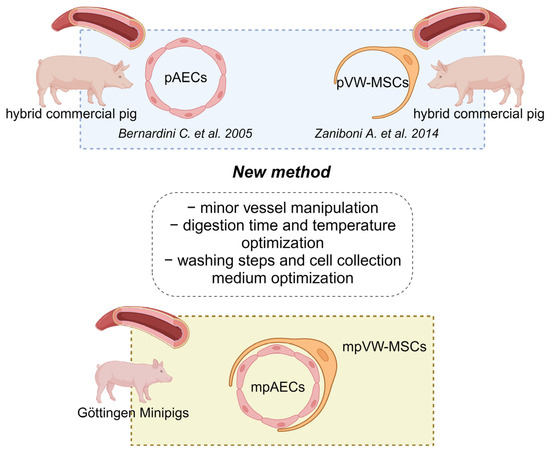
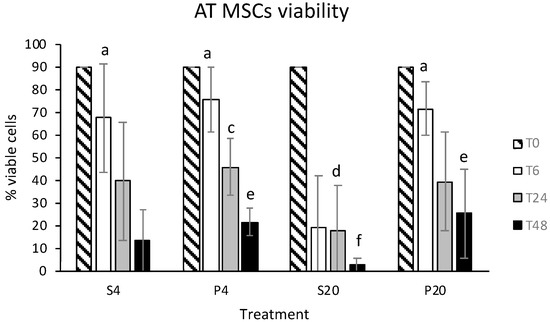
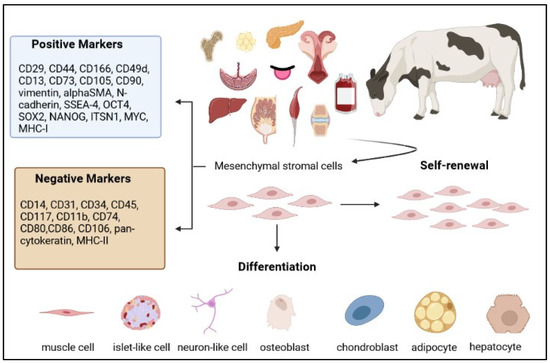
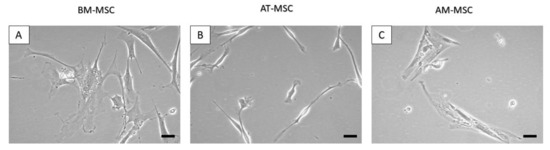
Stem Cells in Domestic Animals: Applications in Health and Production
A topical collection in Animals (ISSN 2076-2615). This collection belongs to the section "Veterinary Clinical Studies".
Viewed by 81259Editors
Interests: mesenchymal stromal cells; veterinary cell therapy; assisted reproduction; cell banking; animal reproduction
Special Issues, Collections and Topics in MDPI journals
Interests: regenerative medicine, mesenchymal stromal cells; cryopreservation; animal reproduction; fertility; assisted reproductive techniques
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
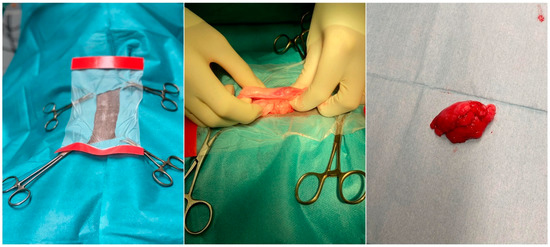
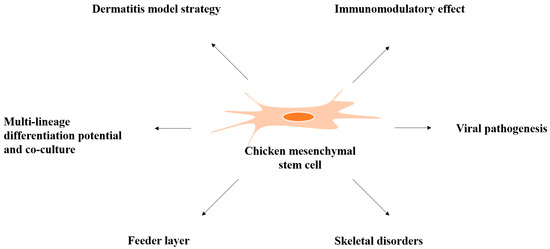
Over the past decade, stem cell research has emerged as an area of major interest for its potential applications, both in human and veterinary medicine. The effective management of companion and sport animals requires sophisticated new treatments and preventive strategies. Furthermore, also in livestock species, stem cell therapy could be used to treat several medical conditions that negatively affect meat and milk production or reproductive efficiency. In the era of antibiotic resistance, stem cells modified with therapeutic agents might be employed to combat mastitis in dairy cow, sheep, goat or buffalo, with enormous benefits also for human health. Reproductive performances could be improved by the integration of stem cells into the in vitro embryo production system, representing in another way the importance of stem cells in addressing commercial goals. Moreover, the importance of domestic animals as models for human diseases has been recognized. The quantity of tissue available for decellularization is greater than that in small animal models such as mice, and larger animal species more closely resembling some human behavior can be used for translational applications in cell therapy. Domestic animals are also models for stem cell secretome studies and clinical trials.
Prof. Dr. Eleonora Iacono
Dr. Barbara Merlo
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Animals is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Stem cells
- Domestic animals
- Cellular therapy
- Translational medicine
- Cell culture
- Secretome