biomaterials 1988, 9, 5–13. [Google Scholar] [CrossRef]
- DeFife, K.M.; Yun, J.K.; Azeez, A.; Stack, S.; Ishihara, K.; Nakabayashi, N.; Colton, E.; Anderson, J.M. Adhesion and cytokine production by monocytes on poly(2-methacryloyloxyethyl phosphorylcholine-co-alkyl methacrylate)-coated polymers. J. Biomed. Mater. Res. 1995, 29, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Gu, H.; Luan, H.; Mo, Z.; Song, I.; Fan, Y. Biological and Physical Properties of a Modification Silicone Liner. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 12110. [Google Scholar] [CrossRef]
- Luis, E.; Pan, H.M.; Bastola, A.K.; Bajpai, R.; Sing, S.L.; Song, J.; Yeong, W.Y. 3D Printed Silicone Meniscus Implants: Influence of the 3D Printing Process on Properties of Silicone Implants. Polymers 2020, 12, 2136. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long Term Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.; Tamayo, L.; Gulppi, M.; Rabagliati, F.; Flores, M.; Urzúa, M.; Azócar, M.; Zagal, J.H.; Encinas, M.V.; Zhou, X.; et al. Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles. Molecules 2018, 23, 2747. [Google Scholar] [CrossRef]
- Shibata, Y.; Yamashita, Y.; Tsuru, K.; Ishihara, K.; Fukazawa, K.; Ishikawa, K. Preventive effects of a phospholipid polymer coating on PMMA on biofilm formation by oral streptococci. Appl. Surf. Sci. 2016, 390, 602–607. [Google Scholar] [CrossRef]
- Azari, S.; Zou, L. Fouling resistant zwitterionic surface modification of reverse osmosis membranes using amino acid l-cysteine. Desalination 2013, 324, 79–86. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Kojima, M.; Ishihara, K.; Watanabe, A.; Nakabayashi, N. Interaction between phospholipids and biocompatible polymers containing a phosphorylcholine moiety. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Amsterdam, The Netherlands, 1991; pp. 69–72. ISBN 9780080451541. [Google Scholar]
- Ishihara, K.; Tsuji, T.; Kurosaki, T.; Nakabayashi, N. Hemocompatibility on graft copolymers composed of poly(2-methacryloyloxyethyl phosphorylcholine) side chain and poly(n-butyl methacrylate) backbone. J. Biomed. Mater. Res. 1994, 28, 225–232. [Google Scholar] [CrossRef]
- Ishihara, K.; Fukumoto, K.; Iwasaki, Y.; Nakabayashi, N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials 1999, 20, 1553–1559. [Google Scholar] [CrossRef]
- Woitschach, F.; Kloss, M.; Schlodder, K.; Rabes, A.; Mörke, C.; Oschatz, S.; Senz, V.; Borck, A.; Grabow, N.; Reisinger, E.C.; et al. The Use of Zwitterionic Methylmethacrylat Coated Silicone Inhibits Bacterial Adhesion and Biofilm Formation of Staphylococcus aureus. Front. Bioeng. Biotechnol. 2021, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef]
- Maheswari, P.; Barghava, P.; Mohan, D. Preparation, morphology, hydrophilicity and performance of poly (ether-ether-sulfone) incorporated cellulose acetate ultrafiltration membranes. J. Polym. Res. 2013, 20, 1–17. [Google Scholar] [CrossRef]
- Feldman, D.L.; Mogelesky, T.C. Use of Histopaque for isolating mononuclear cells from rabbit blood. J. Immunol. Methods 1987, 102, 243–249. [Google Scholar] [CrossRef]
- Johnston, L.; Harding, S.A.; La Flamme, A.C. Comparing methods for ex vivo characterization of human monocyte phenotypes and in vitro responses. Immunobiology 2015, 220, 1305–1310. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Bonder, C.S.; Hart, P.H.; Davies, K.V.; Burkly, L.C.; Finlay-Jones, J.J.; Woodcock, J.M. Characterization of IL-4 receptor components expressed on monocytes and monocyte-derived macrophages: Variation associated with differential signaling by IL-4. Growth Factors 2001, 19, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.A.; Baganizi, D.R.; Sahu, R.; Singh, S.R.; Dennis, V.A. SOCS Proteins as Regulators of Inflammatory Responses Induced by Bacterial Infections: A Review. Front. Microbiol. 2017, 8, 2431. [Google Scholar] [CrossRef]
- Vijaya Bhaskar, T.B.; Ma, N.; Lendlein, A.; Roch, T. The interaction of human macrophage subsets with silicone as a biomaterial. Clin. Hemorheol. Microcirc. 2015, 61, 119–133. [Google Scholar] [CrossRef]
- Qin, X.-H.; Senturk, B.; Valentin, J.; Malheiro, V.; Fortunato, G.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Cell-Membrane-Inspired Silicone Interfaces that Mitigate Proinflammatory Macrophage Activation and Bacterial Adhesion. Langmuir 2019, 35, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.E.; Whyte, C.S.; Gordon, P.; Barker, R.N.; Rees, A.J.; Wilson, H.M. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology 2014, 141, 96–110. [Google Scholar] [CrossRef]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.-D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef]
- Song, C.-L.; Li, Q.; Yu, Y.-P.; Wang, G.; Wang, J.-P.; Lu, Y.; Zhang, J.-C.; Diao, H.-Y.; Liu, J.-G.; Liu, Y.-H.; et al. Study of novel coating strategy for coronary stents: Simutaneous coating of VEGF and anti-CD34 antibody. Rev. Bras. Cir. Cardiovasc. 2015, 30, 159–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, Y.J.; Kim, K.-C.; Heo, J.-Y.; Jing, K.; Lee, K.E.; Hwang, J.S.; Lim, K.; Jo, D.-Y.; Ahn, J.P.; Kim, J.-M.; et al. Induction of Angiogenesis by Matrigel Coating of VEGF-Loaded PEG/PCL-Based Hydrogel Scaffolds for hBMSC Transplantation. Mol. Cells 2015, 38, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Aubin, H.; Steinbrink, M.; Schiffer, F.; Assmann, A.; Weisel, R.D.; Matsui, Y.; Li, R.-K.; Lichtenberg, A.; Akhyari, P. Bioactive coating of decellularized vascular grafts with a temperature-sensitive VEGF-conjugated hydrogel accelerates autologous endothelialization in vivo. J. Tissue Eng. Regen. Med. 2018, 12, e513–e522. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woitschach, F.; Kloss, M.; Schlodder, K.; Borck, A.; Grabow, N.; Reisinger, E.C.; Sombetzki, M. In Vitro Study of the Interaction of Innate Immune Cells with Liquid Silicone Rubber Coated with Zwitterionic Methyl Methacrylate and Thermoplastic Polyurethanes. Materials 2021, 14, 5972. https://doi.org/10.3390/ma14205972
Woitschach F, Kloss M, Schlodder K, Borck A, Grabow N, Reisinger EC, Sombetzki M. In Vitro Study of the Interaction of Innate Immune Cells with Liquid Silicone Rubber Coated with Zwitterionic Methyl Methacrylate and Thermoplastic Polyurethanes. Materials. 2021; 14(20):5972. https://doi.org/10.3390/ma14205972
Chicago/Turabian StyleWoitschach, Franziska, Marlen Kloss, Karsten Schlodder, Alexander Borck, Niels Grabow, Emil C. Reisinger, and Martina Sombetzki. 2021. "In Vitro Study of the Interaction of Innate Immune Cells with Liquid Silicone Rubber Coated with Zwitterionic Methyl Methacrylate and Thermoplastic Polyurethanes" Materials 14, no. 20: 5972. https://doi.org/10.3390/ma14205972
APA StyleWoitschach, F., Kloss, M., Schlodder, K., Borck, A., Grabow, N., Reisinger, E. C., & Sombetzki, M. (2021). In Vitro Study of the Interaction of Innate Immune Cells with Liquid Silicone Rubber Coated with Zwitterionic Methyl Methacrylate and Thermoplastic Polyurethanes. Materials, 14(20), 5972. https://doi.org/10.3390/ma14205972






